Abstract
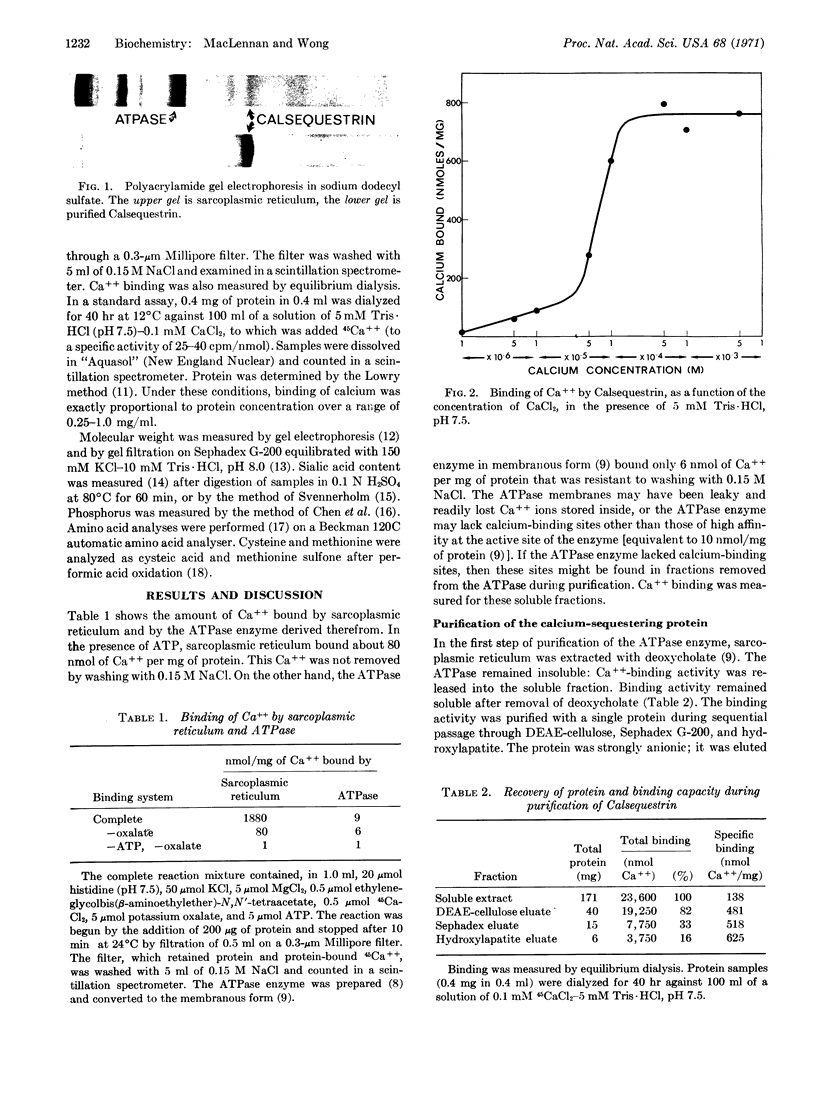
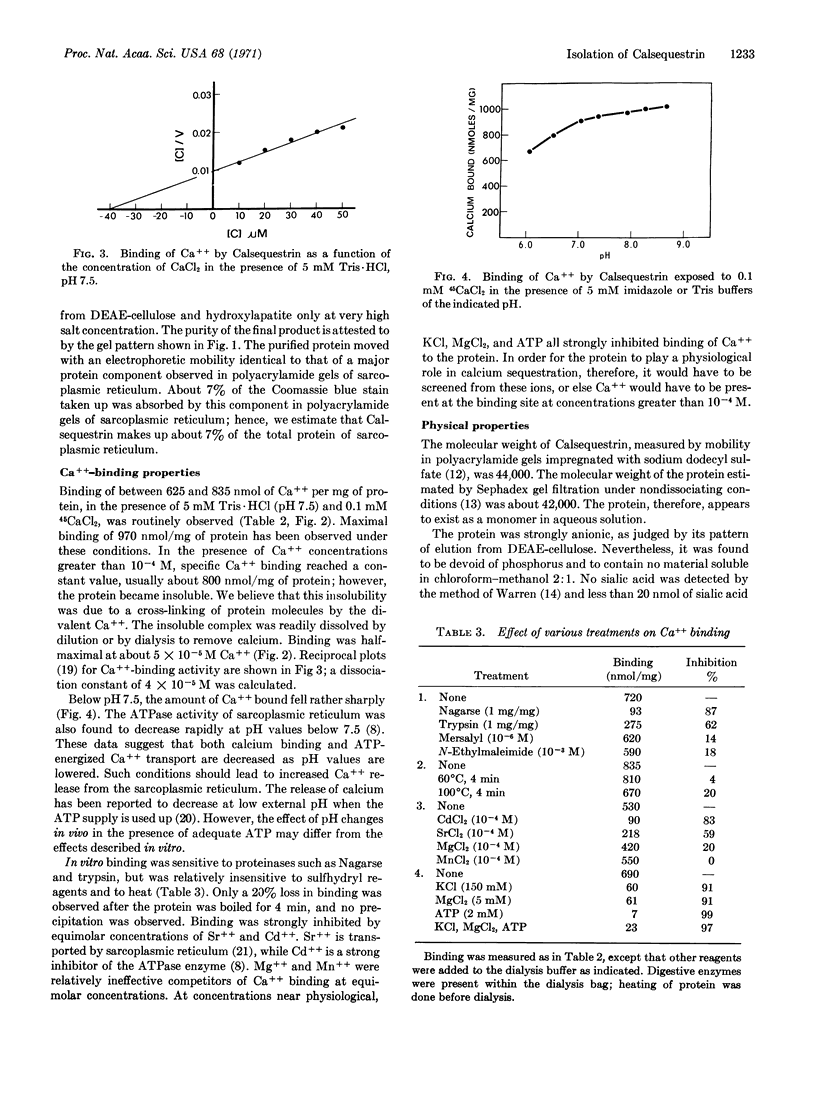
An acidic protein has been extracted from sarcoplasmic reticulum with KCl and deoxycholate. The protein, which remains soluble after extraction, has been highly purified by fractionation on DEAE-cellulose, Sephadex, and hydroxylaptite. It has a molecular weight of 44,000 and contains 392 amino acid residues per molecule, of which 146 are either glutamic or aspartic acid. No phosphorus, sialic acid, or lipid has been detected in the preparation. The protein has been shown to bind up to 970 nmol of Ca++ per mg (43 mol/mol) at pH 7.5, with an apparent dissociation constant of 4 × 10-5 M. Preliminary data indicate that the protein is unique to sarcoplasmic reticulum and that it is hydrophobically bonded on the interior of these vesicles. The protein is believed to play a role in sequestering calcium within sarcoplasmic reticulum. The name Calsequestrin is suggested for the protein.
Keywords: rabbit, deoxycholate, column chromatography, transport
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews P. Estimation of the molecular weights of proteins by Sephadex gel-filtration. Biochem J. 1964 May;91(2):222–233. doi: 10.1042/bj0910222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho A. P. Binding of cations by microsomes from rabbit skeletal muscle. J Cell Physiol. 1966 Feb;67(1):73–83. doi: 10.1002/jcp.1040670109. [DOI] [PubMed] [Google Scholar]
- Carvalho A. P., Leo B. Effects of ATP on the interaction of Ca++, Mg++, and K+ with fragmented sarcoplasmic reticulum isolated from rabbit skeletal muscle. J Gen Physiol. 1967 May;50(5):1327–1352. doi: 10.1085/jgp.50.5.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen A., Selinger Z. Calcium binding properties of sarcoplasmic reticulum membranes. Biochim Biophys Acta. 1969 Jun 3;183(1):27–35. doi: 10.1016/0005-2736(69)90126-6. [DOI] [PubMed] [Google Scholar]
- Ebashi S., Endo M. Calcium ion and muscle contraction. Prog Biophys Mol Biol. 1968;18:123–183. doi: 10.1016/0079-6107(68)90023-0. [DOI] [PubMed] [Google Scholar]
- Ebashi S., Lipmann F. ADENOSINE TRIPHOSPHATE-LINKED CONCENTRATION OF CALCIUM IONS IN A PARTICULATE FRACTION OF RABBIT MUSCLE. J Cell Biol. 1962 Sep 1;14(3):389–400. doi: 10.1083/jcb.14.3.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HASSELBACH W., MAKINOSE M. [The calcium pump of the "relaxing granules" of muscle and its dependence on ATP-splitting]. Biochem Z. 1961;333:518–528. [PubMed] [Google Scholar]
- Hanes C. S. Studies on plant amylases: The effect of starch concentration upon the velocity of hydrolysis by the amylase of germinated barley. Biochem J. 1932;26(5):1406–1421. doi: 10.1042/bj0261406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwant W. O., Seeman P. The displacement of membrane calcium by a local anesthetic (chlorpromazine). Biochim Biophys Acta. 1969;193(2):338–349. doi: 10.1016/0005-2736(69)90194-1. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lehninger A. L. A soluble, heat-labile, high-affinity Ca2 plus-binding factor extracted from rat liver mitochondria. Biochem Biophys Res Commun. 1971 Jan 22;42(2):312–318. doi: 10.1016/0006-291x(71)90104-5. [DOI] [PubMed] [Google Scholar]
- MARTONOSI A., FERETOS R. SARCOPLASMIC RETICULUM. II. CORRELATION BETWEEN ADENOSINE TRIPHOSPHATASE ACTIVITY AND CA++ UPTAKE. J Biol Chem. 1964 Feb;239:659–668. [PubMed] [Google Scholar]
- MacLennan D. H. Purification and properties of an adenosine triphosphatase from sarcoplasmic reticulum. J Biol Chem. 1970 Sep 10;245(17):4508–4518. [PubMed] [Google Scholar]
- MacLennan D. H., Seeman P., Iles G. H., Yip C. C. Membrane formation by the adenosine triphosphatase of sarcoplasmic reticulum. J Biol Chem. 1971 Apr 25;246(8):2702–2710. [PubMed] [Google Scholar]
- Nakamaru Y., Schwartz A. Possible control of intracellular calcium metabolism by [H+]: sarcoplasmic reticulum of skeletal and cardiac muscle. Biochem Biophys Res Commun. 1970 Nov 25;41(4):830–836. doi: 10.1016/0006-291x(70)90157-9. [DOI] [PubMed] [Google Scholar]
- Pardee A. B. Membrane transport proteins. Proteins that appear to be parts of membrane transport systems are being isolated and characterized. Science. 1968 Nov 8;162(3854):632–637. doi: 10.1126/science.162.3854.632. [DOI] [PubMed] [Google Scholar]
- Pardee A. B. Purification and properties of a sulfate-binding protein from Salmonella typhimurium. J Biol Chem. 1966 Dec 25;241(24):5886–5892. [PubMed] [Google Scholar]
- Sandow A. Excitation-contraction coupling in skeletal muscle. Pharmacol Rev. 1965 Sep;17(3):265–320. [PubMed] [Google Scholar]
- Sandow A. Skeletal muscle. Annu Rev Physiol. 1970;32:87–138. doi: 10.1146/annurev.ph.32.030170.000511. [DOI] [PubMed] [Google Scholar]
- Sommer J. R., Hasselbach W. The effect of glutaraldehyde and formaldehyde on the calcium pump of the sarcoplasmic reticulum. J Cell Biol. 1967 Sep;34(3):902–905. doi: 10.1083/jcb.34.3.902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WARREN L. The thiobarbituric acid assay of sialic acids. J Biol Chem. 1959 Aug;234(8):1971–1975. [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Yasui B., Fuchs F., Briggs F. N. The role of the sulfhydryl groups of tropomyosin and troponin in the calcium control of actomyosin contractility. J Biol Chem. 1968 Feb 25;243(4):735–742. [PubMed] [Google Scholar]




