Abstract
The intercellular transfer of plasma membrane patches, also known as trogocytosis, has a strong impact on the function and fate of immune cells. We have recently shown that natural killer (NK) cells undergo fratricide following the trogocytosis-mediated acquisition of tumor-derived NKG2D ligands. Malignant cells may therefore employ trogocytosis to escape NKG2D-mediated immune responses.
Keywords: NK cells, NKG2D, trogocytosis
The immune system can operate a multipronged control on tumor progression, implying that malignant cells must develop multiple immunoregulatory strategies to evade antitumor immune responses. An improved understanding of the complex interplay between malignant cells and immune cells is therefore crucial for development of effective anticancer immunotherapies. Natural killer (NK) cells play a pivotal role in cancer immunosurveillance by recognizing and eliminating abnormal cells in an MHC-unrestricted manned and without the need for prior sensitization. The NK cell-mediated killing of neoplastic cells depends on the integration of signals generated by inhibitory and activating receptors. Inhibitory receptors for MHC class I molecules allow NK cells to spare normal cells, whereas NK cells can attack abnormal cells that have lost MHC class I expression upon “missing-self” recognition. Among NK-cell activating receptors, killer cell lectin-like receptor subfamily K, member 1 (KLRK1, best known as NKG2D) is a major mediator of antitumor immune responses through a process that is known as “induced-self” recognition. In this setting, the expression of NKG2D ligands (NKG2DLs) is induced by cellular stress, infection or malignant transformation. Thus, NKG2D can recognize potentially dangerous cells and transmit an activatory signal via the adaptor proteins hematopoietic cell signal transducer (HCST, best known as DAP10), in both humans and mice, and TYRO protein tyrosine kinase binding protein (Tyrobp, best known as DAP12), in mice but not humans. Such a signal stimulates NK cells to produce cytokines and release cytotoxic granules that contain perforin and granzymes. The indispensable role of NKG2D in cancer immunosurveillance has been demonstrated in NKG2D-deficient mice, which show an increased incidence of prostate adenocarcinoma and an accelerated progression of Eμ-myc induced lymphomas.1 However, tumors can develop even when the NKG2D-dependent immunosurveillance system is intact. Indeed, NKG2DLs have been detected in a wide variety of human tumor biopsies, suggesting that neoplastic cells can overwhelm and/or escape from NKG2D-mediated antitumor immunity through several mechanisms.
We have previously demonstrated that NKG2DLs on malignant cells downregulated NKG2D expression on NK cells.2 In human cancer cells, the NKG2DL MHC class I-related molecules A and B (MICA and MICB) can be processed to soluble forms that inhibit NKG2D expression on by cytotoxic T lymphocytes (CTLs).3 However, in this study the downregulation of NKG2D was observed with a concentration of recombinant soluble MICA of 100 ng/mL,3 which is much higher than the average level that this protein attains in the serum of advanced cancer patients. The downregulation of NKG2D is also mediated by other immunosuppressive factors such as transforming growth factor β (TGFβ), which is produced by cancer cells, regulatory T cells and myeloid-derived suppressor cells. This said, the downregulation of NKG2D alone may not completely explain the capacity of cancer cells to evade immunosurveillance. In this regard, some NK cells have been shown to undergo rapid apoptosis upon contacting malignant cells that express NKG2DLs.4 Thus, we hypothesized that the NKG2D-NKG2DL interaction could induce the activation-induced demise of NK cells. At odds with the FAS-dependent activation-induced death of T lymphocytes; however, we could not observe the induction of NKG2DL expression on activated NK cells. Moreover, the antibody-dependent cross-linking of NKG2D did not provoke NK cell death, indicating that NKG2D does not directly transmit a lethal signal. Instead, we found that the death of NK cells is mediated by a fratricidal process triggered by NKG2D-NKG2DL interactions.5 The fratricide of immune cells has first been observed in CTLs. In this setting, upon interaction with antigen-presenting cells (APCs), CTLs can acquire peptide-MHC complexes and hence become susceptible to lysis by neighboring CTLs.6 Likewise, we showed that upon the NKG2D-mediated recognition of cancer cells, NK cells can rapidly acquire NKG2DLs in a cell-to-cell contact-dependent manner via a process recently termed trogocytosis. Thus, NK cells “dressed” with tumor-derived NKG2DLs are rapidly lysed by tumor-naïve NK cells, both in vitro and in vivo (Fig. 1). Because the acquisition of tumor-derived NKG2DLs by NK cells requires the driving force of clathrin-dependent endocytosis,5 we proposed that such an NK-cell fratricide may represent an intentional negative feedback regulation of NK cells. Alternatively, neoplastic cells may provide NKG2DLs to NK cells as part of an immune escape mechanism. It will be important to assess whether the perturbation of NK-cell fratricide affects tumor growth in vivo.
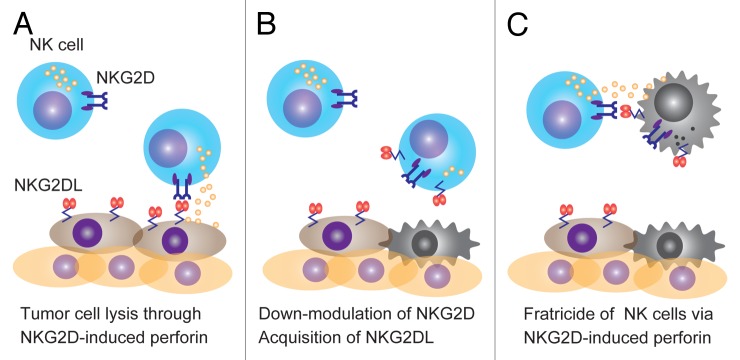
Figure 1. NKG2D-mediated fratricidal regulation of natural killer cells. (A) Natural killer (NK) cells recognize NKG2D ligand (NKG2DL)-expressing malignant cells and attack them with cytotoxic granules containing perforin. (B) NKG2D-NKG2DL interactions result in the downregulation of NKG2D as well as in the rapid acquisition of NKG2DL by NK cells. (C) NKG2DL-dressed NK cells are eliminated by fratricide in an NKG2D- and perforin-dependent manner.
Trogocytosis, the process whereby 2 cells can exchange patches of their plasma membranes, was first described 40 y ago for mouse T cells that acquired MHC class II molecules from B cells. However, the physiological relevance of this phenomenon has remained unclear for many years. Recently, trogocytosis between immune cells has been proposed to regulate immune responses.7,8 For instance, dendritic cells (DCs) can acquire MHC class I molecules from other DCs, and these so-called “cross-dressed” DCs drive the proliferation of memory CD8+ T cells.7 We have also previously demonstrated that NK cell-DC interactions result in MHC class II-dressed NK cells, which suppress T-cell responses by inducing anergy.8 Of note, trogocytosis is not limited to immune cells, as human ovarian cancer cells have been shown to develop chemoresistance by acquiring the P-glycoprotein from stromal cells.9 Thus, trogocytosis provide various cells with novel biological functions that cannot be detected by the analysis of gene expression profiles. Such dynamic intercellular communication may constitutively take place within the tumor microenvironment, a setting in which diverse immune cells accumulate and coordinately create an immunoregulatory network that favors disease progression.10 Upon the contact between cancer and NK cells, the trogocytic transfer of NKG2DLs from the former to the latter could dampen NK cell-mediated antitumor functions by inducing NKG2D downregulation as well as NK-cell fratricide. Further investigation is required to elucidate the molecular mechanisms that underlie trogocytosis-mediated immune regulation in the tumor microenvironment. These studies will provide novel insights into the complex interplay between malignant and immune cells.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Citation: Nakamura K, Nakayama M, Kawano M, Ishii T, Harigae H, Ogasawara K. NK cell-fratricide: Dynamic crosstalk between NK cells and cancer cells. OncoImmunology 2013; 2:e26529; 10.4161/onci.26529
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/26529
References
- 1.Guerra N, Tan YX, Joncker NT, Choy A, Gallardo F, Xiong N, Knoblaugh S, Cado D, Greenberg NM, Raulet DH. NKG2D-deficient mice are defective in tumor surveillance in models of spontaneous malignancy. Immunity. 2008;28:571–80. doi: 10.1016/j.immuni.2008.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ogasawara K, Hamerman JA, Hsin H, Chikuma S, Bour-Jordan H, Chen T, Pertel T, Carnaud C, Bluestone JA, Lanier LL. Impairment of NK cell function by NKG2D modulation in NOD mice. Immunity. 2003;18:41–51. doi: 10.1016/S1074-7613(02)00505-8. [DOI] [PubMed] [Google Scholar]
- 3.Groh V, Wu J, Yee C, Spies T. Tumour-derived soluble MIC ligands impair expression of NKG2D and T-cell activation. Nature. 2002;419:734–8. doi: 10.1038/nature01112. [DOI] [PubMed] [Google Scholar]
- 4.Taga K, Yamauchi A, Kabashima K, Bloom ET, Muller J, Tosato G. Target-induced death by apoptosis in human lymphokine-activated natural killer cells. Blood. 1996;87:2411–8. [PubMed] [Google Scholar]
- 5.Nakamura K, Nakayama M, Kawano M, Amagai R, Ishii T, Harigae H, Ogasawara K. Fratricide of natural killer cells dressed with tumor-derived NKG2D ligand. Proc Natl Acad Sci U S A. 2013;110:9421–6. doi: 10.1073/pnas.1300140110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang JF, Yang Y, Sepulveda H, Shi W, Hwang I, Peterson PA, Jackson MR, Sprent J, Cai Z. TCR-Mediated internalization of peptide-MHC complexes acquired by T cells. Science. 1999;286:952–4. doi: 10.1126/science.286.5441.952. [DOI] [PubMed] [Google Scholar]
- 7.Wakim LM, Bevan MJ. Cross-dressed dendritic cells drive memory CD8+ T-cell activation after viral infection. Nature. 2011;471:629–32. doi: 10.1038/nature09863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nakayama M, Takeda K, Kawano M, Takai T, Ishii N, Ogasawara K. Natural killer (NK)-dendritic cell interactions generate MHC class II-dressed NK cells that regulate CD4+ T cells. Proc Natl Acad Sci U S A. 2011;108:18360–5. doi: 10.1073/pnas.1110584108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rafii A, Mirshahi P, Poupot M, Faussat AM, Simon A, Ducros E, Mery E, Couderc B, Lis R, Capdet J, et al. Oncologic trogocytosis of an original stromal cells induces chemoresistance of ovarian tumours. PLoS One. 2008;3:e3894. doi: 10.1371/journal.pone.0003894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kerkar SP, Restifo NP. Cellular constituents of immune escape within the tumor microenvironment. Cancer Res. 2012;72:3125–30. doi: 10.1158/0008-5472.CAN-11-4094. [DOI] [PMC free article] [PubMed] [Google Scholar]


