Abstract
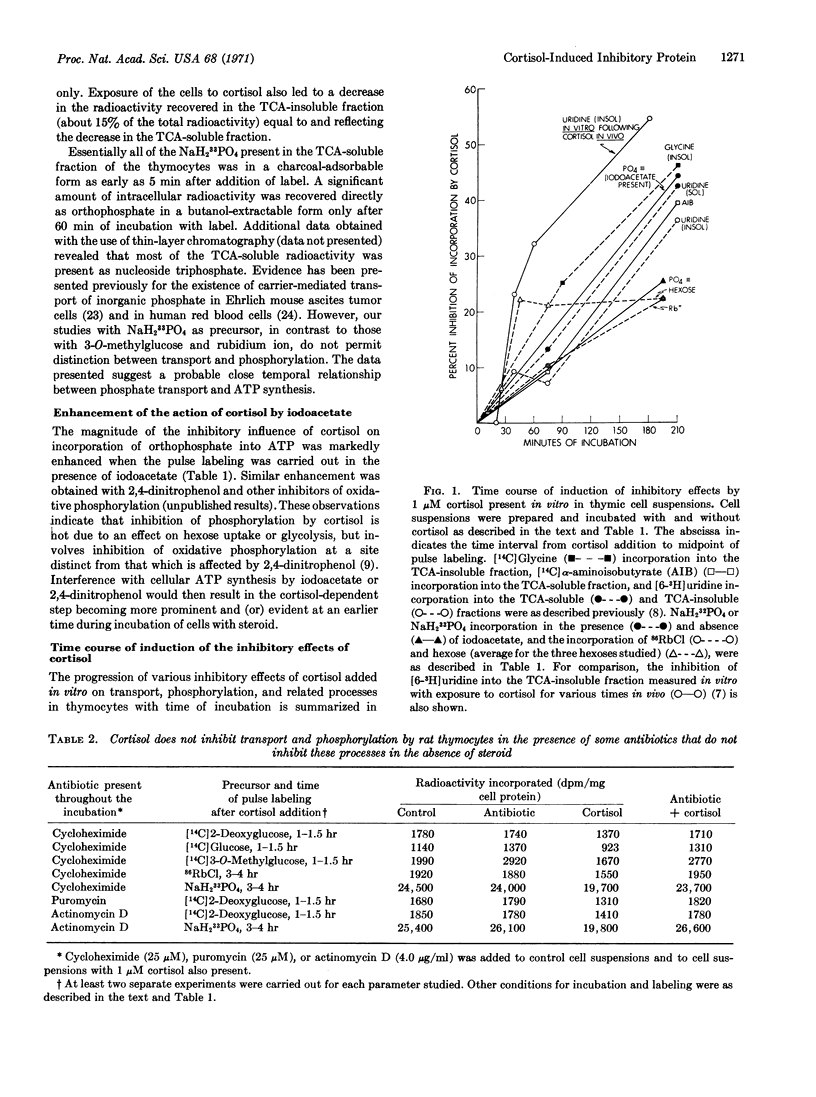
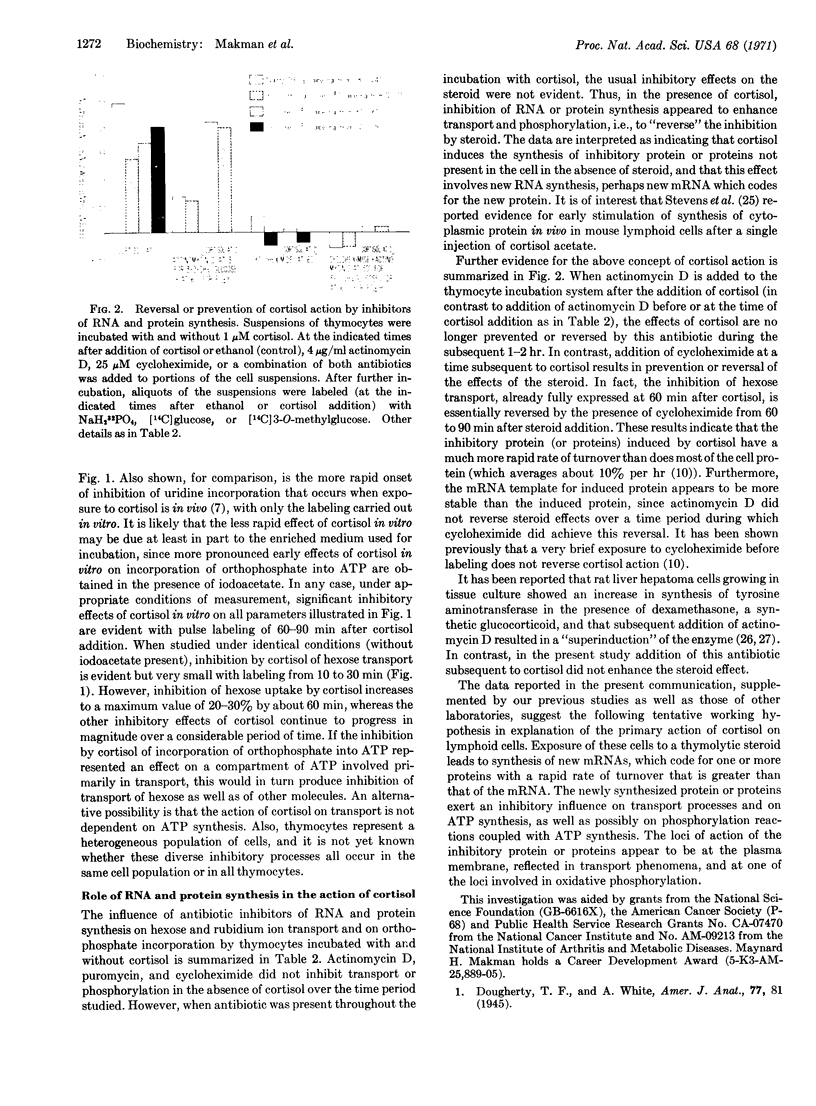
Studies of intact rat thymocytes incubated in vitro with cortisol, actinomycin D, puromycin, and cycloheximide indicate that distinct inhibitory effects of cortisol on transport and phosphorylation are due to an action on mRNA synthesis with consequent induction of synthesis of protein(s) with inhibitory influence. Incubation of thymocytes with cortisol results in inhibition of the rate of labeled orthophosphate incorporation into ATP and the entry of rubidium ion and hexoses into the cells. Continuing protein synthesis is required for the progressive and persistent manifestation of the inhibitory effects of the steroid. RNA synthesis is also required during the initial phase of incubation of cells with cortisol, but significant inhibitory effects of cortisol, once initiated, are evident for at least 60-120 min after addition of actinomycin D. In contrast, addition of cycloheximide some time after cortisol results in prevention or reversal of the effects of the steroid. In the absence of cortisol, the antibiotics exert relatively little effect on orthophosphate incorporation and on the transport processes studied. It is suggested that the sequence of events leading to dissolution of thymocytes exposed to cortisol is initiated by the synthesis of mRNA coding for inhibitory protein(s) with more rapid turnover rates than that of the mRNA, and that these events are modulated by the relative sensitivity of different cellular processes to the protein inhibitor(s).
Keywords: messenger RNA, rubidium transport, hexose transport
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARTLETT G. R. Methods for the isolation of glycolytic intermediated by column chromatography with ion exchange resins. J Biol Chem. 1959 Mar;234(3):459–465. [PubMed] [Google Scholar]
- EAGLE H. Amino acid metabolism in mammalian cell cultures. Science. 1959 Aug 21;130(3373):432–437. doi: 10.1126/science.130.3373.432. [DOI] [PubMed] [Google Scholar]
- HELMREICH E., EISEN H. N. The distribution and utilization of glucose in isolated lymph node cells. J Biol Chem. 1959 Aug;234(8):1958–1965. [PubMed] [Google Scholar]
- KIPNIS D. M., CORI C. F. Studies of tissue permeability. V. The penetration and phosphorylation of 2-deoxyglucose in the rat diaphragm. J Biol Chem. 1959 Jan;234(1):171–177. [PubMed] [Google Scholar]
- Levinson C. Phosphate partition and transport in the Ehrlich mouse ascites tumor cell. Biochim Biophys Acta. 1966 Jun 8;120(2):292–298. doi: 10.1016/0926-6585(66)90349-9. [DOI] [PubMed] [Google Scholar]
- MARSH B. B. The estimation of inorganic phosphate in the presence of adenosine triphosphate. Biochim Biophys Acta. 1959 Apr;32:357–361. doi: 10.1016/0006-3002(59)90607-9. [DOI] [PubMed] [Google Scholar]
- Makman M. H., Dvorkin B., White A. Alterations in protein and nucleic acid metabolism of thymocytes produced by adrenal steroids in vitro. J Biol Chem. 1966 Apr 10;241(7):1646–1648. [PubMed] [Google Scholar]
- Makman M. H., Dvorkin B., White A. Influence of cortisol on the utilization of precursors of nucleic acids and protein by lymphoid cells in vitro. J Biol Chem. 1968 Apr 10;243(7):1485–1497. [PubMed] [Google Scholar]
- Makman M. H., Nakagawa S., Dvorkin B., White A. Inhibitory effects of cortisol and antibiotics on substrate entry and ribonucleic acid synthesis in rat thymocytes in vitro. J Biol Chem. 1970 May 25;245(10):2556–2563. [PubMed] [Google Scholar]
- Makman M. H., Nakagawa S., White A. Studies of the mode of action of adrenal steroids on lymphocytes. Recent Prog Horm Res. 1967;23:195–227. doi: 10.1016/b978-1-4831-9826-2.50008-8. [DOI] [PubMed] [Google Scholar]
- Miller J. F., Osoba D. Current concepts of the immunological function of the thymus. Physiol Rev. 1967 Jul;47(3):437–520. doi: 10.1152/physrev.1967.47.3.437. [DOI] [PubMed] [Google Scholar]
- Mosher K. M., Young D. A., Munck A. Evidence for irreversible, actinomycin D-sensitive, and temperature-sensitive steps following binding of cortisol to glucocorticoid receptors and preceding effects on glucose metabolism in rat thymus cells. J Biol Chem. 1971 Feb 10;246(3):654–659. [PubMed] [Google Scholar]
- Munck A. Steroid concentration and tissue integrity as factors determining the physiological significance of effects of adrenal steroids in vitro. Endocrinology. 1965 Aug;77(2):356–360. doi: 10.1210/endo-77-2-356. [DOI] [PubMed] [Google Scholar]
- Reel J. R., Kenney F. T. "Superinduction" of tyrosine transaminase in hepatoma cell cultures: differential inhibition of synthesis and turnover by actionomycin D. Proc Natl Acad Sci U S A. 1968 Sep;61(1):200–206. doi: 10.1073/pnas.61.1.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SOLS A., CRANE R. K. Substrate specificity of brain hexokinase. J Biol Chem. 1954 Oct;210(2):581–595. [PubMed] [Google Scholar]
- Stevens W., Bedke C., Dougherty T. F. Effects of cortisol acetate on various aspects of cellular metabolism in mouse lymphatic tissue. J Reticuloendothel Soc. 1967 Jul;4(4):254–283. [PubMed] [Google Scholar]
- Tomkins G. M., Gelehrter T. D., Granner D., Martin D., Jr, Samuels H. H., Thompson E. B. Control of specific gene expression in higher organisms. Expression of mammalian genes may be controlled by repressors acting on the translation of messenger RNA. Science. 1969 Dec 19;166(3912):1474–1480. doi: 10.1126/science.166.3912.1474. [DOI] [PubMed] [Google Scholar]
- WHITE A. Effects of steroids on aspects of the metabolism and functions of the lymphocyte: a hypothesis of the cellular mechanisms in antibody formation and related immune phenomena. Ann N Y Acad Sci. 1958 Sep 5;73(1):79–104. doi: 10.1111/j.1749-6632.1959.tb40793.x. [DOI] [PubMed] [Google Scholar]
- Young D. A. Glucocorticoid action on rat thymus cells. Interrelationships between carbohydrate, protein, and adenine nucleotide metabolism and cortisol effects on these functions in vitro. J Biol Chem. 1969 Apr 25;244(8):2210–2217. [PubMed] [Google Scholar]



