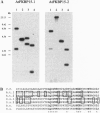
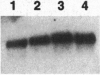
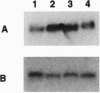
Abstract
Immunophilins are intracellular receptors for the immunosuppressants cyclosporin A, FK506, and rapamycin. In addition to their use in organ transplantation, these natural products have been used to investigate signaling pathways in yeast, plant, and mammalian cells. We have recently described the identification of an immunosuppressant-sensitive signaling pathway in and the purification of several immunophilins from Vicia faba plants. We now report the molecular characterization of a 15 kDa FK506- and rapamycin-binding protein from V. faba (VfFKBP15). The amino acid sequence deduced from the cDNA starts with a signal peptide of 22 hydrophobic amino acids. The core region of VfFKBP15 is most similar to yeast and mammalian FKBP13 localized in the endoplasmic reticulum (ER). In addition, VfFKBP15 has a carboxyl-terminal sequence that is ended with SSEL, a putative ER retention signal. These findings suggest that VfFKBP15 is a functional homolog of FKBP13 from other organisms. Interestingly, two distinct cDNAs corresponding to two isoforms of FKBP15 have been cloned from Arabidopsis and also identified from rice data base, suggesting that pFKBP15 (plant FKBP15) is encoded by a small gene family in plants. This adds to the diversity of plant FKBP members even with the same subcellular localization and is in contrast with the situation in mammalian and yeast systems in which only one FKBP13 gene has been found. Like the mammalian and yeast FKBP13, the recombinant VfFKBP15 protein has rotamase activity that is inhibited by both FK506 and rapamycin with a Ki value of 30 nM and 0.9 nM, respectively, illustrating that VfFKBP15 binds rapamycin in preference over FK506. The mRNA of VfFKBP15 is ubiquitously expressed in various plant tissues including leaves, stems, and roots, consistent with the ER localization of the protein. Levels of VfFKBP15 mRNA are elevated by heat shock, suggesting a possible role for this FKBP member under stress conditions.
Full text
PDF


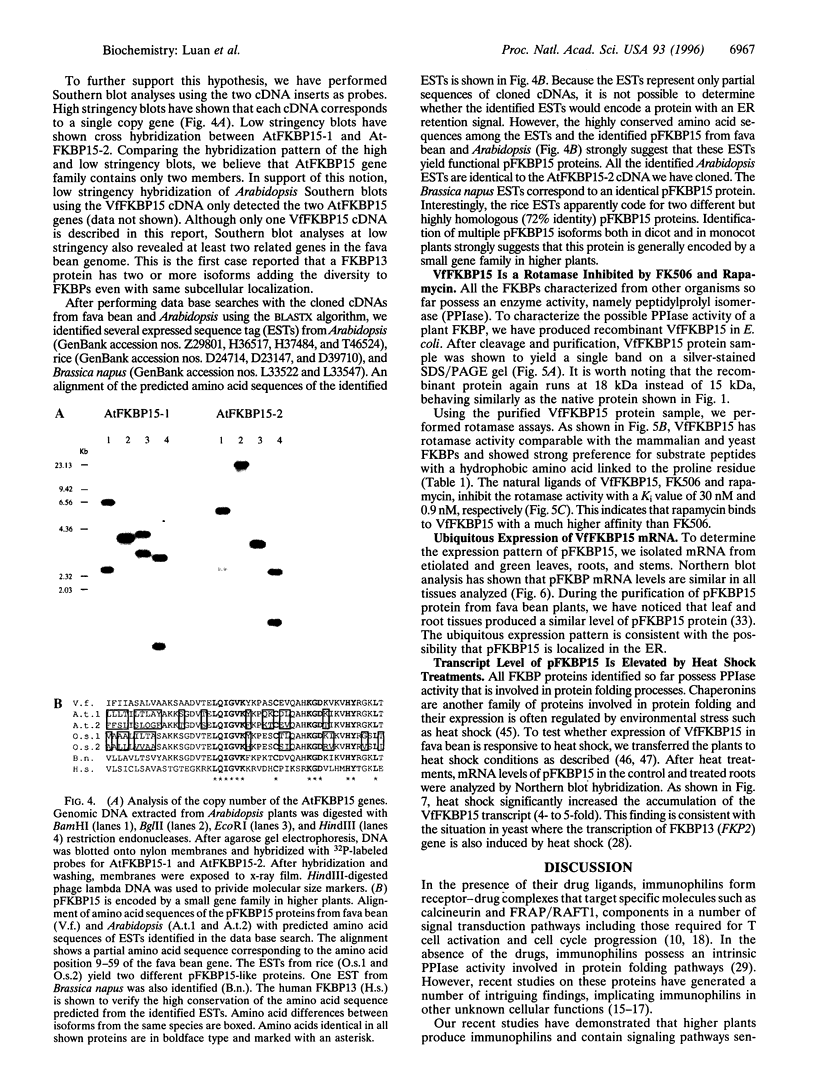

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albers M. W., Williams R. T., Brown E. J., Tanaka A., Hall F. L., Schreiber S. L. FKBP-rapamycin inhibits a cyclin-dependent kinase activity and a cyclin D1-Cdk association in early G1 of an osteosarcoma cell line. J Biol Chem. 1993 Oct 25;268(30):22825–22829. [PubMed] [Google Scholar]
- Bram R. J., Hung D. T., Martin P. K., Schreiber S. L., Crabtree G. R. Identification of the immunophilins capable of mediating inhibition of signal transduction by cyclosporin A and FK506: roles of calcineurin binding and cellular location. Mol Cell Biol. 1993 Aug;13(8):4760–4769. doi: 10.1128/mcb.13.8.4760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brillantes A. B., Ondrias K., Scott A., Kobrinsky E., Ondriasová E., Moschella M. C., Jayaraman T., Landers M., Ehrlich B. E., Marks A. R. Stabilization of calcium release channel (ryanodine receptor) function by FK506-binding protein. Cell. 1994 May 20;77(4):513–523. doi: 10.1016/0092-8674(94)90214-3. [DOI] [PubMed] [Google Scholar]
- Brown E. J., Albers M. W., Shin T. B., Ichikawa K., Keith C. T., Lane W. S., Schreiber S. L. A mammalian protein targeted by G1-arresting rapamycin-receptor complex. Nature. 1994 Jun 30;369(6483):756–758. doi: 10.1038/369756a0. [DOI] [PubMed] [Google Scholar]
- Cameron A. M., Steiner J. P., Roskams A. J., Ali S. M., Ronnett G. V., Snyder S. H. Calcineurin associated with the inositol 1,4,5-trisphosphate receptor-FKBP12 complex modulates Ca2+ flux. Cell. 1995 Nov 3;83(3):463–472. doi: 10.1016/0092-8674(95)90124-8. [DOI] [PubMed] [Google Scholar]
- Clipstone N. A., Crabtree G. R. Identification of calcineurin as a key signalling enzyme in T-lymphocyte activation. Nature. 1992 Jun 25;357(6380):695–697. doi: 10.1038/357695a0. [DOI] [PubMed] [Google Scholar]
- Fischer G., Wittmann-Liebold B., Lang K., Kiefhaber T., Schmid F. X. Cyclophilin and peptidyl-prolyl cis-trans isomerase are probably identical proteins. Nature. 1989 Feb 2;337(6206):476–478. doi: 10.1038/337476a0. [DOI] [PubMed] [Google Scholar]
- Galat A., Lane W. S., Standaert R. F., Schreiber S. L. A rapamycin-selective 25-kDa immunophilin. Biochemistry. 1992 Mar 3;31(8):2427–2434. doi: 10.1021/bi00123a031. [DOI] [PubMed] [Google Scholar]
- Gething M. J., Sambrook J. Protein folding in the cell. Nature. 1992 Jan 2;355(6355):33–45. doi: 10.1038/355033a0. [DOI] [PubMed] [Google Scholar]
- Heitman J., Movva N. R., Hall M. N. Targets for cell cycle arrest by the immunosuppressant rapamycin in yeast. Science. 1991 Aug 23;253(5022):905–909. doi: 10.1126/science.1715094. [DOI] [PubMed] [Google Scholar]
- Hendrickson B. A., Zhang W., Craig R. J., Jin Y. J., Bierer B. E., Burakoff S., DiLella A. G. Structural organization of the genes encoding human and murine FK506-binding protein (FKBP) 13 and comparison to FKBP1. Gene. 1993 Dec 8;134(2):271–275. doi: 10.1016/0378-1119(93)90106-d. [DOI] [PubMed] [Google Scholar]
- Higgins D. G., Sharp P. M. Fast and sensitive multiple sequence alignments on a microcomputer. Comput Appl Biosci. 1989 Apr;5(2):151–153. doi: 10.1093/bioinformatics/5.2.151. [DOI] [PubMed] [Google Scholar]
- Hung D. T., Schreiber S. L. cDNA cloning of a human 25 kDa FK506 and rapamycin binding protein. Biochem Biophys Res Commun. 1992 Apr 30;184(2):733–738. doi: 10.1016/0006-291x(92)90651-z. [DOI] [PubMed] [Google Scholar]
- Jayaraman T., Brillantes A. M., Timerman A. P., Fleischer S., Erdjument-Bromage H., Tempst P., Marks A. R. FK506 binding protein associated with the calcium release channel (ryanodine receptor). J Biol Chem. 1992 May 15;267(14):9474–9477. [PubMed] [Google Scholar]
- Jin Y. J., Albers M. W., Lane W. S., Bierer B. E., Schreiber S. L., Burakoff S. J. Molecular cloning of a membrane-associated human FK506- and rapamycin-binding protein, FKBP-13. Proc Natl Acad Sci U S A. 1991 Aug 1;88(15):6677–6681. doi: 10.1073/pnas.88.15.6677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Y. J., Burakoff S. J., Bierer B. E. Molecular cloning of a 25-kDa high affinity rapamycin binding protein, FKBP25. J Biol Chem. 1992 Jun 5;267(16):10942–10945. [PubMed] [Google Scholar]
- Jin Y. J., Burakoff S. J. The 25-kDa FK506-binding protein is localized in the nucleus and associates with casein kinase II and nucleolin. Proc Natl Acad Sci U S A. 1993 Aug 15;90(16):7769–7773. doi: 10.1073/pnas.90.16.7769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kieber J. J., Rothenberg M., Roman G., Feldmann K. A., Ecker J. R. CTR1, a negative regulator of the ethylene response pathway in Arabidopsis, encodes a member of the raf family of protein kinases. Cell. 1993 Feb 12;72(3):427–441. doi: 10.1016/0092-8674(93)90119-b. [DOI] [PubMed] [Google Scholar]
- Ko K., Bornemisza O., Kourtz L., Ko Z. W., Plaxton W. C., Cashmore A. R. Isolation and characterization of a cDNA clone encoding a cognate 70-kDa heat shock protein of the chloroplast envelope. J Biol Chem. 1992 Feb 15;267(5):2986–2993. [PubMed] [Google Scholar]
- Kunz J., Henriquez R., Schneider U., Deuter-Reinhard M., Movva N. R., Hall M. N. Target of rapamycin in yeast, TOR2, is an essential phosphatidylinositol kinase homolog required for G1 progression. Cell. 1993 May 7;73(3):585–596. doi: 10.1016/0092-8674(93)90144-f. [DOI] [PubMed] [Google Scholar]
- Lebeau M. C., Massol N., Herrick J., Faber L. E., Renoir J. M., Radanyi C., Baulieu E. E. P59, an hsp 90-binding protein. Cloning and sequencing of its cDNA and preparation of a peptide-directed polyclonal antibody. J Biol Chem. 1992 Mar 5;267(7):4281–4284. [PubMed] [Google Scholar]
- Liu J., Albers M. W., Wandless T. J., Luan S., Alberg D. G., Belshaw P. J., Cohen P., MacKintosh C., Klee C. B., Schreiber S. L. Inhibition of T cell signaling by immunophilin-ligand complexes correlates with loss of calcineurin phosphatase activity. Biochemistry. 1992 Apr 28;31(16):3896–3901. doi: 10.1021/bi00131a002. [DOI] [PubMed] [Google Scholar]
- Liu J., Farmer J. D., Jr, Lane W. S., Friedman J., Weissman I., Schreiber S. L. Calcineurin is a common target of cyclophilin-cyclosporin A and FKBP-FK506 complexes. Cell. 1991 Aug 23;66(4):807–815. doi: 10.1016/0092-8674(91)90124-h. [DOI] [PubMed] [Google Scholar]
- Lorenz M. C., Heitman J. TOR mutations confer rapamycin resistance by preventing interaction with FKBP12-rapamycin. J Biol Chem. 1995 Nov 17;270(46):27531–27537. doi: 10.1074/jbc.270.46.27531. [DOI] [PubMed] [Google Scholar]
- Luan S., Albers M. W., Schreiber S. L. Light-regulated, tissue-specific immunophilins in a higher plant. Proc Natl Acad Sci U S A. 1994 Feb 1;91(3):984–988. doi: 10.1073/pnas.91.3.984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luan S., Li W., Rusnak F., Assmann S. M., Schreiber S. L. Immunosuppressants implicate protein phosphatase regulation of K+ channels in guard cells. Proc Natl Acad Sci U S A. 1993 Mar 15;90(6):2202–2206. doi: 10.1073/pnas.90.6.2202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman T., de Bruijn F. J., Green P., Keegstra K., Kende H., McIntosh L., Ohlrogge J., Raikhel N., Somerville S., Thomashow M. Genes galore: a summary of methods for accessing results from large-scale partial sequencing of anonymous Arabidopsis cDNA clones. Plant Physiol. 1994 Dec;106(4):1241–1255. doi: 10.1104/pp.106.4.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen J. B., Foor F., Siekierka J. J., Hsu M. J., Ramadan N., Morin N., Shafiee A., Dahl A. M., Brizuela L., Chrebet G. Yeast FKBP-13 is a membrane-associated FK506-binding protein encoded by the nonessential gene FKB2. Proc Natl Acad Sci U S A. 1992 Aug 15;89(16):7471–7475. doi: 10.1073/pnas.89.16.7471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Keefe S. J., Tamura J., Kincaid R. L., Tocci M. J., O'Neill E. A. FK-506- and CsA-sensitive activation of the interleukin-2 promoter by calcineurin. Nature. 1992 Jun 25;357(6380):692–694. doi: 10.1038/357692a0. [DOI] [PubMed] [Google Scholar]
- Partaledis J. A., Berlin V. The FKB2 gene of Saccharomyces cerevisiae, encoding the immunosuppressant-binding protein FKBP-13, is regulated in response to accumulation of unfolded proteins in the endoplasmic reticulum. Proc Natl Acad Sci U S A. 1993 Jun 15;90(12):5450–5454. doi: 10.1073/pnas.90.12.5450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peattie D. A., Harding M. W., Fleming M. A., DeCenzo M. T., Lippke J. A., Livingston D. J., Benasutti M. Expression and characterization of human FKBP52, an immunophilin that associates with the 90-kDa heat shock protein and is a component of steroid receptor complexes. Proc Natl Acad Sci U S A. 1992 Nov 15;89(22):10974–10978. doi: 10.1073/pnas.89.22.10974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pich U., Schubert I. Midiprep method for isolation of DNA from plants with a high content of polyphenolics. Nucleic Acids Res. 1993 Jul 11;21(14):3328–3328. doi: 10.1093/nar/21.14.3328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatini D. M., Erdjument-Bromage H., Lui M., Tempst P., Snyder S. H. RAFT1: a mammalian protein that binds to FKBP12 in a rapamycin-dependent fashion and is homologous to yeast TORs. Cell. 1994 Jul 15;78(1):35–43. doi: 10.1016/0092-8674(94)90570-3. [DOI] [PubMed] [Google Scholar]
- Schreiber S. L. Chemistry and biology of the immunophilins and their immunosuppressive ligands. Science. 1991 Jan 18;251(4991):283–287. doi: 10.1126/science.1702904. [DOI] [PubMed] [Google Scholar]
- Schreiber S. L. Immunophilin-sensitive protein phosphatase action in cell signaling pathways. Cell. 1992 Aug 7;70(3):365–368. doi: 10.1016/0092-8674(92)90158-9. [DOI] [PubMed] [Google Scholar]
- Smith D. B., Johnson K. S. Single-step purification of polypeptides expressed in Escherichia coli as fusions with glutathione S-transferase. Gene. 1988 Jul 15;67(1):31–40. doi: 10.1016/0378-1119(88)90005-4. [DOI] [PubMed] [Google Scholar]
- Stan R., McLaughlin M. M., Cafferkey R., Johnson R. K., Rosenberg M., Livi G. P. Interaction between FKBP12-rapamycin and TOR involves a conserved serine residue. J Biol Chem. 1994 Dec 23;269(51):32027–32030. [PubMed] [Google Scholar]
- Standaert R. F., Galat A., Verdine G. L., Schreiber S. L. Molecular cloning and overexpression of the human FK506-binding protein FKBP. Nature. 1990 Aug 16;346(6285):671–674. doi: 10.1038/346671a0. [DOI] [PubMed] [Google Scholar]
- Tai P. K., Albers M. W., Chang H., Faber L. E., Schreiber S. L. Association of a 59-kilodalton immunophilin with the glucocorticoid receptor complex. Science. 1992 May 29;256(5061):1315–1318. doi: 10.1126/science.1376003. [DOI] [PubMed] [Google Scholar]
- Wang H., Goffreda M., Leustek T. Characteristics of an Hsp70 homolog localized in higher plant chloroplasts that is similar to DnaK, the Hsp70 of prokaryotes. Plant Physiol. 1993 Jul;102(3):843–850. doi: 10.1104/pp.102.3.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng X. F., Florentino D., Chen J., Crabtree G. R., Schreiber S. L. TOR kinase domains are required for two distinct functions, only one of which is inhibited by rapamycin. Cell. 1995 Jul 14;82(1):121–130. doi: 10.1016/0092-8674(95)90058-6. [DOI] [PubMed] [Google Scholar]