Abstract
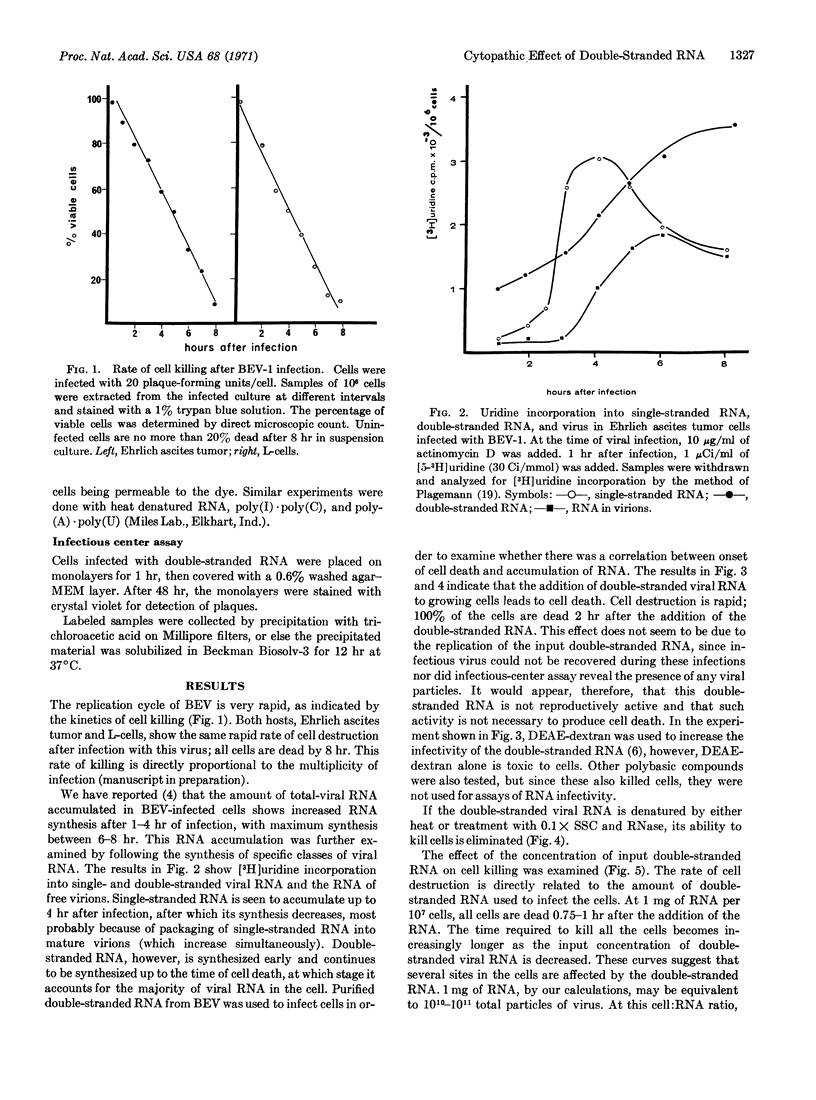
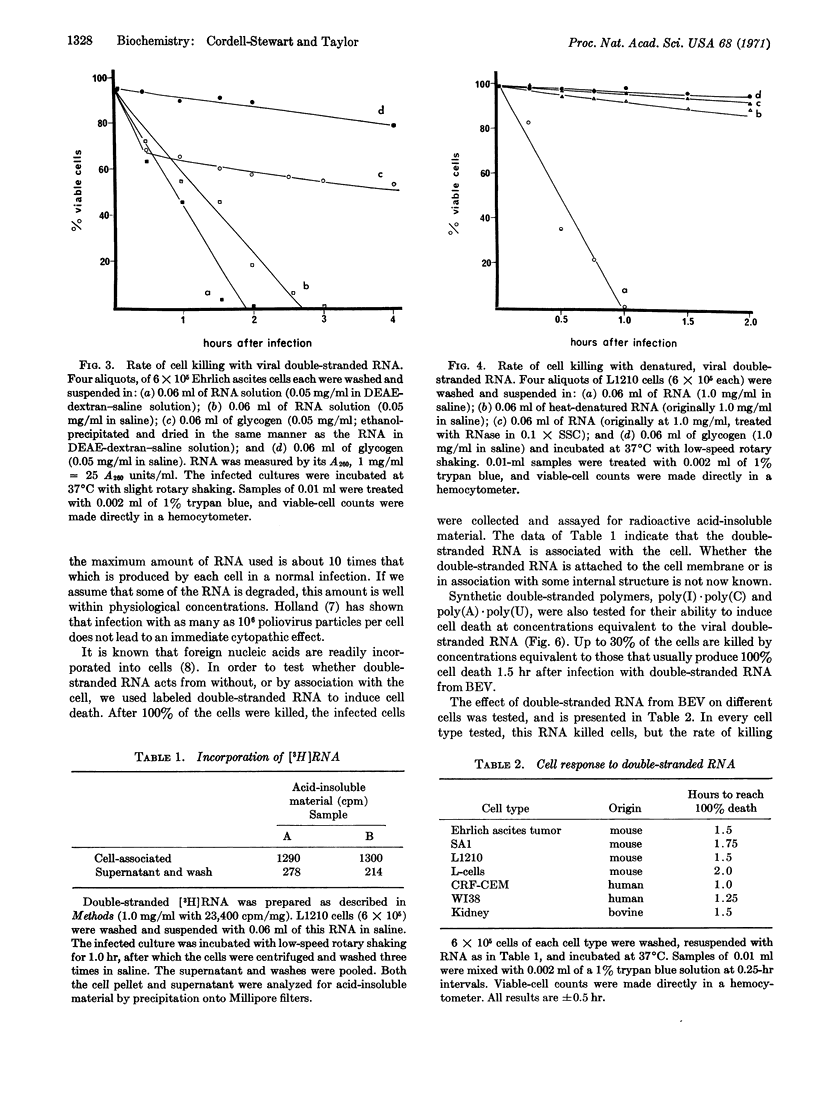
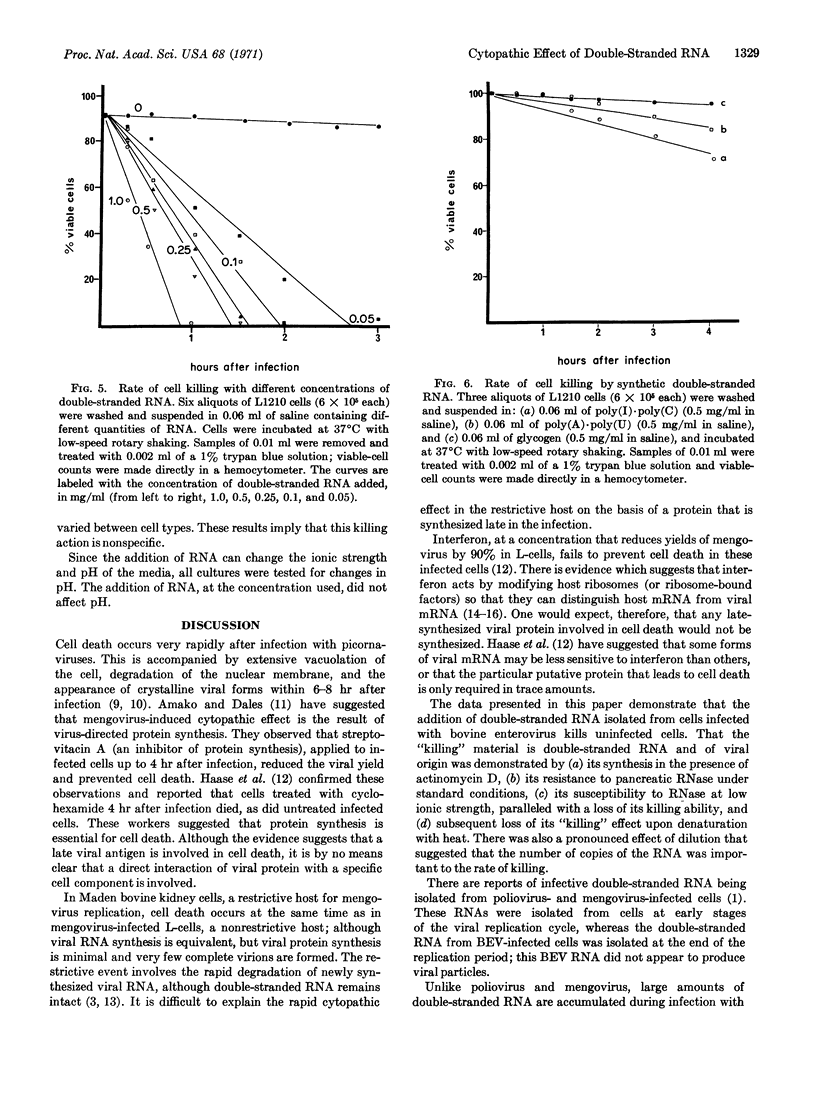
During bovine enterovirus infection of Ehrlich ascites tumor cells, large amounts of double-stranded RNA accumulate. Addition of this double-stranded RNA to uninfected cells leads to rapid cell death. This is not a result of infectious virus production. Neither single-stranded RNA nor heat-denatured double-stranded RNA has this effect. Similar experiments with synthetic double-stranded polymers, poly(I)·poly(C) and poly(A)·poly(U), show that they are only slightly toxic at the concentrations used. The effect of the double-stranded RNA is nonspecific for cells of different origins. The implications of this finding in relation to the cytopathic effects of picornavirus and to cancer chemotherapy are discussed.
Keywords: bovine enterovirus, picornavirus, interferon, tumor regression
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amako K., Dales S. Cytopathology of Mengovirus infection. I. Relationship between cellular disintegration and virulence. Virology. 1967 Jun;32(2):184–200. doi: 10.1016/0042-6822(67)90269-3. [DOI] [PubMed] [Google Scholar]
- Amako K., Dales S. Cytopathology of Mengovirus infection. II. Proliferation of membranous cisternae. Virology. 1967 Jun;32(2):201–215. doi: 10.1016/0042-6822(67)90270-x. [DOI] [PubMed] [Google Scholar]
- Arlinghaus R. B., Polatnick J., Vande Woude G. F. Studies on foot-and-mouth disease virus ribonucleic acid synthesis. Virology. 1966 Nov;30(3):541–550. doi: 10.1016/0042-6822(66)90129-2. [DOI] [PubMed] [Google Scholar]
- Baltimore D., Girard M., Darnell J. E. Aspects of the synthesis of poliovirus RNA and the formation of virus particles. Virology. 1966 Jun;29(2):179–189. doi: 10.1016/0042-6822(66)90024-9. [DOI] [PubMed] [Google Scholar]
- Bishop J. M., Koch G. Purification and characterization of poliovirus-induced infectious double-stranded ribonucleic acid. J Biol Chem. 1967 Apr 25;242(8):1736–1743. [PubMed] [Google Scholar]
- Carter W. A., Levy H. B. Ribosomes: effect of interferon on their interaction with rapidly labeled cellular and viral RNA's. Science. 1967 Mar 10;155(3767):1254–1257. doi: 10.1126/science.155.3767.1254. [DOI] [PubMed] [Google Scholar]
- Ehrenfeld E., Hunt T. Double-stranded poliovirus RNA inhibits initiation of protein synthesis by reticulocyte lysates. Proc Natl Acad Sci U S A. 1971 May;68(5):1075–1078. doi: 10.1073/pnas.68.5.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOLLAND J. J. INHIBITION OF HOST CELL MACROMOLECULAR SYNTHESIS BY HIGH MULTIPLICITIES OF POLIOVIRUS UNDER CONDITIONS PREVENTING VIRUS SYNTHESIS. J Mol Biol. 1964 Apr;8:574–581. doi: 10.1016/s0022-2836(64)80012-7. [DOI] [PubMed] [Google Scholar]
- Haase A. T., Levy H., Baron S., Kasel J. A. Mengovirus-induced cytopathic effect in L-cells: protective effect of interferon. J Virol. 1969 Oct;4(4):490–495. doi: 10.1128/jvi.4.4.490-495.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch G., Quintrell N., Bishop J. M. An agar cell-suspension plaque assay for isolated viral RNA. Biochem Biophys Res Commun. 1966 Aug 12;24(3):304–309. doi: 10.1016/0006-291x(66)90155-0. [DOI] [PubMed] [Google Scholar]
- LWOFF A., DULBECCO R., VOGT M., LWOFF M. Kinetics of the release of poliomyelitis virus from single cells. Virology. 1955 May;1(1):128–139. doi: 10.1016/0042-6822(55)90010-6. [DOI] [PubMed] [Google Scholar]
- Larson V. M., Clark W. R., Hilleman M. R. Influence of synthetic (poly I:C) and viral double-stranded ribonucleic acids on adenovirus 12 oncogenesis in hamsters. Proc Soc Exp Biol Med. 1969 Jul;131(3):1002–1011. doi: 10.3181/00379727-131-34028. [DOI] [PubMed] [Google Scholar]
- Levy H. B., Law L. W., Rabson A. S. Inhibition of tumor growth by polyinosinic-polycytidylic acid. Proc Natl Acad Sci U S A. 1969 Feb;62(2):357–361. doi: 10.1073/pnas.62.2.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy H. B., Riley F. The effect of polyinosinic:polycytidylic acid on tumor metabolism. Proc Soc Exp Biol Med. 1970 Oct;135(1):141–145. doi: 10.3181/00379727-135-35006. [DOI] [PubMed] [Google Scholar]
- Marcus P. I., Salb J. M. Molecular basis of interferon action: inhibition of viral RNA translation. Virology. 1966 Nov;30(3):502–516. doi: 10.1016/0042-6822(66)90126-7. [DOI] [PubMed] [Google Scholar]
- Ralph R. K. Double-stranded viral RNA. Adv Virus Res. 1969;15:61–158. doi: 10.1016/s0065-3527(08)60874-x. [DOI] [PubMed] [Google Scholar]
- Taylor M. W., Cordell B., Souhrada M., Prather S. Viruses as an aid to cancer therapy: regression of solid and ascites tumors in rodents after treatment with bovine enterovirus. Proc Natl Acad Sci U S A. 1971 Apr;68(4):836–840. doi: 10.1073/pnas.68.4.836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor M. W., Davidson J. N., Land C., Wall R. Virus-induced regression of tumor growth. J Natl Cancer Inst. 1970 Mar;44(3):515–519. [PubMed] [Google Scholar]
- Wall R., Taylor M. W. Host-dependent restriction of mengovirus replication. J Virol. 1969 Nov;4(5):681–687. doi: 10.1128/jvi.4.5.681-687.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wall R., Taylor M. W. Mengovirus RNA synthesis in productive and restrictive cell lines. Virology. 1970 Sep;42(1):78–86. doi: 10.1016/0042-6822(70)90240-0. [DOI] [PubMed] [Google Scholar]
- YOON C. H. INCORPORATION OF EXOGENOUS RNA INTO NELSON'S ASCITES CELLS IN VITRO. Exp Cell Res. 1965 May;38:386–397. doi: 10.1016/0014-4827(65)90412-x. [DOI] [PubMed] [Google Scholar]


