Abstract
Context
Annual ultrasound (US) may detect small, node-negative breast cancers not seen on mammography (M). MRI may depict additional breast cancers beyond mammography and ultrasound (M+US).
Objective
Determine supplemental cancer detection yield of ultrasound and MRI in women at elevated risk for breast cancer.
Design, Setting, Participants
From April 2004 to February 2006, 2809 women at 21 sites with elevated cancer risk and dense breasts consented to three annual independent screens with mammography and ultrasound in randomized order. After 3 rounds of mammography and US screening, 703 women from 14 sites consented to a single MRI.
Main Outcome Measures
Cancer detection rate (yield), sensitivity, specificity, positive predictive value of biopsies performed (PPV3 – rate of malignancy among cases positive on screening, who actually underwent biopsy), interval cancer rate. The diagnosis of breast cancer was based on a biopsy showing in situ or infiltrating ductal carcinoma or infiltrating lobular carcinoma in the breast or axillary lymph nodes. Reference standard was defined as a combination of pathology and 12-month follow-up and was available for 2662 women (7473 M+US screens) and 612 MRI participants.
Results
The 2662 patients underwent 7473 mammograms and US, with 110 women having 111 breast cancers detected, of which 33 were detected on mammography only, 32 on US only, 26 on both mammography and US, and 9 on MRI after mammography and US. Eleven were not detected by any imaging modality. Supplemental incidence-screening US identified 3.7 cancers per 1000 women-screens (95% CI 2.1 to 5.8, p<.001). Sensitivity, specificity, and PPV3 for M +US were 57/75 (0.76, 95% CI 0.65 to 0.85), 3987/4739 (0.84, 95% CI 0.83 to 0.85), and 55/339 (0.16, 95% CI 0.12 to 0.21); and for mammography alone 39/75 (0.52, 95% CI 0.40 to 0.64), 4325/4739 (0.91,95% 0.90 to 0.92), and 37/97 (0.38, 95% CI 0.28 to 0.49) (p<.001 all comparisons). Of 612 analyzable MRI participants, 16 (2.6%) had breast cancer diagnosed. Supplemental yield of MRI was 14.7 per 1000 (95% CI 3.5 to 25.9, p=.004). Sensitivity, specificity, and PPV3 for MRI+M+US were 16/16 (1.00, 95% CI 0.79 to 1.00), 390/596 (0.65, 95% CI 0.61 to 0.69), and 15/81 (0.19, 95% CI 0.11 to 0.29); and for M+US 7/16 (0.44, 95% CI 0.20 to 0.70, p=.004), 503/596 (0.84, 95% CI 0.81 to 0.87, p <.001), and 7/38 (0.18, 95% CI 0.08 to 0.34, p= .98) for M+US. Number of screens needed to detect one cancer was 127(95%CI 99 to 167) for mammography; 234(95%CI 173 to 345) for supplemental ultrasound, and 68 (95%CI 39 to 286) for MRI after negative M+US.
Conclusions
The addition of screening ultrasound or MRI to mammography in women at increased risk of breast cancer resulted in a higher cancer detection yield but also an increase in false positive findings.
INTRODUCTION
Six previous single-center studies1–6 and three multicenter trials7–9 have shown supplemental screening breast ultrasound significantly increases detection of node-negative invasive breast cancer in women with mammographically dense breast tissue on the first, prevalence screen, consistently increasing cancer detection (yield) by 3.5 per 1000 in single-center studies and 4.2 to 4.4 per 1000 in multicenter trials. The vast majority of cancers seen only on ultrasound have been node-negative invasive breast cancers. Until now, it was unclear whether or not there was a detection benefit to continuing ultrasound screening annually (i.e. incidence screening).
A substantial majority of ACRIN 6666 participants were at intermediate risk for breast cancer, with over half having personal history of breast cancer (PHBC).7 While there was evidence from prior studies10–13 that MRI provided considerable detection benefit even after combined ultrasound and mammography in high-risk women, the combination of ultrasound and mammography might still identify the vast majority of cancers when they are node negative, at much lower cost to the health care system than MRI, particularly in screening women with a lower prevalence of disease. A substudy of ACRIN 6666 participants was therefore undertaken to assess the rate and stage of cancers detected with a single screening MRI.
METHODS
Study Design
Participants were asymptomatic women with heterogeneously dense or extremely dense breast tissue14 and at least one other risk factor for breast cancer (Table 1) who presented for routine annual mammography. Race and ethnic group were self-assigned based on pre-assigned fixed categories.
Table 1.
Participant Characteristics
| Analysis Set Screen 1 (N=2659) |
Analysis Set Screen 2 (N=2493) |
Analysis Set Screen 3 (N=2321) |
MR Analysis Set (N=612) |
||
|---|---|---|---|---|---|
| Age at scan (yrs) | Median (range) | 55.0 (25–91) | 56.0 (26–92) | 57.0 (27–93) | 57.0 (27–87) |
| Mean (SD) | 55.2 (10.1) | 56.4 (9.9) | 57.7 (9.8) | 56.8 (9.5) | |
| Age group at scan, n (%) | Age < 40 | 134 (5.0) | 89 (3.6) | 65 (2.8) | 17 (2.8) |
| 40 ≤ Age ≤ 49 | 627 (23.6) | 514 (20.6) | 392 (16.9) | 114 (18.6) | |
| 50 ≤ Age ≤ 69 | 1678 (63.1) | 1644 (65.9) | 1597 (68.8) | 429 (70.1) | |
| Age > 69 | 220 (8.3) | 246 (9.9) | 267 (11.5) | 52 (8.5) | |
| Race or Ethnicity, n (%) | White | 2467 (92.8) | 2316 (92.9) | 2162 (93.1) | 576 (94.1) |
| Hispanic or Latino | 265 (10.0) | 233 (9.3) | 209 (9.0) | 83 (13.6) | |
| Black or African American | 91 (3.4) | 85 (3.4) | 77 (3.3) | 11 (1.8) | |
| Native Hawaiian or other Pacific Islander | 4 (0.2) | 3 (0.1) | 4 (0.2) | 1 (0.2) | |
| Asian | 90 (3.4) | 82 (3.3) | 71 (3.1) | 22 (3.6) | |
| American Indian or Alaskan Native | 4 (0.2) | 4 (0.2) | 4 (0.2) | 1 (0.2) | |
| Unknown | 11 (0.4) | 11 (0.4) | 11 (0.5) | 1 (0.2) | |
| Menopausal Status, n (%) | Premenopausala | 609 (22.9) | 554 (22.2) | 502 (21.6) | 155 (25.3) |
| Perimenopausalb | 182 (6.8) | 170 (6.8) | 158 (6.8) | 37 (6.0) | |
| Postmenopausalc | 1362 (51.2) | 1294 (51.9) | 1208 (52.0) | 316 (51.6) | |
| Surgical Menopause | 484 (18.2) | 454 (18.2) | 432 (18.6) | 103 (16.8) | |
| Unknown | 22 (0.8) | 21 (0.8) | 21 (0.9) | 1 (0.2) | |
| Personal History of Breast Cancer (Regardless of Other Risk Factors), n (%) | Yesd | 1426 (53.6) | 1331 (53.4) | 1253 (54.0) | 275 (44.9) |
| Visually-Estimated Breast Density at scan, n (%) | <25% | 47 (1.8) | 47 (1.9) | 34 (1.5) | 7 (1.1) |
| 26–40% | 278 (10.5) | 236 (9.5) | 196 (8.4) | 61 (10.0) | |
| 41–60% | 824 (31.0) | 792 (31.8) | 774 (33.3) | 191 (31.2) | |
| 61–80% | 994 (37.4) | 976 (39.1) | 920 (39.6) | 253 (41.3) | |
| Greater than 80% | 515 (19.4) | 442 (17.7) | 395 (17.0) | 100 (16.3) | |
| Unknown | 1 (0.0) | 0 (0.0) | 2 (0.1) | 0 (0.0) | |
| Primary Risk Factor,e n (%) | Mutation in BRCA-1 or BRCA-2 | 23 (0.9) | 20 (0.8) | 18 (0.8) | 3 (0.5) |
| History of Prior Chest and/or Mediastinal and/or Axillary Irradiation | 8 (0.3) | 6 (0.2) | 6 (0.3) | 2 (0.3) | |
| Personal History of Breast Cancer | 1413 (53.1) | 1321 (53.0) | 1244 (53.6) | 273 (44.6) | |
| Lifetime Risk, Gail/Claus Model ≥ 25%e | 504 (19.0) | 460 (18.5) | 425 (18.3) | 135 (22.1) | |
| Five-Year Risk, Gail Model ≥ 2.5% | 406 (15.3) | 391 (15.7) | 366 (15.8) | 113 (18.5) | |
| Five-Year Risk, Gail Model ≥ 1.7% and Extremely Dense Breasts | 225 (8.5) | 217 (8.7) | 195 (8.4) | 70 (11.4) | |
| ADH/ALH/LCIS or Atypical Papilloma | 80 (3.0) | 78 (3.1) | 67 (2.9) | 16 (2.6) |
Premenopausal was defined as last menstrual period within prior 30 days.
Perimenopausal was defined as last menstrual period > 30 days and < 12 months prior.
Postmenopausal was defined as last menstrual period at least 12 months prior.
869/1426 (71.1%) of women with PHBC had lumpectomy and radiation therapy for the affected breast(s) on study.
Participants with multiple risk factors were determined to have a primary risk factor using the following hierarchy: Mutation in BRCA-1 or BRCA-2, history of prior chest and/or mediastinal and/or axillary irradiation, personal history of breast cancer, lifetime risk, Gail model ≥ 25%, five-year risk, Gail model ≥ 2.5%, five-year risk, Gail model ≥ 1.7% and extremely dense breasts, and finally, prior biopsy showing atypical ductal hyperplasia (ADH), atypical lobular hyperplasia (ALH), lobular 1carcinoma in situ, or atypical papilloma.
One participant's eligibility is based on a recalculated Gail score, where the original score was missing.
Each participant underwent mammographic and physician-performed ultrasonographic screening examinations in randomized order, with the interpreting radiologist for each examination masked to results of the other study, at 0 months (screen 1), 12 months (screen 2), and 24 months (screen 3). The randomization process has been previously described,7 and initial randomization order was maintained for subsequent screening rounds. If recommendation from either screening test was other than routine annual screening, the test was considered positive and a qualified site investigator then recorded an integrated interpretation by reviewing study mammogram and ultrasound together, and clinical management was based on integrated interpretation. If both modalities recommended routine annual follow-up, no integration was performed. Cancers positive only on a given modality refers to those not visible on any other modality. Sensitivity of a modality alone refers to the number of cancers that would have been detected if only that modality had been used, and includes some cancers that were also visible on the other modality.
To be eligible for the MRI substudy, women had to have completed the third round of annual screening ultrasound and mammography per protocol7 and undergo contrast-enhanced breast MRI within 8 weeks of the 24 month (third) screening mammogram. Interpretation of each of screening mammography, screening US, and MRI was blinded to results of the other examinations. A separate integrated breast-level interpretation across all modalities (mammography, ultrasound, MRI) was then performed which determined clinical management. We have previously reported on the slightly greater risk and younger age of women accepting vs. declining MRI.15 Women enrolled at sites in the MRI substudy were less likely to have PHBC; no other systematic differences were noted across sites.
Web-based data capture and quality monitoring were conducted by the ACRIN biostatistics and data management center. The study was HIPAA-compliant and received institutional review board approval from all participating sites, ACRIN and National Cancer Institute Cancer Imaging Program approval, and data and safety monitoring committee review every six months.
Participants
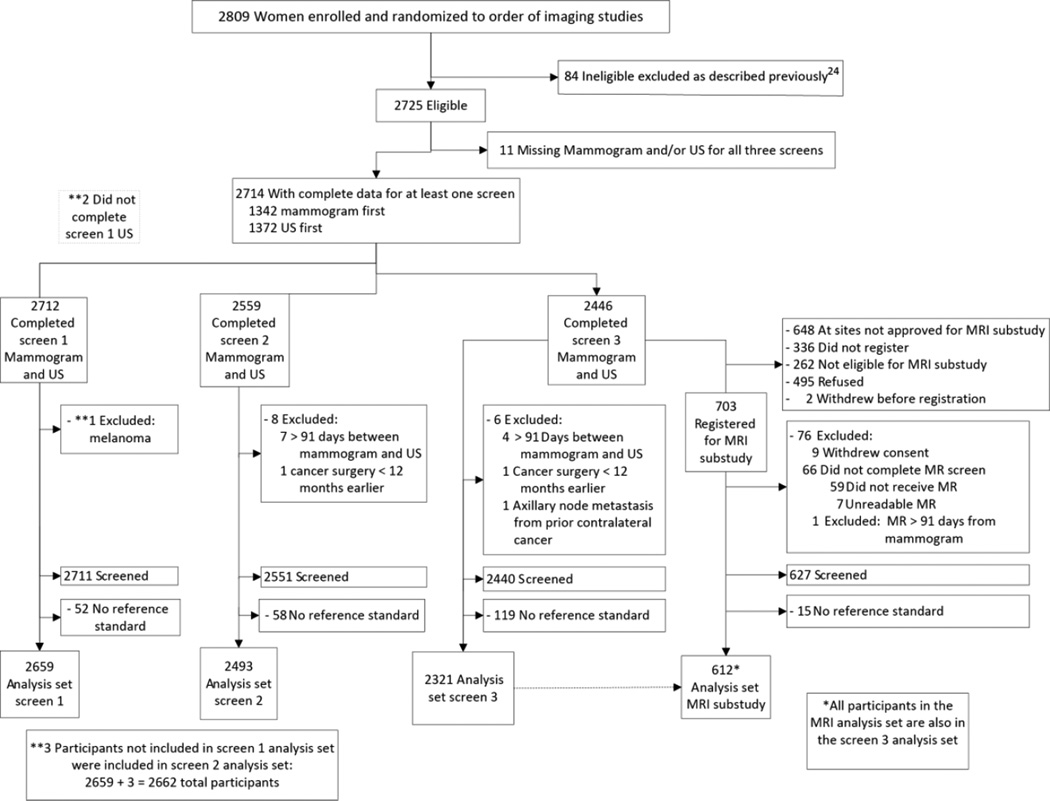
Among the 21 sites, 2809 women were recruited between April 2004 and February 2006, of whom 2725 were eligible (Figure 1). Women aged at least 25 years presenting for routine mammography were eligible to participate if they met study definitions of elevated risk (Table 1) and had heterogeneously dense or extremely dense parenchyma14 in at least one quadrant, either by prior mammography report or review of prior mammograms. Women were excluded if they were pregnant or lactating, had known metastatic disease, signs or symptoms of breast disease, breast surgery within prior 12 months, or breast implants. For the MRI substudy, women also could not have contraindications to MRI [pacemaker, aneurysm clip, other metallic implant, weight > 300 lb, or renal impairment (glomerular filtration rate of <30 mL/min/1.73 m2 or dialysis regimen)]. Participants provided written informed consent at their initial visit and again for the MRI substudy (when applicable).
Figure 1.
Flowchart of protocol. Participants with negative results on both mammography and ultrasound were imputed as having negative results on integrated reading: 1844 in year one; 1922 in year two; 1912 in year three. Reference standard was the most severe of biopsy results within 365 days of mammographic screening and/or clinical follow-up at one year. Biopsies prompted by an early subsequent screening examination were attributed to that subsequent screen.
Screening methods are detailed in the online supplement. Expanded 7-point BI-RADS27,29,30 assessment scale was used: 1, negative; 2, benign; 3, probably benign; 4a, low suspicion; 4b, intermediate suspicion; 4c, moderate suspicion; and 5, highly suggestive of malignancy.
Reference Standard
Reference standard was the most severe of biopsy results within 365 days of mammographic screening and/or clinical follow-up at one year. Each mammographic screen was targeted 365 days after the previous mammographic screening. A complete examination of all study breasts performed more than 11 full months after the previous screen was considered the next annual screen; only 88/7473 (1.2%) visits occurred before 11 months. The absence of a known diagnosis of cancer on participant interview, review of medical records, or both, at least 11 full months (330 days) after mammographic screening, was considered disease negative, as were 7 cases of prophylactic mastectomies with no evidence of cancer at pathology. Biopsy results showing breast cancer (in situ or infiltrating ductal carcinoma or infiltrating lobular carcinoma) in the breast or axillary lymph nodes were considered disease positive.
Statistical Methods
The primary unit of analysis was the participant. Participant’s BI-RADS score was derived as the maximum breast level BI-RADS or the score from the cancer side when only one breast had cancer. In keeping with planned revisions to BI-RADS (Edward A. Sickles, MD, Professor of Radiology, University of California, San Francisco, personal written communication 11/29/09), a screening BI-RADS assessment score of 3, 4a, 4b, 4c, or 5 was considered test positive, provided the recommendation was for other than routine screening. This differs from the definition of test positive used in our initial publication of the first screen wherein an assessment of 4a or higher was considered test positive29: results of the first screen have been reanalyzed and included herein. For a participant diagnosed with cancer, the breast(s) with cancer were excluded from analysis for the next annual screen.
The cancer detection rate (yield, i.e., the proportion of women with a positive screen test and positive reference standard), sensitivity, specificity, recall rate, PPV1 (malignancy rate among cases positive on screening), short-term follow-up rate, biopsy rate and AUC (area under the empirical ROC curve using BI-RADS scores) were reported. PPV3 is defined as rate of malignancy among cases positive on screening who underwent biopsy of the same lesion.14 Interval cancers were defined as those diagnosed because of a clinical abnormality such as a lump, skin thickening, or pathologic nipple discharge, in the interval between prescribed screenings (i.e. less than 365 days after the last screening mammogram).
Single-year estimates of yield, sensitivity, specificity, recall rate, PPV1, short-term follow-up rate, biopsy rate and PPV3, were determined as simple proportions with exact 95% CIs (Clopper-Pearson).16 The 95% CIs for differences in yield, sensitivity, specificity, recall rate, short-term follow-up rate and biopsy rate were calculated per Fleiss et al17 and p-values for the above comparisons were based on McNemar’s test statistic. The 95% CIs and p-values for differences in PPV1 and PPV3 were calculated using bootstrap resampling method18. All inferences for incidence screens were based on bootstrap resampling method. Estimates, 95% CIs and P-values related to AUC were derived by using method of Delong et al19 for empirical ROC curves. Results in participants with PHBC were compared to those without by the bootstrap method. All p-values were reported as two-sided, with 0.05 set as threshold for significance. All analyses were implemented in SAS 9.2 (Cary, NC).
RESULTS
Participant Demographic Information
There were 2659, 2493, and 2321 eligible women with reference standard for the first, second, and third annual mammogram and ultrasound screens respectively (Figure 1, Table 1). Participant demographics at enrollment were previously reported.7 Median age at enrollment was 55 years (range 25–91). Approximately 29% of women were under age 50 at enrollment and 23% were premenopausal (Table 1). Nearly 54% of women had PHBC. Median age of 612 MRI participants was 57 years (range 27–87); 21% were under age 50 at scan, 25% were premenopausal, and 45% had PHBC.
Cancer Detection
A total of 110 participants were diagnosed with breast cancer over three years of study, with one woman diagnosed by mammography in year one again diagnosed in the contralateral breast in year 3 (by MRI only) and counted as separate events, i.e. total of 111 participant-cancer events. Of 111 diagnoses, 89 (80%) were invasive (Table 2). Fifty-nine of 111 (53%) were seen on mammography, including 33 (30%) seen only on mammography; 32 (29%) additional only on US; 9 (8%) only on MRI after both mammography and US; and 11 (10%) not seen on imaging. Of 32 cancers seen only on US, 30 (94%) were invasive, with median size 10 mm (range 2–40), and 26/27 (96%) of those staged were node negative.
Table 2.
Summary of Cancer Detection and Characteristics for 2662 Unique Participants Screened Three Years with Mammography and Physician-Performed Ultrasound and 612 Participants Screened also with MRI
| Seen on Mammography only |
Seen on Mammography and US |
Seen on US only before MRI |
Not seen on imaging |
Seen on MRI only | Total | |
|---|---|---|---|---|---|---|
| Number of participants | 2662 | 2662 | 2662 | NA | 612 | NA |
| Number of screens | 7473 | 7473 | 7473 | NA | 612 | NA |
| Number of cancers | 33 | 26 | 32 | 11 | 9 | 111 |
| Invasive cancers, n (%) | 18 (55) | 23 (88) | 30 (94) | 10 (91) | 8 (89) | 89 (80) |
| Median size invasive tumor, mm (range) | 11.5 (1–55) | 16.0 (3–0) | 10.0 (2–40) | 8.5 (2–13) | 8.5 (1–25) | 12.0 (1–55) |
| Nodal staging availablea | 15 | 15 | 27 | 6 | 7 | 70 |
| Node positive, n (%) | 5(33) | 7(47) | 1(4) | 0(0) | 0(0) | 13(19) |
| Cancer Type and grade, n (%) | ||||||
| IDC | 17 (52) | 16 (62) | 24 (75) | 8 (73) | 7 (78) | 72 (65) |
| High grade | 7 (21) | 4 (15) | 6 (19) | 2 (18) | 2 (22)b | 21 (19) |
| Intermediate grade | 6 (18) | 8 (31) | 7 (22) | 1 (9) | 1 (11) | 23 (21) |
| Low grade | 3 (9) | 4 (15) | 11 (34) | 3 (27) | 4 (44) | 25 (23) |
| Grade cannot be assessed | 1 (3) | 0 | 0 | 2 (18) | 0 | 3 (3) |
| ILC | 1 (3) | 5 (19)c | 5 (16) | 1 (9) | 0 | 12 (11) |
| Mixed IDC and ILC | 0 | 2 (8)d | 1 (3)d | 1 (9) | 1 (11) | 5 (5) |
| DCIS | 15 (45) | 3 (12) | 2 (6) | 1 (9) | 1 (11) | 22 (20) |
| High grade | 2 (6) | 0 | 1 (3) | 1 (9) | 0 | 4 (4) |
| Intermediate grade | 11 (33) | 3 (12) | 1 (3) | 0 | 0 | 15 (14) |
| Low grade | 2 (6) | 0 | 0 | 0 | 1 (11) | 3 (3) |
Abbreviations used: IDC = invasive ductal carcinoma; ILC = invasive lobular carcinoma; DCIS = ductal carcinoma in situ; grade was collected only for IDC and DCIS
Axillary nodal status could not be assessed for 14 participants with PHBC where nodes had previously been removed, nor for one with a personal history of Hodgkin’s disease and prior nodal treatment. Node status was not determined for one participant over age 80 as it would not affect treatment planning. For three participants without nodal staging, the reason was unknown.
Includes one T1mic tumor, with grade based on the DCIS grade
Includes one ILC with DCIS for which grade of the ILC is missing
Includes one mixed IDC-ILC with associated intermediate nuclear grade DCIS
A total of 16/612 (2.6%) MRI participants were diagnosed with breast cancer, with 12/16 (75%) invasive. 9/16 (56%) were seen only on MRI after negative mammography and US: 8/9 (89%) were invasive, with median size 8.5 mm (range 1–25), and all 7 with staging were node negative (Table 2). Two invasive cancers detected by US and not mammography in the MRI substudy were also seen on MRI.
Supplemental Cancer Detection Yield
Supplemental US increased cancer detection with each annual screen beyond that of mammography, adding detection of 5.3 cancers per 1000 women in year one (95%CI 2.1 to 8.4; p<.001 vs. mammography alone); 3.7 per 1000 per year in years two and three (95%CI 2.1 to 5.8, p<.001 vs. mammography alone) (Table 3); and averaging 4.3 per 1000 for each of three rounds of annual screening. Addition of MRI increased cancer detection, with supplemental cancer detection yield of 14.7 per 1000 women (95%CI 3.5 to 25.9, p=.004 vs. M+US) (Table 4). Number of screens needed to detect one cancer was 127(95%CI 99 to 167) for mammography; 234(95%CI 173 to 345) for supplemental ultrasound and 68 (95%CI 39 to 286) for MRI after negative M+US.
Table 3.
Screening Performance in 2662 Unique Participants Screened Three Years with Mammography and Physician-Performed Ultrasound
| Mammography (M) alone | Combined Mammography and US (M + US) |
Difference of (M + US) and M alone |
Ultrasound (US) alone | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Est | (No/ Total) | 95% CI | Est | (No/ Total) | 95% CI | Est | 95% CI | pvalue | Est | (No/ Total) | 95% CI | ||
|
Yield, per 1000 (N/Total) |
Screen 1 | 7.5 | (20/ 2659) | (4.6, 11.6) | 12.8 | (34/ 2659) | (8.9, 17.8) | 5.3 | (2.1, 8.4) | <0.001 | 9.0 | (24/ 2659) | (5.8, 13.4) |
| Screen 23a | 8.1 | (39/ 4814) | (5.8, 11.1) | 11.8 | (57/ 4814) | (9.0, 15.3) | 3.7 | (2.1, 5.8) | <0.001 | 7.1 | (34/ 4814) | (4.9, 9.9) | |
| AUC | Screen 1 | 0.74 | (0.63, 0.84) | 0.94 | (0.89, 0.99) | 0.20 | (0.10, 0.30) | <0.001 | 0.76 | (0.66, 0.87) | |||
| Screen 2 | 0.75 | (0.65, 0.86) | 0.89 | (0.82, 0.97) | 0.14 | (0.03, 0.25) | 0.012 | 0.71 | (0.58, 0.84) | ||||
| Screen 3 | 0.72 | (0.64, 0.81) | 0.82 | (0.74, 0.89) | 0.10 | (0.00, 0.18) | 0.041 | 0.62 | (0.52, 0.72) | ||||
|
Sensitivity, % (N/Total) |
Screen 1 | 55.6 | (20/ 36) | (38.1, 72.1) | 94.4 | (34/ 36) | (81.3, 99.3) | 38.9 | (20.2, 57.6) | <0.001 | 66.7 | (24/ 36) | (49.0, 81.4) |
| Screen 23 | 52.0 | (39/ 75) | (40.2, 63.7) | 76.0 | (57/ 75) | (64.7, 85.1) | 24.0 | (14.7, 33.3) | <0.001 | 45.3 | (34/ 75) | (33.8, 57.3) | |
|
Specificity, % (N/Total) |
Screen 1 | 89.1 | (2337/ 2623) | (87.8, 90.3) | 74.3 | (1950/ 2623) | (72.6, 76.0) | −14.8 | (−16.3, −13.2) | <0.001 | 79.8 | (2092/ 2623) | (78.2, 81.3) |
| Screen 23 | 91.3 | (4325/ 4739) | (90.4, 92.1) | 84.1 | (3987/ 4739) | (83.1, 85.2) | −7.1 | (−8.0, −6.3) | <0.001 | 89.9 | (4258/ 4739) | (89.0, 90.7) | |
|
Recall Rate, % (N/Total) |
Screen 1 | 11.5 | (306/ 2659) | (10.3, 12.8) | 26.6 | (707/ 2659) | (24.9, 28.3) | 15.1 | (13.5, 16.6) | <0.001 | 20.9 | (555/ 2659) | (19.3, 22.5) |
| Screen 23 | 9.4 | (453/ 4814) | (8.6, 10.3) | 16.8 | (809/ 4814) | (15.8, 17.9) | 7.4 | (6.6, 8.2) | <0.001 | 10.7 | (515/ 4814) | (9.8, 11.6) | |
|
PPV1,b % (N/Total) |
Screen 1 | 6.5 | (20/ 306) | (4.0, 9.9) | 4.8 | (34/ 707) | (3.4, 6.7) | −1.7 | (−3.7, 0.1) | 0.073 | 4.3 | (24/ 555) | (2.8, 6.4) |
| Screen 23 | 8.6 | (39/ 453) | (6.2, 11.6) | 7.0 | (57/ 809) | (5.4, 9.0) | −1.6 | (−3.1, −0.2) | 0.038 | 6.6 | (34/ 515) | (4.6, 9.1) | |
|
Short-term Follow-up Rate, % (N/Total) |
Screen 1 | 3.2 | (84/ 2659) | (2.5, 3.9) | 13.8 | (368/ 2659) | (12.5, 15.2) | 10.7 | (9.5, 11.9) | <0.001 | 11.1 | (296/ 2659) | (10.0, 12.4) |
| Screen 23 | 1.6 | (76/ 4814) | (1.2, 2.0) | 5.3 | (256/ 4814) | (4.7, 6.0) | 3.7 | (3.2, 4.3) | <0.001 | 3.9 | (190/ 4814) | (3.4, 4.5) | |
| Biopsy Rate, % (N/Total) | Screen 1 | 2.4 | (65/ 2659) | (1.9, 3.1) | 10.2 | (272/ 2659) | (9.1, 11.4) | 7.8 | (6.7, 8.8) | <0.001 | 8.8 | (233/ 2659) | (7.7, 9.9) |
| Screen 23 | 2.0 | (97/ 4814) | (1.6, 2.5) | 7.0 | (339/ 4814) | (6.3, 7.8) | 5.0 | (4.4, 5.7) | <0.001 | 5.5 | (266/ 4814) | (4.9, 6.2) | |
|
PPV3,c % (N/Total) |
Screen 1 | 29.2 | (19/ 65) | (18.6, 41.8) | 11.4 | (31/ 272) | (7.9, 15.8) | −17.8 | (−26.7, −9.3) | <0.001 | 9.0 | (21/ 233) | (5.7, 13.4) |
| Screen 23 | 38.1 | (37/ 97) | (28.5, 48.6) | 16.2 | (55/ 339) | (12.5, 20.6) | −21.9 | (−28.7, −14.7) | <0.001 | 11.7 | (31/ 266) | (8.1, 16.1) | |
Screen 23 refers to incidence screens in years 2 and 3 (i.e. at 12 and 24 months after study entry respectively).
PPV1 is defined as the malignancy rate among women with a positive screening test (i.e. recalled from screening for further testing or short-interval follow-up).
PPV3 is defined as the malignancy rate among women with a positive screening test who underwent biopsy of the same lesion.
Table 4.
Screening Performance in 612 Participants Screened with MRI after Three Annual Screens with Mammography and Ultrasound
| (M+US+MR) - (M+US) | (M+MRI) - M | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| M+US | M+US+MR | Est | 95% CI | P | M | M + MRI | Est | 95% CI | P | MRI | |
|
Yield, per 1000 (No/Total) (95% CI) |
11.4 (7/612) (4.6, 23.4) |
26.1 (16/612) (15.0, 42.1) |
14.7 | (3.5, 25.9) | 0.004 | 8.2 (5/612) (2.7, 19.0) |
26.1 (16/612) (15.0, 42.1) |
18.0 | (5.8, 30.1) | <.001 | 22.9 (14/612) (12.6, 38.1) |
|
AUC (95% CI) |
0.69 (0.55, 0.83) |
0.95 (0.91, 0.99) |
0.26 | (0.11, 0.42) | <.001 | 0.63 (0.47, 0.78) |
0.94 (0.90, 0.98) |
0.31 | (0.16, 0.46) | <.001 | 0.87 (0.75, 0.98) |
|
Sensitivity, % (No/Total) (95% CI) |
43.8 (7/16) (19.8, 70.1) |
100.0 (16/16) (79.4, 100.0) |
56.3 | (25.7, 86.8) | 0.004 | 31.3 (5/16) (11.0, 58.7) |
100.0 (16/16) (79.4, 100.0) |
68.8 | (39.8, 97.7) | <.001 | 87.5 (14/16) (61.7, 98.4) |
|
Specifity, % (No/Total) (95% CI) |
84.4 (503/596) (81.2, 87.2) |
65.4 (390/596) (61.5, 69.3) |
−19.0 | (−22.3, −15.6) | <.001 | 92.1 (549/596) (89.7, 94.1) |
70.6 (421/596) (66.8, 74.3) |
−21.5 | (−24.9, −18.0) | <.001 | 75.7 (451/596) (72.0, 79.1) |
|
Recall Rate, % (No/Total) (95% CI) |
16.3 (100/612) (13.5, 19.5) |
36.3 (222/612) (32.5, 40.2) |
19.9 | (16.6, 23.3) | <.001 | 8.5 (52/612) (6.4, 11.0) |
31.2 (191/612) (27.6, 35.0) |
22.7 | (19.2, 26.2) | <.001 | 26.0 (159/612) (22.5, 29.6) |
|
PPV1, % (No/Total) (95% CI) |
7.0 (7/100) (2.9, 13.9) |
7.2 (16/222) (4.2, 11.4) |
0.2 | (−3.8, 4.0) | 0.917 | 9.6 (5/52) (3.2, 21.0) |
8.4 (16/191) (4.9, 13.2) |
−1.2 | (−8.0, 4.6) | 0.701 | 8.8 (14/159) (4.9, 14.3) |
|
Short-term follow up rate, % (No/Total) (95% CI) |
4.6 (28/612) (3.1, 6.5) |
19.6 (120/612) (16.5, 23.0) |
15.0 | (12.0, 18.0) | <.001 | 0.5 (3/612) (0.1, 1.4) |
16.3 (100/612) (13.5, 19.5) |
15.8 | (12.8, 18.9) | <.001 | 15.8 (97/612) (13.0, 19.0) |
|
Biopsy rate, % (No/Total) (95% CI) |
6.2 (38/612) (4.4, 8.4) |
13.2 (81/612) (10.7, 16.2) |
7.0 | (4.8, 9.2) | <.001 | 1.6 (10/612) (0.8, 3.0) |
9.6 (59/612) (7.4, 12.3) |
8.0 | (5.7, 10.3) | <.001 | 8.5 (52/612) (6.4, 11.0) |
|
PPV3, % (No/Total) (95% CI) |
18.4 (7/38) (7.7, 34.3) |
18.5 (15/81) (10.8, 28.7) |
0.1 | (−8.8, 8.8) | 0.983 | 50.0 (5/10) (18.7, 81.3) |
25.4 (15/59) (15.0, 38.4) |
−24.6 | (−51.2, 3.7) | 0.081 | 23.1 (12/52) (12.5, 36.8) |
p-value that observed difference of combined mammography, US, and MRI vs. mammography and US occurred by chance
p-value that observed difference of combined mammography and MRI vs. mammography alone occurred by chance
Yield is the cancer detection rate
PPV1 is defined as the malignancy rate among women with a positive screening test (i.e. recalled from screening for further testing or short-interval follow-up).
PPV3 is defined as the malignancy rate among women with a positive screening test who underwent biopsy of the same lesion.
Sensitivity, Specificity, and AUC
Sensitivity of combined mammography and US was 57/75(0.76, 95%CI 0.65 to 0.85) for combined screens two and three, higher than mammography alone [39/75(0.52, 95%CI 0.40 to 0.64), p<.001]. Specificity of combined mammography and US was 3987/4739 (0.84, 95%CI 0.83 to 0.85) for incidence screens, lower than specificity of mammography alone [4325/4739 (0.91, 95%CI 0.90 to 0.92), p<.001] (Table 3).
For 612 MRI participants, sensitivity increased from 7/16(0.44, 95%CI 0.20 to 0.70) with combined mammography and ultrasound to 16/16(1.00, 95%CI 0.79 to 1.00) with addition of MRI(p=.004). Specificity was reduced to 390/596(0.65, 95%CI 0.61 to 0.69) after MRI, compared to combined M+US at 503/596(0.84, 95%CI 0.81 to 0.87, p <.001) (Table 4).
Overall AUC increased in each year when US was added to mammography (Table 3). Adding MRI lowered apparent performance of M+US since more cancers were identified by MRI (Table 4).
Additional Biopsies and PPV3
PPV3 of combined mammography and US was 31/272(0.11, 95%CI 0.08 to 0.16) for year 1 and 55/339(0.16, 95%CI 0.12 to 0.21) for incidence screens (years two and three combined), These values were significantly lower than those of mammography alone (19/65 [0.29, 95%CI 0.19 to 0.42] and 37/97[0.38, 95%CI 0.28 to 0.49] respectively, p<.001 for both) (Table 3). Percentage of women undergoing biopsy after mammography and US decreased from 272/2659 (10.2%, 95%CI 9.1 to 11.4%) in year 1 to 339/4814 (7.0%, 95%CI 6.3 to 7.8%) for incidence screens (p<.001). The biopsy rates after mammography alone were 65/2659 (2.4%, 95%CI 1.9 to 3.1%) in year 1 and 97/4814 (2.0%, 95%CI 1.6 to 2.5%) for incidence screens.
For 612 MRI participants, rate of biopsy after full workup of M+US was 38/612(6.2%, 95%CI 4.4 to 8.4%), which increased to 81/612(13.2%, 95% CI10.7 to 16.2%) with addition of MRI (p<.001). PPV3 after M+US was 7/38(0.18, 95%CI 0.08 to 0.34) and with addition of MRI was 15/81(0.19, 95%CI 0.11 to 0.29, p=.98) (Table 4).
Interval Cancers
Of 20 women with cancer not seen on either mammography or US in three annual rounds, 9 were among the 612 participants who also had MRI screening in year three, with cancer detected on MRI. Another 9 cancers were identified because of clinical abnormalities in interval between screens (interval cancer rate 8.1%): two had clinical findings in year one; four in year two; and three in year three. One participant was found to have high-grade DCIS because of off-study computer-assisted detection applied to mammogram (revealing calcifications) after year 3 interpretation had been recorded. One participant with BRCA-1 mutation had MRI off study six months after the third screen and was found to have a 7 mm node-negative grade III IDC.
Women with Personal History of Breast Cancer (PHBC)
A total of 1426/2659(54%) of participants had PHBC at study entry and underwent 4010 screens; 59/1426 (4.1%) were diagnosed with cancer (28 only ipsilateral and 29 only contralateral to the original cancer; 2 bilateral). Supplemental yield of US was the same in women with PHBC and in women without PHBC (online Table 5A), as was the absolute increase in sensitivity due to added ultrasound. Supplemental US was less likely to prompt unnecessary recall or biopsy in women with PHBC than those without (online Table 5A). . The supplemental yield of MRI in women with or without PHBC in MR substudy is detailed online (online Table 5B). Supplemental MRI was less likely to prompt unnecessary recall or biopsy in women with PHBC than those without (online Table 5B).
DISCUSSION
In this study, annual supplemental incidence screening ultrasound detected an additional 3.7 cancers per 1000 women per year screened beyond mammography alone. The majority of cancers seen only on US were node-negative invasive cancers; invasive lobular carcinoma and low-grade invasive ductal carcinoma were overrepresented among such cancers.
One of the major concerns about screening is the harm of extra testing and biopsies for women who do not have cancer.20 As has been observed with mammography21 and MRI,11,22–24 the risk of false positives decreased significantly with annual screening ultrasound in this study compared to the first screen. However, there still remained a substantial rate of biopsies prompted only by incidence screening ultrasound, averaging 5.0% (242/4814) of women, with only 7.4% (18/242) of those biopsied only due to ultrasound found to have cancer.
In a separate analysis of ACRIN 6666 participants, MRI was significantly less tolerable than mammography or ultrasound.25 Only 58% of ACRIN 6666 participants offered a screening MRI at no out-of-pocket cost accepted the invitation.15 These barriers are in addition to high costs of MRI equipment, contrast, and examination, as well as high rates of induced testing including biopsy.
Contrast-enhanced MRI has been recommended for supplemental screening of women at high risk for breast cancer, defined as those women with a lifetime risk of 20 to 25% or greater based on family history or prior mantle radiation to the chest.26 Across nine series, the supplemental yield of MRI after mammography in high-risk women was 11 per 1000 (reviewed in 27), and was 14 per 1000 among the subset who also had screening ultrasound.11–13,24 Similar results were observed in this study of women who were mostly at intermediate risk for breast cancer.
Fewer studies have evaluated MRI in women at intermediate risk, including women with PHBC, prior atypical biopsy or lobular carcinoma in situ (LCIS), intermediate family history of breast cancer (lifetime risk of 15–20% per the ACS guidelines26), or women whose only risk factor is dense breasts. Recent studies collectively suggest that supplemental MRI screening may be reasonable for women with PHBC and also found false positives to be less frequent than in women with a family history of breast cancer.28–30
For high-risk women unable to undergo MRI,15 and for intermediate-risk women with dense breasts, including those with PHBC, this study supports the use of supplemental screening with ultrasound in addition to mammography. With either MRI or US, the risks of false positives, including unnecessary biopsies, were lower for supplemental screening in women with PHBC than in women without. The outcomes in terms of staging, node-positive disease, and interval cancer rates achieved in this study after three years of programmatic screening with both ultrasound and mammography were comparable to benchmarks from studies which included MRI.10–13,24
If screening ultrasound were to be adopted for women with dense breasts who are not candidates for MRI, there would be obstacles to its implementation. These include the availability of only one current procedural terminology (CPT) code, 76645, for breast ultrasound, with low reimbursement (2010 Medicare reimbursement averaged a global fee of $89.85 to $91.83,31 which does not cover the costs of physicians performing and interpreting a thorough screening examination). While supplemental cancer detection rates with technologist-performed screening US were similar to physician-performed US in one series,4 there remains a shortage of qualified breast US technologists.
There are a few limitations to this study. Additional node-negative invasive cancers were found by adding screening ultrasound to mammography in each incidence screen, and increasing detection of such cancers correlates with mortality reduction;32 however, we did not have a control group with no ultrasound performed in which to compare clinical outcomes, and mortality was not assessed. In Japan, the ongoing J-START trial of biennial mammography, with or without technologist-performed screening ultrasound, will follow outcomes to mortality reduction.33 We only performed a single screening MRI, and false positives would be expected to decrease in subsequent years.11,22 Not all sites in the original ACRIN 6666 protocol were able to offer MRI.
SUMMARY
In summary, the cancer detection benefit to supplemental screening ultrasound seen on the first screen persisted with each annual screen. Rates of biopsy for findings seen only on ultrasound remained substantial on incidence screens, representing 5% of women, with only 7.4% of those women found to have cancer. Risks of false positives were lower in women with PHBC than in women without.
As has been seen in other studies10,11,24,34, MRI significantly increased detection of early breast cancer beyond that seen with mammography or mammography combined with ultrasound. The 56% absolute increase in cancer detection seen in the MRI substudy (16/16 vs. 7/16) was greater than the 34% absolute increase in invasive cancer detection (71/89 vs. 41/89) seen by adding annual ultrasound to mammography in the main ACRIN 6666 study. However, given the low (clinically-detected) interval cancer rate of only 8% in the main ACRIN 6666 protocol, and the fact that all interval cancers remained node-negative at diagnosis, it is unclear that the added cost and reduced tolerability of screening MRI are justified in women at intermediate risk for breast cancer in lieu of supplemental screening with ultrasound. Despite its higher sensitivity, addition of screening MRI rather than ultrasound to mammography in broader populations of women at intermediate risk with dense breasts may not be appropriate, particularly when the current high false positive rates, cost, and reduced tolerability of MRI are considered.
Supplementary Material
Acknowledgements
Funding/Support: The study was funded by the Avon Foundation and grants CA 80098 and CA 79778 from the National Cancer Institute.
Role of the Sponsors: The Avon Foundation was not involved in the design or conduct of the study; collection, management, analysis, or interpretation of the data; or preparation, review, or approval of the manuscript. The trial was conducted by the American College of Radiology Imaging Network, a member of the National Cancer Institute’s Clinical Trials Cooperative Groups Program, and was developed and carried out adhering to the standard cooperative group processes. These processes include review of and input about the trial design from the NCI’s Cancer Therapy Evaluation Program (CTEP). Upon CTEP’s approval of the research protocol, the NCI was not involved in the design or conduct of the study; collection, management, analysis, or interpretation of the data; or preparation, review, or approval of the manuscript.
Abbreviations used
- ACRIN
American College of Radiology Imaging Network.
Footnotes
Presented at the 2009 Radiologic Society of North America Scientific Assembly Trial Registration Clinicaltrials.gov identifier: NCT 00072501.
The full protocol is available online at: http://acrin.org/Portals/0/Protocols/6666/Protocol-ACRIN%206666%20Admin%20Update%2011.30.07.pdf
Author Contributions: Drs. Zhang and Cormack had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Berg, Zhang, Mendelson, Pisano.
Acquisition of data: Berg, Mendelson, Lehrer, Böhm-Vélez, Pisano, Jong, Evans, Morton, Mahoney, Hovanessian-Larsen, Barr, Farria, Gabrielli.
Analysis and interpretation of data: Berg, Zhang, Cormack, Pisano, Marques, Adams, Yeh.
Drafting of the manuscript: Berg, Zhang.
Critical revision of the manuscript for important intellectual content: Berg, Zhang, Mendelson, Lehrer, Böhm-Vélez, Pisano, Jong, Evans, Morton, Mahoney, Hovanessian-Larsen, Barr, Farria, Gabrielli.
Statistical analysis: Zhang, Cormack, Marques, Adams, Yeh.
Obtained funding: Berg.
Administrative, technical, or material support: Berg, Mendelson, Gabrielli.
Study supervision: Berg, Zhang.
Financial Disclosures: Dr Berg reports that she has served as a consultant to Naviscan, Inc. and SuperSonic Imagine, has received research support from Naviscan Inc., has prepared educational materials for Gamma Medica, has a research grant from Hologic, Inc., and is on the medical advisory board of Philips. Dr Mendelson reports that she is a member of the scientific advisory boards of MediPattern, Hologic, and Siemens and has received equipment support from Philips and research support from SuperSonic Imagine and Siemens. Dr Böhm-Vélez reports that she is a member of the scientific advisory board of Philips, does clinical validation studies for Philips Ultrasound, and is on the speakers bureau of Dilon. Dr Pisano reports that her laboratory received research support from GE Healthcare, Konica Minolta, Sectra AB, Naviscan Inc., Koning, Zumatek, Inc., equipment grants from R2 and iCAD, is a board member of ACR Imaging Metrix and NextRay, Inc., and a stockholder inNextRay, Inc. Dr Jong reports that she is a consultant to and receives research support from GE Healthcare. Dr Evans reports that he is a member of the scientific advisory board of Hologic. Dr Mahoney reports that she is a consultant to Ethicon EndoSurgery and SenoRx and on the scientific advisory board of Hologic and receives research support from Naviscan, Inc.. Dr Larsen reports that she receives equipment support from Naviscan Inc. Dr Barr reports that he is a member of the ultrasound advisory boards of and has received equipment support, research support, and speakers fees from Siemens and Philips, an equipment grant from SuperSonic, Inc., and a research grant from Bracco. The remaining coauthors report no financial disclosures.
ACRIN 6666 Site Investigators: Allegheny-Singer Research Institute, Pittsburgh, Pennsylvania: William R. Poller, MD, principal investigator (PI), Michelle Huerbin, research associate (RA); American Radiology Services–Johns Hopkins Green Spring, Baltimore, Maryland: Wendie A. Berg, MD, PhD (PI), Barbara E. Levit, RT(RA), Kathy Wetzel (RA); Beth Israel Deaconess Medical Center, Boston, Massachusetts: Janet K. Baum, MD, and Valerie J. Fein-Zachary, MD (PIs), Suzette M. Kelleher, BA (RA); CERIM, Buenos Aires: Daniel E. Lehrer, MD(PI), Maria S. Ostertag (RA); Duke University Medical Center, Durham, North Carolina: Mary Scott Soo, MD (PI), Brenda N. Prince, RT (RA); Mayo Clinic, Rochester, Minnesota: Marilyn J. Morton, DO (PI), Lori M.
Johnson, AAS (RA); Feinberg School of Medicine, Northwestern University, Chicago, Illinois: Ellen B. Mendelson, MD (PI), Marysia Kalata, AA (RA); Radiology Associates of Atlanta, Atlanta, Georgia: Handel Reynolds,MD(PI), Y. Suzette Wheeler, RN, MSHA (RA); Radiology Consultants/Forum Health, Youngstown, Ohio: Richard G. Barr, MD, PhD (PI), Marilyn J. Mangino, RN (RA); Radiology Imaging Associates, Denver, Colorado: A. Thomas Stavros,MD(PI), Margo Valdez (RA); Sunnybrook Health Sciences Centre, University of Toronto, Toronto, Ontario, Canada: Roberta A. Jong,MD(PI), Julie H. Lee, BSC (RA); Thomas Jefferson University Hospital, Philadelphia, Pennsylvania: Catherine W. Piccoli, MD, and Christopher R.B. Merritt, MS,MD(PIs), Colleen Dascenzo (RA); David Geffen School of Medicine at University of California Los Angeles Medical Center, Los Angeles: Anne C. Hoyt, MD (PI), Roslynn Marzan, BS (RA); University of Cincinnati Medical Center, Cincinnati, Ohio: Mary C. Mahoney, MD (PI), Monene M. Kamm, AS (RA); University of North Carolina, Chapel Hill: Etta D. Pisano, MD (PI), Laura A. Tuttle, MA (RA), Keck School of Medicine, University of Southern California, Los Angeles: Linda H. Larsen, MD(PI), Christina E. Kiss, AA (RA); University of Texas M. D. Anderson Cancer Center, Houston: Gary J. Whitman, MD (PI), Sharon R. Rice, AA (RA); University of Texas Southwestern Medical Center, Dallas: W. Phil Evans, MD (PI), Kimberly T. Taylor, AA (RA); Washington University School of Medicine, St. Louis, Missouri: Dione M. Farria, MD, MPH (PI), Darlene J. Bird, RT, AS (RA); and Weinstein Imaging Associates, Pittsburgh, Pennsylvania: Marcela Böhm-Vélez, MD, (PI), Antoinette Cockroft (RA).
Additional Contributions: The authors thank Jeffrey Blume, PhD, Vanderbilt University, Nashville, TN, for his contributions to initial study design and supervision and Robert A. Smith, PhD, American Cancer Society, Atlanta, GA, for review and helpful discussions. We especially thank Marydale DeBor, JD, Chief Advisor Avon Breast Cancer Crusade 1993–2003, who was instrumental in securing the support within the Avon Foundation which made this study possible, and Marc Hurlbert, PhD, of the Avon Foundation for his continued vision and unwavering support. We are indebted to the many investigators, coinvestigators, and research associates at the clinical sites. We appreciate the efforts of ACRIN Data Management and Imaging staff, and we especially thank Cynthia Olson, MBA, MHS, for administrative oversight. We thank Eric Berns, PhD, University of Colorado, Denver, CO, for ultrasound image quality control and R. Edward Hendrick, PhD, University of Colorado, Denver, CO, for MRI imaging quality control. No one was compensated beyond their usual salary for their efforts for this study. We are especially grateful to the 2809 women who enrolled in this study.
References
- 1.Buchberger W, Niehoff A, Obrist P, DeKoekkoek-Doll P, Dunser M. Clinically and mammographically occult breast lesions: detection and classification with high-resolution sonography. Semin Ultrasound CT MR. 2000;21(4):325–336. doi: 10.1016/s0887-2171(00)90027-1. [DOI] [PubMed] [Google Scholar]
- 2.Crystal P, Strano SD, Shcharynski S, Koretz MJ. Using sonography to screen women with mammographically dense breasts. AJR Am J Roentgenol. 2003 Jul;181(1):177–182. doi: 10.2214/ajr.181.1.1810177. [DOI] [PubMed] [Google Scholar]
- 3.Gordon PB, Goldenberg SL. Malignant breast masses detected only by ultrasound. A retrospective review [see comments] Cancer. 1995;76(4):626–630. doi: 10.1002/1097-0142(19950815)76:4<626::aid-cncr2820760413>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 4.Kaplan SS. Clinical utility of bilateral whole-breast US in the evaluation of women with dense breast tissue. Radiology. 2001 Dec;221(3):641–649. doi: 10.1148/radiol.2213010364. [DOI] [PubMed] [Google Scholar]
- 5.Kolb TM, Lichy J, Newhouse JH. Comparison of the performance of screening mammography, physical examination, and breast US and evaluation of factors that influence them: An analysis of 27,825 patient evaluations. Radiology. 2002 Oct;225(1):165–175. doi: 10.1148/radiol.2251011667. [DOI] [PubMed] [Google Scholar]
- 6.Leconte I, Feger C, Galant C, et al. Mammography and subsequent whole-breast sonography of nonpalpable breast cancers: the importance of radiologic breast density. AJR Am J Roentgenol. 2003 Jun;180(6):1675–1679. doi: 10.2214/ajr.180.6.1801675. [DOI] [PubMed] [Google Scholar]
- 7.Berg WA, Blume JD, Cormack JB, et al. Combined screening with ultrasound and mammography vs mammography alone in women at elevated risk of breast cancer. JAMA. 2008 May 14;299(18):2151–2163. doi: 10.1001/jama.299.18.2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Corsetti V, Ferrari A, Ghirardi M, et al. Role of ultrasonography in detecting mammographically occult breast carcinoma in women with dense breasts. Radiol Med (Torino) 2006 Apr;111(3):440–448. doi: 10.1007/s11547-006-0040-5. [DOI] [PubMed] [Google Scholar]
- 9.Tohno E, Ueno E, Watanabe H. Ultrasound screening of breast cancer. Breast Cancer. 2009;16(1):18–22. doi: 10.1007/s12282-008-0082-8. [DOI] [PubMed] [Google Scholar]
- 10.Kuhl C, Weigel S, Schrading S, et al. Prospective multicenter cohort study to refine management recommendations for women at elevated familial risk of breast cancer: the EVA trial. J Clin Oncol. 2010 Mar 20;28(9):1450–1457. doi: 10.1200/JCO.2009.23.0839. [DOI] [PubMed] [Google Scholar]
- 11.Kuhl CK, Schrading S, Leutner CC, et al. Mammography, breast ultrasound, and magnetic resonance imaging for surveillance of women at high familial risk for breast cancer. J Clin Oncol. 2005 Nov 20;23(33):8469–8476. doi: 10.1200/JCO.2004.00.4960. [DOI] [PubMed] [Google Scholar]
- 12.Lehman CD, Isaacs C, Schnall MD, et al. Cancer yield of mammography, MR, and US in high-risk women: prospective multi-institution breast cancer screening study. Radiology. 2007 Aug;244(2):381–388. doi: 10.1148/radiol.2442060461. [DOI] [PubMed] [Google Scholar]
- 13.Sardanelli F, Podo F, D'Agnolo G, et al. Multicenter comparative multimodality surveillance of women at genetic-familial high risk for breast cancer (HIBCRIT study): interim results. Radiology. 2007 Mar;242(3):698–715. doi: 10.1148/radiol.2423051965. [DOI] [PubMed] [Google Scholar]
- 14.D'Orsi CJ, Bassett LW, Berg WA, et al. Breast Imaging Reporting and Data System, BI-RADS: Mammography. 4th edition. Reston: American College of Radiology; 2003. [DOI] [PubMed] [Google Scholar]
- 15.Berg WA, Blume JD, Adams AM, et al. Reasons women at elevated risk of breast cancer refuse breast MR imaging screening: ACRIN 6666. Radiology. 2010 Jan;254(1):79–87. doi: 10.1148/radiol.2541090953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leemis LM, Trivedi KS. A comparison of approximate interval estimators for the bernoulli parameter. Amer Statistician. 1996;50:63–68. [Google Scholar]
- 17.Fleiss JL, Levin B, Paik MC. Statistical Methods for Rates and Proportions. Hoboken: Wiley-Interscience; 2003. [Google Scholar]
- 18.Efron B. Bootstrap Methods: Another Look at the Jackknife. Ann Stat. 1979;7(1):1–26. [Google Scholar]
- 19.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988 Sep;44(3):837–845. [PubMed] [Google Scholar]
- 20.Nelson HD, Tyne K, Naik A, Bougatsos C, Chan BK, Humphrey L. Screening for breast cancer: an update for the U.S Preventive Services Task Force. Ann Intern Med. 2009 Nov 17;151(10):727–737. doi: 10.1059/0003-4819-151-10-200911170-00009. W237–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schell MJ, Yankaskas BC, Ballard-Barbash R, et al. Evidence-based target recall rates for screening mammography. Radiology. 2007 Jun;243(3):681–689. doi: 10.1148/radiol.2433060372. [DOI] [PubMed] [Google Scholar]
- 22.Kriege M, Brekelmans CT, Boetes C, et al. Differences between first and subsequent rounds of the MRISC breast cancer screening program for women with a familial or genetic predisposition. Cancer. 2006 Jun 1;106(11):2318–2326. doi: 10.1002/cncr.21863. [DOI] [PubMed] [Google Scholar]
- 23.Leach MO, Boggis CR, Dixon AK, et al. Screening with magnetic resonance imaging and mammography of a UK population at high familial risk of breast cancer: a prospective multicentre cohort study (MARIBS) Lancet. 2005 May 21–27;365(9473):1769–1778. doi: 10.1016/S0140-6736(05)66481-1. [DOI] [PubMed] [Google Scholar]
- 24.Warner E, Plewes DB, Hill KA, et al. Surveillance of BRCA1 and BRCA2 mutation carriers with magnetic resonance imaging, ultrasound, mammography, and clinical breast examination. Jama. 2004 Sep 15;292(11):1317–1325. doi: 10.1001/jama.292.11.1317. [DOI] [PubMed] [Google Scholar]
- 25.Schleinitz MD, De Palo DM, Blume JD, Cormack JB, Pisano ED, Berg WA. Tolerability of breast cancer screening, diagnostic, and biopsy procedures: Implications for new screening modalities. An ACRIN 6666 Substudy. Quality of Life Res. 2011 submitted. [Google Scholar]
- 26.Saslow D, Boetes C, Burke W, et al. American cancer society guidelines for breast screening with MRI as an adjunct to mammography. CA Cancer J Clin. 2007 Mar-Apr;57(2):75–89. doi: 10.3322/canjclin.57.2.75. [DOI] [PubMed] [Google Scholar]
- 27.Berg WA. Tailored supplemental screening for breast cancer: what now and what next? AJR Am J Roentgenol. 2009 Feb;192(2):390–399. doi: 10.2214/AJR.08.1706. [DOI] [PubMed] [Google Scholar]
- 28.Brennan ME, Houssami N, Lord S, et al. Magnetic resonance imaging screening of the contralateral breast in women with newly diagnosed breast cancer: systematic review and meta-analysis of incremental cancer detection and impact on surgical management. J Clin Oncol. 2009 Nov 20;27(33):5640–5649. doi: 10.1200/JCO.2008.21.5756. [DOI] [PubMed] [Google Scholar]
- 29.Brennan S, Liberman L, Dershaw DD, Morris E. Breast MRI screening of women with a personal history of breast cancer. AJR Am J Roentgenol. 2010 Aug;195(2):510–516. doi: 10.2214/AJR.09.3573. [DOI] [PubMed] [Google Scholar]
- 30.Demartini WB, Kalish GM, Peacock S, Eby PR, Gutierrez RL, Lehman CD. Screening MRI for high risk women: should patients with a treated personal history of breast cancer be screened?; Paper presented at: Radiologic Society of North America; November 28, 2010; Chicago, IL. 2010. [Google Scholar]
- 31. [accessed September 8, 2010]; http://www.cms.gov/PFSlookup/01_Overview.asp#TopOfPage.
- 32.Smith RA, Duffy SW, Gabe R, Tabar L, Yen AM, Chen TH. The randomized trials of breast cancer screening: what have we learned? Radiol Clin North Am. 2004 Sep;42(5):793–806. doi: 10.1016/j.rcl.2004.06.014. v. [DOI] [PubMed] [Google Scholar]
- 33.Ohuchi N, Suzuki A, Sakarai Y, et al. Current Status and Problems of Breast Cancer Screening. JMAJ. 2009;52:45–49. [Google Scholar]
- 34.Sardanelli F, Podo F, Santoro F, et al. Multicenter surveillance of women at high genetic breast cancer risk using mammography, ultrasonography, and contrast-enhanced magnetic resonance imaging (the high breast cancer risk Italian 1 study): final results. Invest Radiol. 2011 Feb;46(2):94–105. doi: 10.1097/RLI.0b013e3181f3fcdf. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.