Abstract
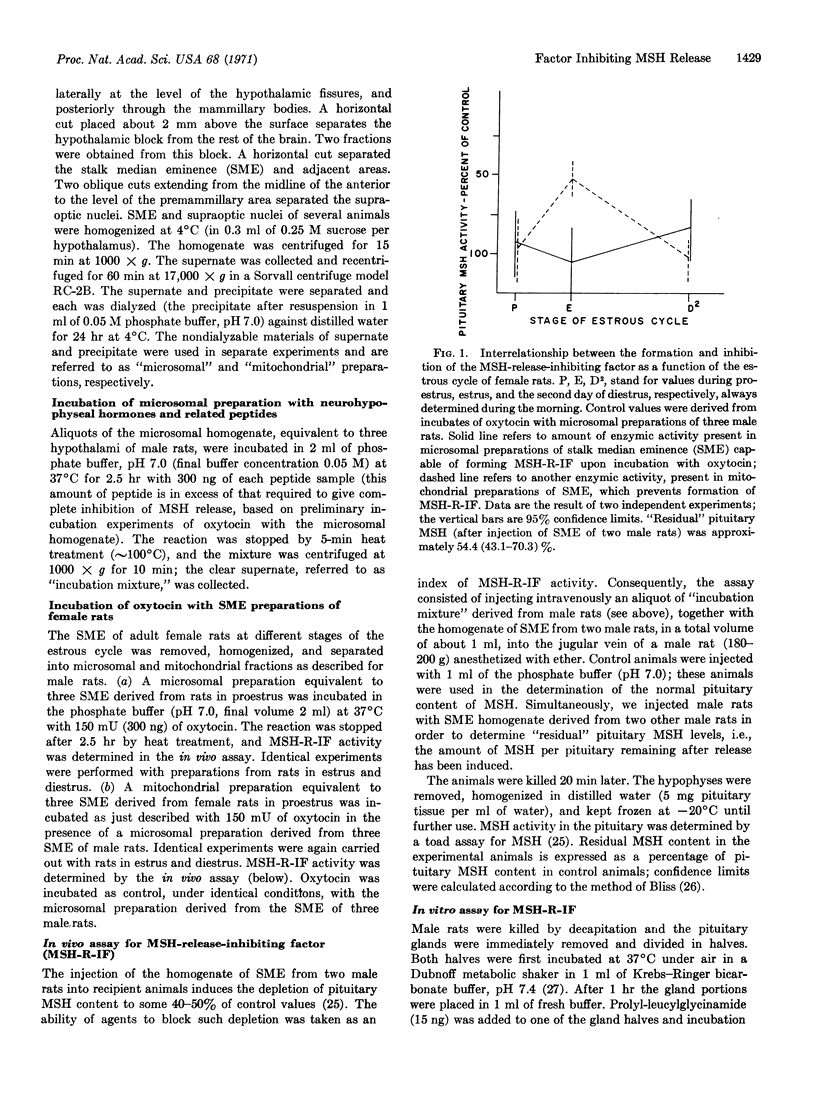
Microsomal preparations from the stalk median eminence of female rats are shown to contain an enzymic activity that is responsible for the formation of MSH-release-inhibiting factor (MSH-R-IF). The amount of this activity remains constant throughout the estrous cycle. The corresponding mitochondrial preparations from the stalk median eminence contain another enzymic principle, estrous cycle-dependent, which competes with the enzyme present in the microsomal preparation for the same “substrate”, and can thereby prevent the formation of MSH-R-IF.
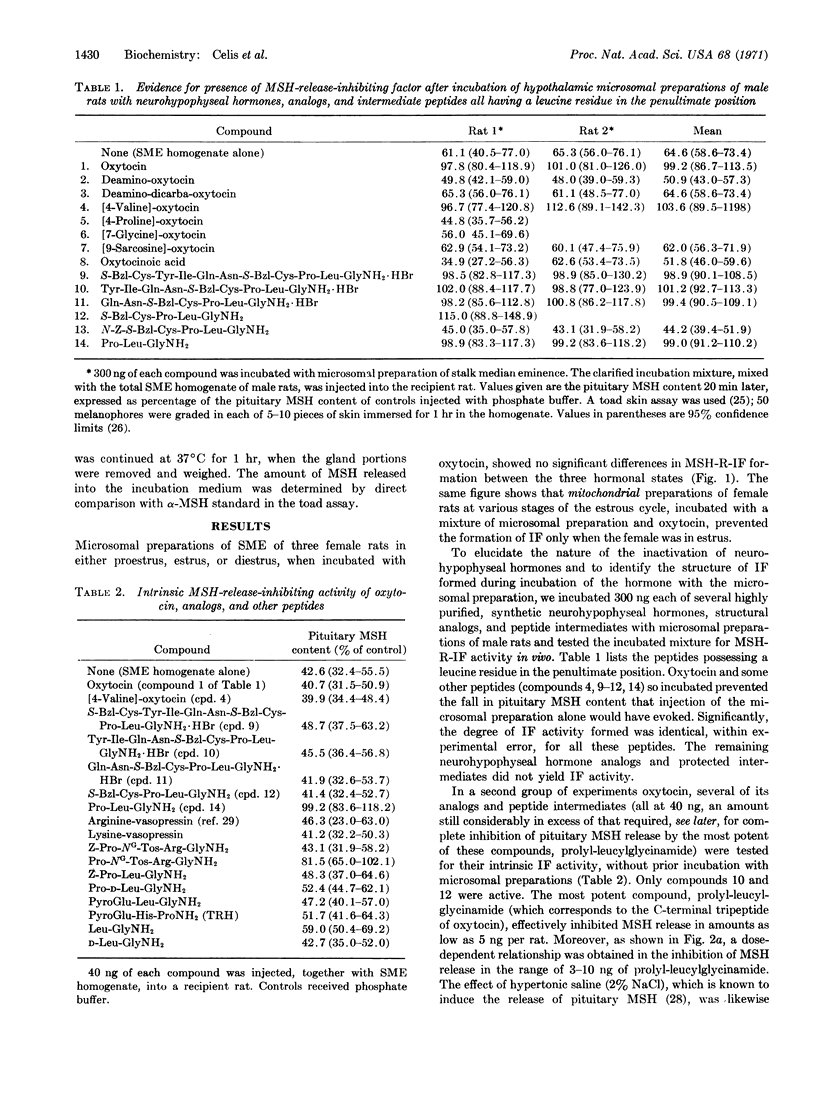
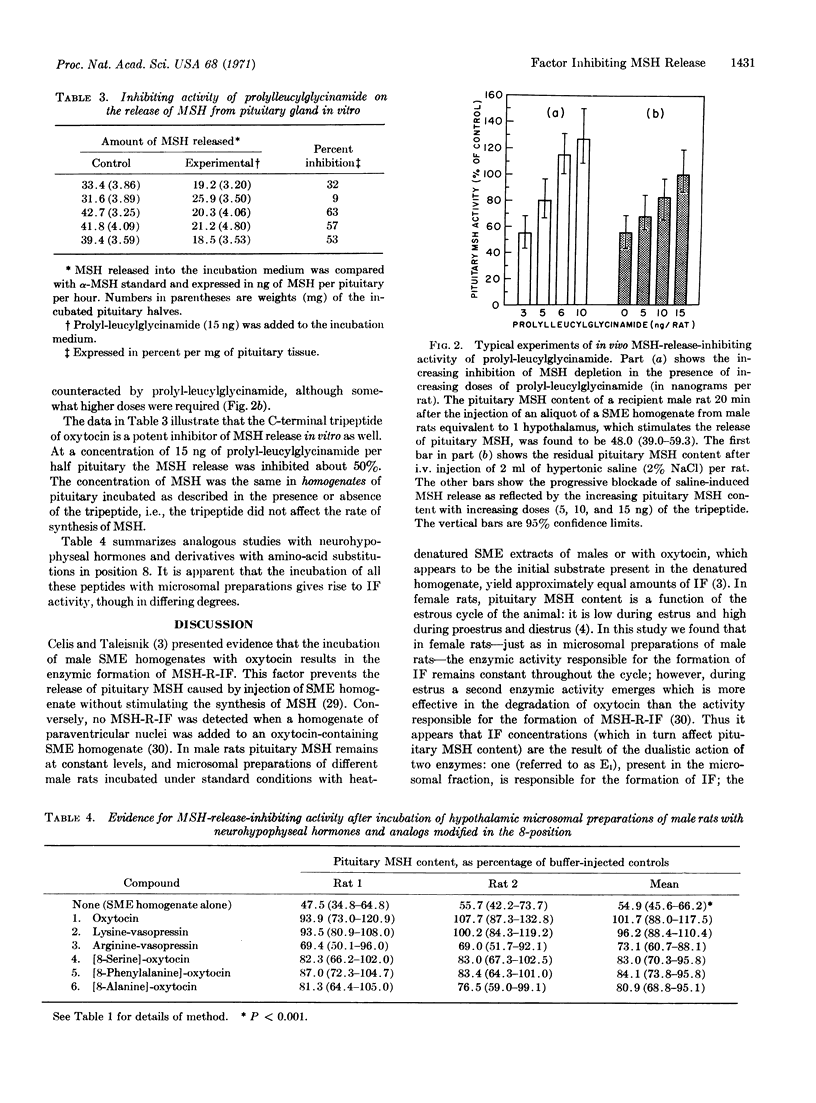
Several neurohypophyseal hormones, analogs, and peptide intermediates have been tested for their intrinsic MSH-R-IF activity and for their ability to be transformed into MSH-R-IF by incubation with microsomal preparations of stalk median eminence from male rats; it is concluded that the enzyme responsible for the formation of MSH-R-IF is an exopeptidase and that the release-inhibiting factor itself is a tripeptide. Oxytocin is converted by the incubation to L-prolyl-L-leucylglycinamide; nanogram amounts of this tripeptide inhibit the release of MSH from the pituitary both in vivo and in vitro.
Keywords: oxytocin, C-terminal tripeptide, estrous cycle, rat
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baxter J. W., Wuu T. C., Manning M., Sawyer W. H. Solid phase synthesis and some pharmacological properties of 8-glutamine-oxytocin: a possible intermediate in the evolution of the neurohypophysial hormones. Experientia. 1969 Nov 15;25(11):1127–1128. doi: 10.1007/BF01900225. [DOI] [PubMed] [Google Scholar]
- Bowers C. Y., Schally A. V., Enzmann F., Boler J., Folkers K. Porcine thyrotropin releasing hormone is (pyro)glu-his-pro(NH2). Endocrinology. 1970 May;86(5):1143–1153. doi: 10.1210/endo-86-5-1143. [DOI] [PubMed] [Google Scholar]
- Brooks C. M., Ishikawa T., Koizumi K., Lu H. H. Activity of neurones in the paraventricular nucleus of the hypothalamus and its control. J Physiol. 1966 Jan;182(1):217–231. doi: 10.1113/jphysiol.1966.sp007820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgus R., Dunn T. F., Desiderio D. M., Ward D. N., Vale W., Guillemin R., Felix A. M., Gillessen D., Studer R. O. Biological activity of synthetic polypeptide derivatives related to the structure of hypothalamic TRF. Endocrinology. 1970 Mar;86(3):573–582. doi: 10.1210/endo-86-3-573. [DOI] [PubMed] [Google Scholar]
- CASH W. D., MAHAFFEY L. M., BUCK A. S., NETTLETON D. E., Jr, ROMAS C., DUVIGNEAUD V. SYNTHESIS AND BIOLOGICAL PROPERTIES OF 9-SARCOSINE OXYTOCIN. J Med Pharm Chem. 1962 May;91:413–423. doi: 10.1021/jm01238a001. [DOI] [PubMed] [Google Scholar]
- Du Vigneaud V., Flouret G., Walter R. Synthesis and some biological properties of 4-valine-oxytocin and 1-deamino-4-valine-oxytocin. J Biol Chem. 1966 May 10;241(9):2093–2096. [PubMed] [Google Scholar]
- Ferrier B. M., Du Vigneaud V. 9-Deamidooxytocin, an analog of the hormone containing a glycine residue in place of the glycinamide residue. J Med Chem. 1966 Jan;9(1):55–57. doi: 10.1021/jm00319a014. [DOI] [PubMed] [Google Scholar]
- Ferrier B. M., Jarvis D., Du Vigneaud V. Deamino-oxytocin. Its isolation by partition chromatography on Sephadex and crystallization from water, and its biological activities. J Biol Chem. 1965 Nov;240(11):4264–4266. [PubMed] [Google Scholar]
- Glass J. D., Dubois B. M., Schwartz I. L., Walter R. Inactivation of neurohypophyseal peptides by a uterine enzyme. Characterization of the hydrolytic cleavage. Endocrinology. 1970 Oct;87(4):730–737. doi: 10.1210/endo-87-4-730. [DOI] [PubMed] [Google Scholar]
- Himmelhoch R., Peterson E. A. Preparation of leucine aminopeptidase free of endopeptidase activity. Biochemistry. 1968 Jun;7(6):2085–2093. doi: 10.1021/bi00846a010. [DOI] [PubMed] [Google Scholar]
- Kastin A. J., Schally A. V. MSH activity in pituitaries of rats treated with hypothalamic extracts. Gen Comp Endocrinol. 1966 Dec;7(3):452–456. doi: 10.1016/0016-6480(66)90066-9. [DOI] [PubMed] [Google Scholar]
- LIGHT A., GREENBERG J. THE SEQUENCE OF 26 AMINO ACID RESIDUES AT THE AMINO TERMINUS OF PAPAIN. J Biol Chem. 1965 Jan;240:258–265. [PubMed] [Google Scholar]
- Meienhofer J., Sano Y. A solid-phase synthesis of [lysine]-vasopressin through a crystalline protected nonapeptide intermediate. J Am Chem Soc. 1968 May 22;90(11):2996–2997. doi: 10.1021/ja01013a067. [DOI] [PubMed] [Google Scholar]
- Meienhofer J., Trzeciak A., Havran R. T., Walter R. A solid-phase synthesis of (8-arginine)-vasopressin through a crystalline protected nonapeptide intermediate and biological properties of the hormone. J Am Chem Soc. 1970 Dec 2;92(24):7199–7202. doi: 10.1021/ja00727a031. [DOI] [PubMed] [Google Scholar]
- Merrifield R. B. Automated synthesis of peptides. Science. 1965 Oct 8;150(3693):178–185. doi: 10.1126/science.150.3693.178. [DOI] [PubMed] [Google Scholar]
- SCHROEDER W. A., SHELTON J. R., SHELTON J. B., CORMICK J. THE AMINO ACID SEQUENCE OF THE ALPHA CHAIN OF HUMAN FETAL HEMOGLOBIN. Biochemistry. 1963 Nov-Dec;2:1353–1357. doi: 10.1021/bi00906a030. [DOI] [PubMed] [Google Scholar]
- SMITH E. L., SPACKMAN D. H. Leucine aminopeptidase. V. Activation, specificity, and mechanism of action. J Biol Chem. 1955 Jan;212(1):271–299. [PubMed] [Google Scholar]
- Sawyer W. H., Baxter J. W., Manning M., Heinicke E., Perks A. M. A fraction resembling oxytocin from Squalus acanthias: pharmacological comparisons with synthetic peptides. Gen Comp Endocrinol. 1970 Aug;15(1):52–58. doi: 10.1016/0016-6480(70)90096-1. [DOI] [PubMed] [Google Scholar]
- Sawyer W. H., Wuu T. C., Baxter J. W., Manning M. 4-Proline analogues of neurohypophysial hormones: hypothetical intermediates in peptide evolution. Endocrinology. 1969 Aug;85(2):385–388. doi: 10.1210/endo-85-2-385. [DOI] [PubMed] [Google Scholar]
- TALEISNIK S., ORIAS R. A MELANOCYTE-STIMULATING HORMONE-RELEASING FACTOR IN HYPOTHALAMIC EXTRACTS. Am J Physiol. 1965 Feb;208:293–296. doi: 10.1152/ajplegacy.1965.208.2.293. [DOI] [PubMed] [Google Scholar]
- Taleisnik S., De Olmos J., Orías R., Tomatis M. E. Effect of hypothalamic lesions on pituitary melanocyte-stimulating hormone. J Endocrinol. 1967 Dec;39(4):485–492. doi: 10.1677/joe.0.0390485. [DOI] [PubMed] [Google Scholar]
- Taleisnik S., Tomatis M. E. Antagonistic effect on melanocyte-stimulating hormone release of two neural tissue extracts. Am J Physiol. 1967 Jan;212(1):157–163. doi: 10.1152/ajplegacy.1967.212.1.157. [DOI] [PubMed] [Google Scholar]
- Taleisnik S., Tomatis M. E. Melanocyte-stimulating hormone-releasing and inhibiting factors in two hypothalamic extracts. Endocrinology. 1967 Oct;81(4):819–825. doi: 10.1210/endo-81-4-819. [DOI] [PubMed] [Google Scholar]
- Tomatis M. E., Taleisnik S. Pituitary melanocyte-stimulating hormone and its hypothalamic factors during the estrous cycle. Acta Physiol Lat Am. 1968;18(1):96–101. [PubMed] [Google Scholar]
- Yamanaka T., Hase S., Sakakibara S., Schwartz I. L., Dubois B. M., Walter R. Crystalline deamino-dicarba-oxytocin. Preparation and some pharmacological properties. Mol Pharmacol. 1970 Sep;6(5):474–480. [PubMed] [Google Scholar]


