Abstract
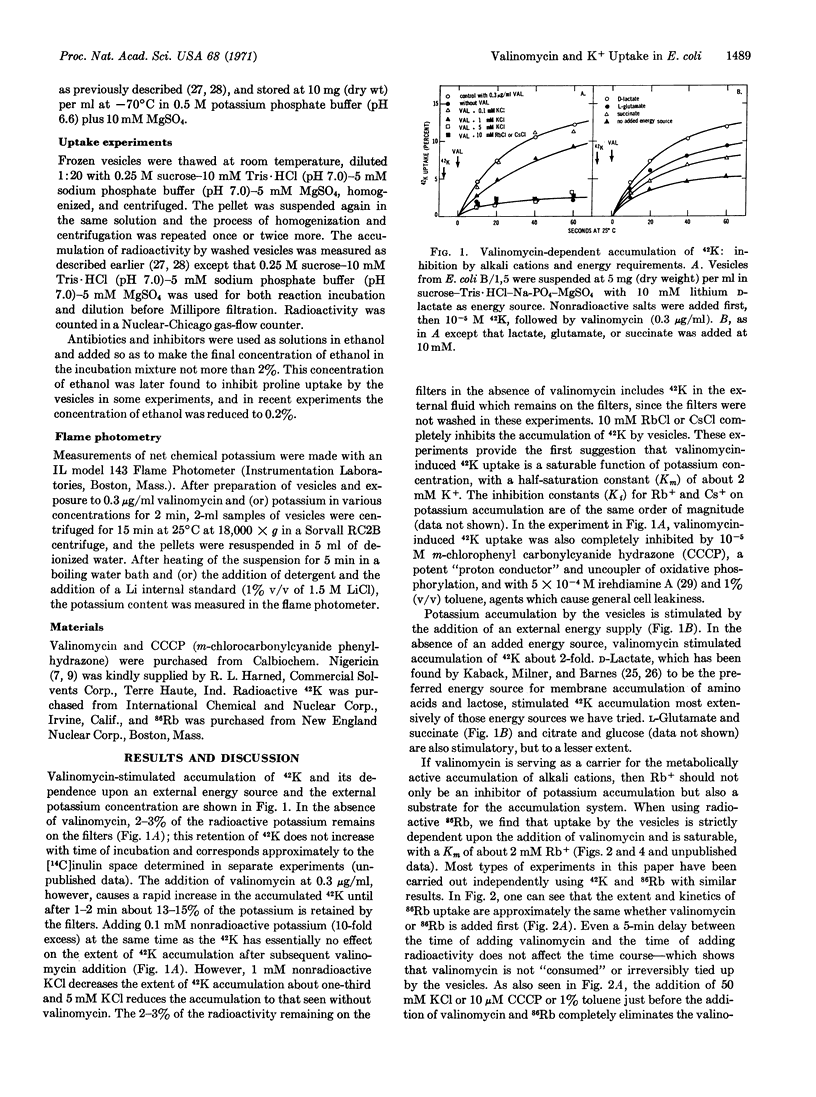
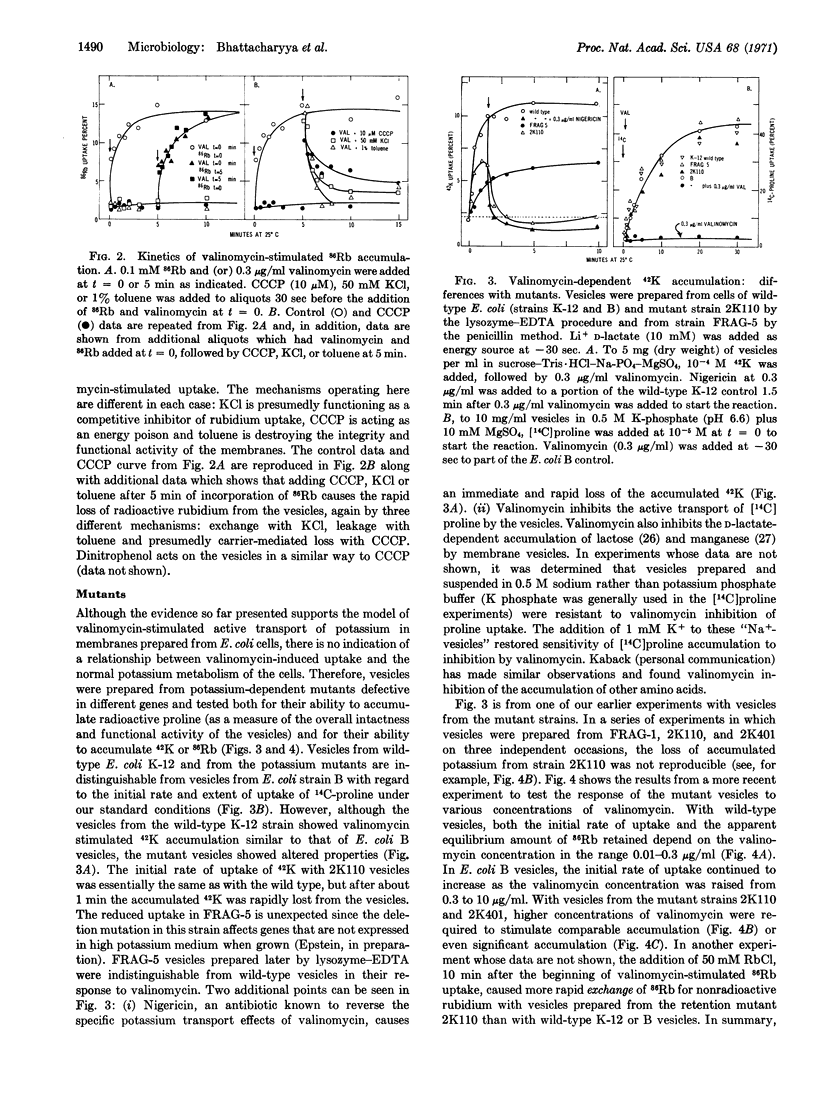
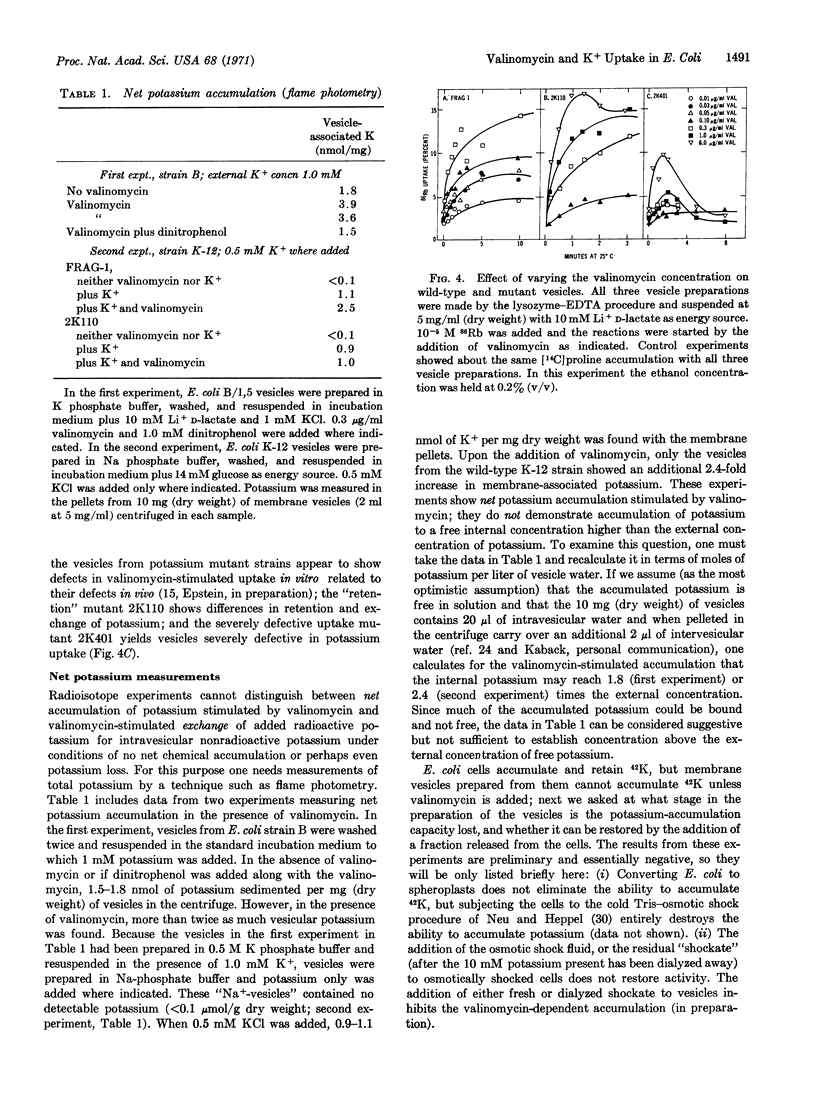
Osmotically shocked Escherichia coli and membrane vesicle ghosts from E. coli cells have lost the ability to accumulate potassium by active transport. The addition of valinomycin to the membrane ghosts restores the capacity to accumulate radioactive 42K and 86Rb by a temperature- and energy-dependent process. Membrane vesicles prepared from mutants of E. coli altered in potassium transport show defects in the valinomycin-stimulated accumulation of 42K that are related to the defects in the intact cells.
Keywords: K-uptake mutants, active transport
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barnes E. M., Jr, Kaback H. R. Beta-galactoside transport in bacterial membrane preparations: energy coupling via membrane-bounded D-lactic dehydrogenase. Proc Natl Acad Sci U S A. 1970 Aug;66(4):1190–1198. doi: 10.1073/pnas.66.4.1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharyya P. Active Transport of Manganese in Isolated Membranes of Escherichia coli. J Bacteriol. 1970 Dec;104(3):1307–1311. doi: 10.1128/jb.104.3.1307-1311.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharyya P., Wendt L., Whitney E., Silver S. Colicin-tolerant mutants of Escherichia coli: resistance of membranes to colicin E1. Science. 1970 May 22;168(3934):998–1000. doi: 10.1126/science.168.3934.998. [DOI] [PubMed] [Google Scholar]
- Blondin G. A., DeCastro A. F., Senior A. E. The isolation and properties of a peptide ionophore from beef heart mitochondria. Biochem Biophys Res Commun. 1971 Apr 2;43(1):28–35. doi: 10.1016/s0006-291x(71)80080-3. [DOI] [PubMed] [Google Scholar]
- CHAPPELL J. B., CROFTS A. R. GRAMICIDIN AND ION TRANSPORT IN ISOLATED LIVER MITOCHONDRIA. Biochem J. 1965 May;95:393–402. doi: 10.1042/bj0950393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damadian R. Ion metabolism in a potassium accumulation mutant of Escherichia coli B. I. Potassium metabolism. J Bacteriol. 1968 Jan;95(1):113–122. doi: 10.1128/jb.95.1.113-122.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein W., Davies M. Potassium-dependant mutants of Escherichia coli K-12. J Bacteriol. 1970 Mar;101(3):836–843. doi: 10.1128/jb.101.3.836-843.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein W., Schultz S. G. Cation Transport in Escherichia coli: V. Regulation of cation content. J Gen Physiol. 1965 Nov 1;49(2):221–234. doi: 10.1085/jgp.49.2.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein W., Schultz S. G. Cation transport in Escherichia coli. VI. K exchange. J Gen Physiol. 1966 Jan;49(3):469–481. doi: 10.1085/jgp.49.3.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harold F. M., Baarda J. R. Effects of nigericin and monactin on cation permeability of Streptococcus faecalis and metabolic capacities of potassium-depleted cells. J Bacteriol. 1968 Mar;95(3):816–823. doi: 10.1128/jb.95.3.816-823.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harold F. M., Baarda J. R. Gramicidin, valinomycin, and cation permeability of Streptococcus faecalis. J Bacteriol. 1967 Jul;94(1):53–60. doi: 10.1128/jb.94.1.53-60.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson P. J., McGivan J. D., Chappell J. B. The action of certain antibiotics on mitochondrial, erythrocyte and artificial phospholipid membranes. The role of induced proton permeability. Biochem J. 1969 Feb;111(4):521–535. doi: 10.1042/bj1110521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaback H. R., Deuel F. Proline uptake by disrupted membrane preparations from Escherichia coli. Arch Biochem Biophys. 1969 Jun;132(1):118–129. doi: 10.1016/0003-9861(69)90343-9. [DOI] [PubMed] [Google Scholar]
- Kaback H. R., Milner L. S. Relationship of a membrane-bound D-(-)-lactic dehydrogenase to amino acid transport in isolated bacterial membrane preparations. Proc Natl Acad Sci U S A. 1970 Jul;66(3):1008–1015. doi: 10.1073/pnas.66.3.1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaback H. R., Stadtman E. R. Proline uptake by an isolated cytoplasmic membrane preparation of Escherichia coli. Proc Natl Acad Sci U S A. 1966 Apr;55(4):920–927. doi: 10.1073/pnas.55.4.920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaback H. R. The role of the phosphoenolpyruvate-phosphotransferase system in the transport of sugars by isolated membrane preparations of Escherichia coli. J Biol Chem. 1968 Jul 10;243(13):3711–3724. [PubMed] [Google Scholar]
- LUBIN M. INTRACELLULAR POTASSIUM AND CONTROL OF PROTEIN SYNTHESIS. Fed Proc. 1964 Sep-Oct;23:994–1001. [PubMed] [Google Scholar]
- LUBOCHINSKY B., MEURY J., STOLKOWSKI J. CIN'ETIQUE DES 'ECHANGES DE POTASSIUM CHEZ L'ESCHERICHIA COLI, SOUCHE B 207, QUI NE PEUT CRO ITRE NORMALEMENT QU'EN PR'ESENCE DE CONCENTRATIONS 'ELEV'EES EN POTASSIUM. C R Hebd Seances Acad Sci. 1964 May 20;258:5106–5109. [PubMed] [Google Scholar]
- Mueller P., Rudin D. O. Development of K+-Na+ discrimination in experimental bimolecular lipid membranes by macrocyclic antibiotics. Biochem Biophys Res Commun. 1967 Feb 21;26(4):398–404. doi: 10.1016/0006-291x(67)90559-1. [DOI] [PubMed] [Google Scholar]
- Neu H. C., Heppel L. A. The release of enzymes from Escherichia coli by osmotic shock and during the formation of spheroplasts. J Biol Chem. 1965 Sep;240(9):3685–3692. [PubMed] [Google Scholar]
- Pressman B. C., Harris E. J., Jagger W. S., Johnson J. H. Antibiotic-mediated transport of alkali ions across lipid barriers. Proc Natl Acad Sci U S A. 1967 Nov;58(5):1949–1956. doi: 10.1073/pnas.58.5.1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tosteson D. C., Andreoli T. E., Tieffenberg M., Cook P. The effects of macrocyclic compounds on cation transport in sheep red cells and thin and thick lipid membranes. J Gen Physiol. 1968 May;51(5 Suppl):373S+–373S+. [PubMed] [Google Scholar]
- Weiden P. L., Epstein W., Schultz S. G. Cation transport in Escherichia coli. VII. Potassium requirement for phosphate uptake. J Gen Physiol. 1967 Jul;50(6):1641–1661. doi: 10.1085/jgp.50.6.1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis D. B., Ennis H. L. Ribonucleic acid and protein synthesis in a mutant of Bacillus subtilis defective in potassium retention. J Bacteriol. 1968 Dec;96(6):2035–2042. doi: 10.1128/jb.96.6.2035-2042.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]


