Abstract
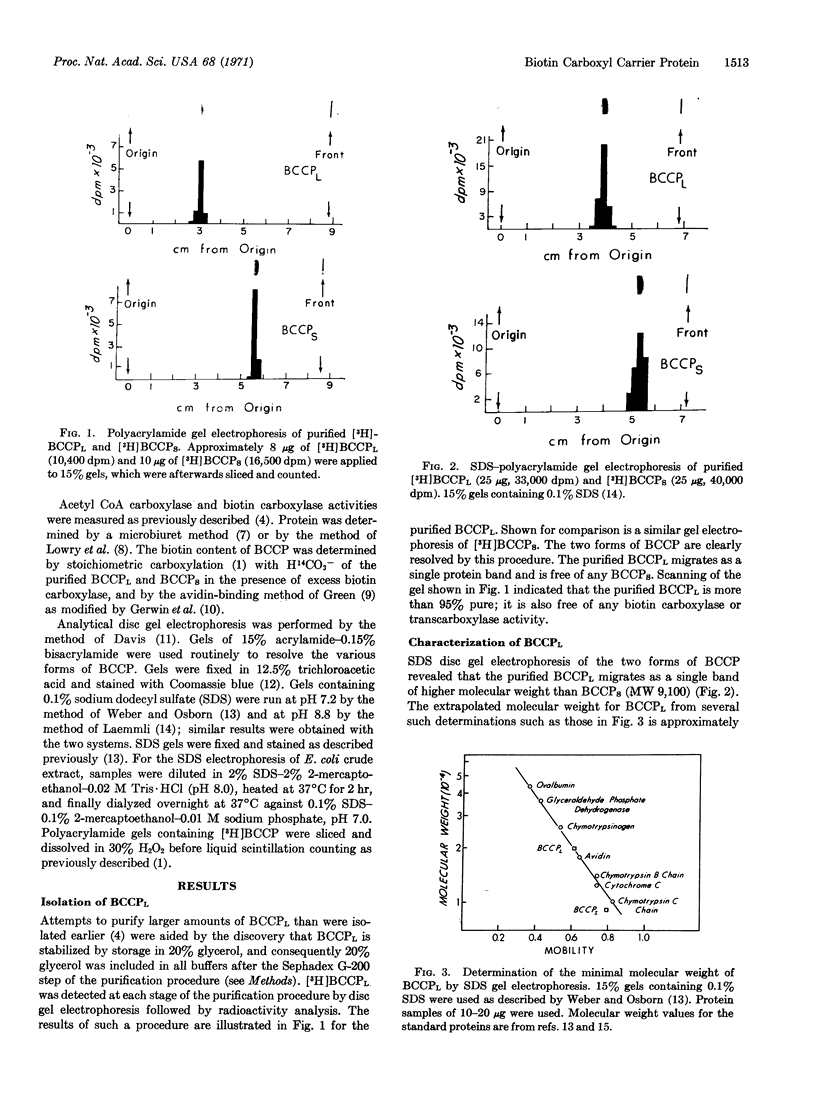
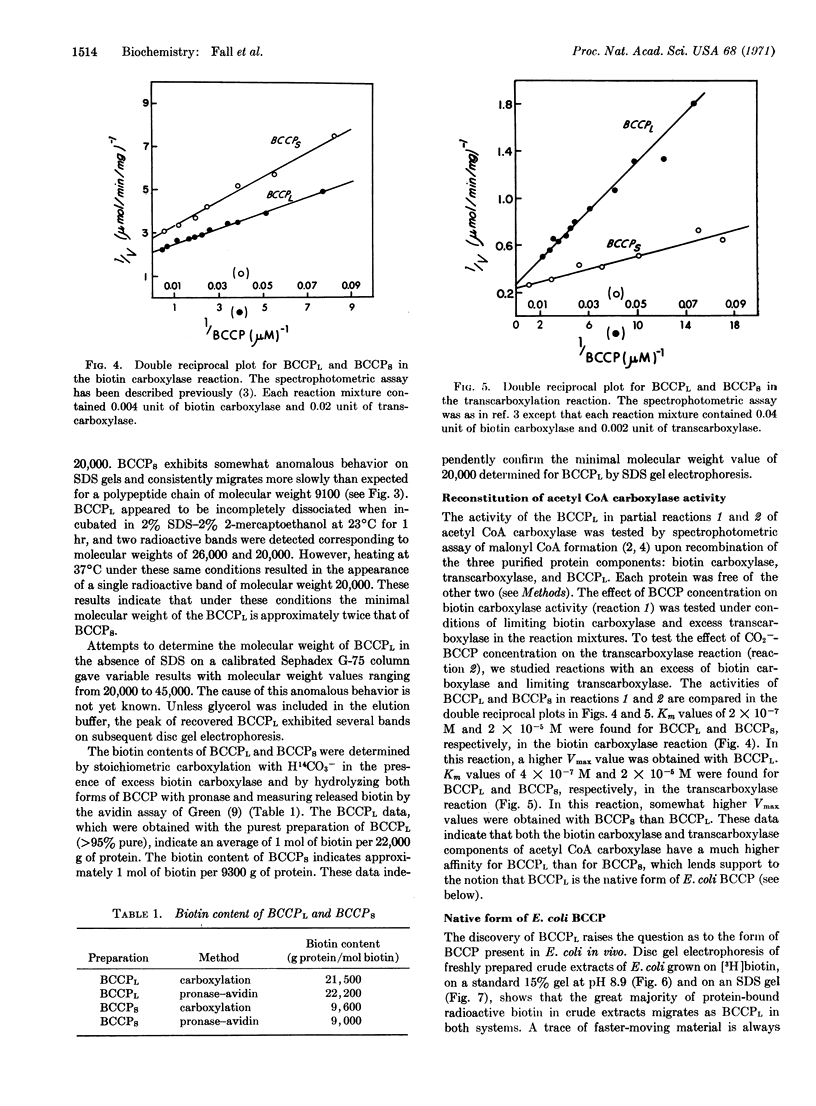
A large form of biotin carboxyl carrier protein (BCCPL) has been isolated from extracts of Escherichia coli. It has a minimal molecular weight of 20,000, according to its behavior on sodium dodecylsulfate-polyacrylamide gel electrophoresis, and contains approximately 1 mol of biotin per 22,000 g of protein. BCCPL exhibits Km values, in the biotin carboxylase and transcarboxylase half-reactions of acetyl CoA carboxylase, of 2 × 10-7 M and 4 × 10-7 M, respectively; these values are 50-100 times lower than those obtained with smaller forms of BCCP previously isolated. Electrophoresis of crude extracts of E. coli indicates that the major biotin-containing protein migrates at the same rate as BCCPL, which suggests that BCCPL is the native form of BCCP in E. coli.
Keywords: high molecular weight, high activity
Full text
PDF
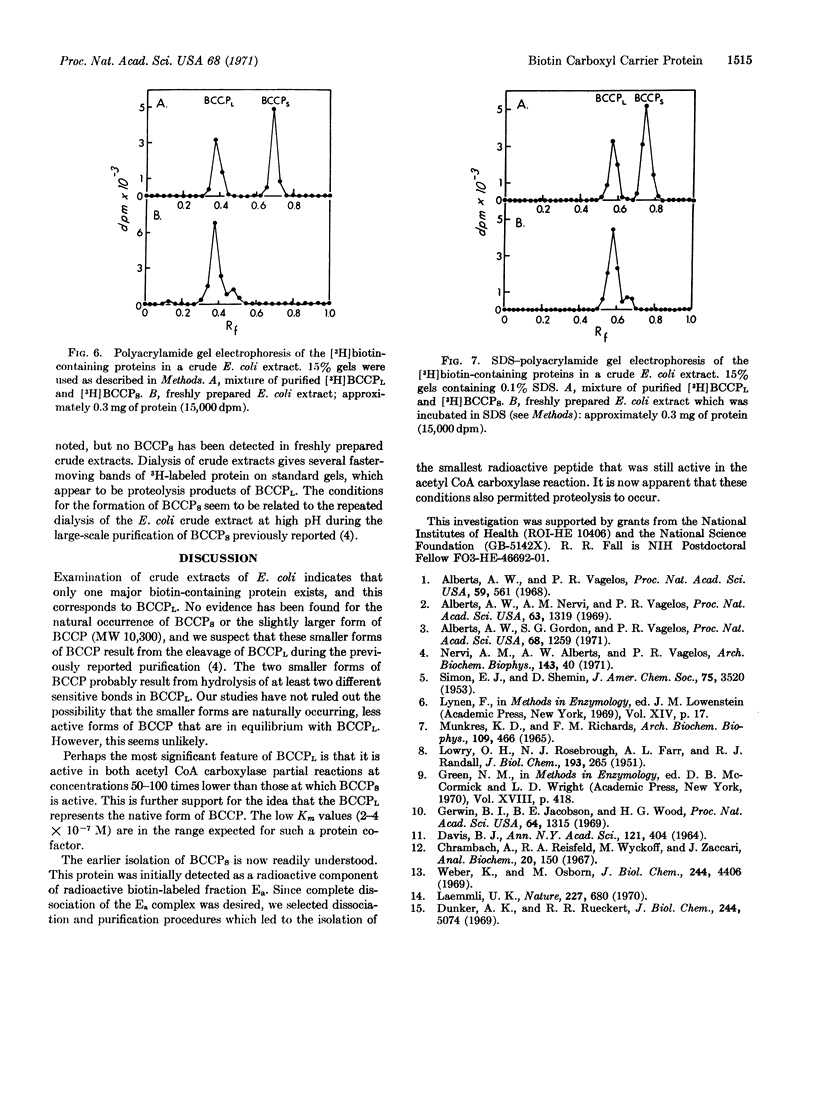
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alberts A. W., Gordon S. G., Vagelos P. R. Acetyl CoA carboxylase: the purified transcarboxylase component. Proc Natl Acad Sci U S A. 1971 Jun;68(6):1259–1263. doi: 10.1073/pnas.68.6.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alberts A. W., Nervi A. M., Vagelos P. R. Acetyl CoA carboxylase, II. Deomonstration of biotin-protein and biotin carboxylase subunits. Proc Natl Acad Sci U S A. 1969 Aug;63(4):1319–1326. doi: 10.1073/pnas.63.4.1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alberts A. W., Vagelos P. R. Acetyl CoA carboxylase. I. Requirement for two protein fractions. Proc Natl Acad Sci U S A. 1968 Feb;59(2):561–568. doi: 10.1073/pnas.59.2.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrambach A., Reisfeld R. A., Wyckoff M., Zaccari J. A procedure for rapid and sensitive staining of protein fractionated by polyacrylamide gel electrophoresis. Anal Biochem. 1967 Jul;20(1):150–154. doi: 10.1016/0003-2697(67)90272-2. [DOI] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Dunker A. K., Rueckert R. R. Observations on molecular weight determinations on polyacrylamide gel. J Biol Chem. 1969 Sep 25;244(18):5074–5080. [PubMed] [Google Scholar]
- Gerwin B. I., Jacobson B. E., Wood H. G. Transcarboxylase. 8. Isolation and properties of a biotin-carboxyl carrier protein. Proc Natl Acad Sci U S A. 1969 Dec;64(4):1315–1322. doi: 10.1073/pnas.64.4.1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- MUNKRES K. D., RICHARDS F. M. THE PURIFICATION AND PROPERTIES OF NEUROSPORA MALATE DEHYDROGENASE. Arch Biochem Biophys. 1965 Mar;109:466–479. doi: 10.1016/0003-9861(65)90391-7. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]