Abstract
Human immunodeficiency virus type 1 is associated with the development of neurocognitive disorders in many infected individuals, including a broad spectrum of motor impairments and cognitive deficits. Despite extensive research, the pathogenesis of HIV-associated neurocognitive disorders (HAND) is still not clear. This review provides a comprehensive view of HAND, including HIV neuroinvasion, HAND diagnosis and different level of disturbances, influence of highly-active antiretroviral therapy to HIV-associated dementia (HAD), possible pathogenesis of HAD, etc. Together, this review will give a thorough and clear understanding of HAND, especially HAD, which will be vital for future research, diagnosis and treatment.
Key words: HIV associated neurocognitive disorders, HIV associated dementia, blood brain barrier, cytokine, chemokine, macrophage, soluble factors
HIV neurobiology and neuroinvasion
Human immunodeficiency virus (HIV) is a member of the genus Lentivirus, part of the family of Retroviridae.1 As is well known, HIV-1 is highly virulent and infective,2 and is responsible for the current AIDS pandemic.3 The HIV-1 genome contains three structural genes (gag, pol and env),4 two regulatory genes (tat and rev) and four accessory genes (nef, vif, vpr and vpu). The HIV-1 genome also consists of at least seven structural elements (LTR, TAR, RRE, PE, SLIP, CRS, and INS).
The initial step of HIV infection is binding of the virions to the CD4 receptor and an additional co-receptor on the cell surface. Twelve chemokine receptors have been recognized acting as HIV co-receptors in cultured cells, but only two appear to play a more definitive role in vivo, CCR5 and CXCR4.5 CCR5 can bind macrophagetropic, non-syncytium-inducing (R5) viruses and it has been suggested to play a more pivotal role in the initiation and spread of HIV infection. This is based on the fact that R5 viruses are predominant not only during the early stages of HIV infection,6 but more than half of HIV-infected individuals uniquely carry CCR5-using HIV strains throughout the course of their infection. Moreover, Individuals homozygous for CCR5-Δ32 mutation, which is a 32bp deletion in the host CCR5 gene, were described to be resistant to HIV infection by R5 strains,7,8 although recent reports based on a single patient suggest that subsequent infection in patients harbouring CCR5-Δ32 can occur via CXCR4 receptor.9 Overall, the CCR5 or macrophage-tropic strains play a crucial role in HIV infection of the central nervous system (CNS).
Possible mechanism of HIV-1 entry into the central nervous system
The R5 viruses are the most common HIV-1 strains isolated from HIV-infected brains,10 which has been reported as the second most frequently infected organ in HIV-infected individuals at autopsy.11 HIV-1 entry into the brain at early phase of the infection can occur by several means,12 including transcytosis; transition by infected endothelial cells, passage through the blood-cerebral spinal fluid (CSF) barrier of the choroid plexus (CPx),13,14 and the Trojan horse model.15,16 Transcytosis pathway is that where brain microvascular endothelial cells (BMVECs) take up HIV-1 particles into vacuoles from the blood side and release them on the brain side of the BMVECs. However, it is estimated that only less than 1% of the taken-up virus can be transmitted through BMVEC.17,18 The second means is still very controversial because it is widely agreed that BMVECs only produce very limited HIV-1, if at all. CSF dissemination from a primary infection of CPx has been proposed as another possible mechanism partially supported by recent studies.19,20 However, our studies,21 together with others,14,22 could not locate any productive HIV infection of the CPx both in vivo and in vitro. The Trojan Horse hypothesis is generally accepted due to the most compelling evidence. 15,16 The details of that model have been elucidated in many reviews.23,24
Although, HIV entry to CNS largely occurs via CCR5 co-receptor, the CXCR4 and CCR3 coreceptors are also reported to play a role in mediating HIV infection of brain. They are expressed in brain microglia although at lower efficiency than CCR5.25 Moreover, HIV co-receptors CCR2, APJ, CX3CR1, STRL33/BONZO, and gpr1 are also expressed in the human brain although so far no defined role for them in mediating HIV CNS infection has been reported. However, CCR2, which is expressed on brain microvascular endothelial cells, has been reported to play a critical role for macrophage transendothelial migration in other neurological inflammatory disease,26 suggesting that it might facilitate HIV-infected leukocytes to transmit through the blood brain barrier (BBB). Another study has shown HIV-1 variants isolated from the infected brain-derived CD4-positive cells expressed a CCR8/TER1, suggesting TER1/CCR8 can function as a co-receptor for HIV-1 CNS infection.27 HIV co-receptor CX3CR1, expressed on microglia, is crucial for sustaining neuron-microglia communication and knockout of CX3CR1 can prevent neuron loss.28 APJ is another co-receptor for some HIV-1 strains, which is expressed in the human brain and in NT2N neurons. Studies have indicated it might play a role in HIV neuropathogenesis.29,30
Impairment of blood brain barrier function in HIV-infected individuals
The alteration of BBB of HIV-1 infected patients has been detected either by MRI or single-proton emission computed tomography or is indicated by the leakage of serum protein, quinolinic acid, metalloproteinase and nitric oxide (NO) in the CSF.31-39 The relative genomic and proteomic changes of HBMEC induced by HIV-1/HIV-infected monocyte-derived macrophages (MDM) have also been found.40,41 Alternation of the BBB function is not only a feature of HIV-1 CNS infection but it has a crucial impact on the pathogenesis of HAD,32 because BBB usually only permits a small percentage of leukocytes to cross without disrupting its integrity, which preclude the circulating monocytes. Therefore, its impairment facilitates penetration of virus and influx of more activated and HIV-1 infected monocytes into the brain, which can spread virus to the resident glia cells, including microglia and astrocytes, and further disrupt the integrity of the BBB. The mechanisms of BBB dysfunction in the course of HIV infection are poorly understood. It has been reported that the plasma lipopolysaccharide (LPS), which can compromise the permeability of BBB, is significantly higher in HIV-1 progressors than LPSinjected HIV-1 seronegative human volunteers.42 In addition, several cellular and viral factors have been demonstrated, including tumor necrosis factor-a (TNF-a) and interleukin-6 (IL-6), HIV-1 TAT and GP120, to influence the monocytes migration across the BBB directly or indirectly. 43-50 Cytokines and chemokines, such as IL-6, IL-10, IFN, CCL-2, CXCL-10, CXCL-1, CXCL-2, CXCL-5 etc., which are associated with the damage of microvascular integrity and the incidence of HAD, have been shown to be up-regulated in the brain and CSF of HIV-1 infected patients.51-54
The central nervous system as a site of HIV-1 reservoir
CNS has been regarded as one of the anatomical HIV reservoirs due to its immunologically privileged status. It is a huge challenge to overcome this difficulty and deliver therapeutic agents into the CNS, especially brain tissue, to treat the CNS disease. It has been reported that the CSF penetration rate of the nucleoside reverse transcriptase inhibitor AZT is 60% compared to the plasma level, and only 11% for 3TC.55,56 Consistent with these reports, other studies have shown the CSF HIV has a slower decay rate, higher evolutionary rate and faster re-bounce rate compared to the HIV in plasma.57-60 In addition, the data on viral genotypic evolution before and after HAART have both shown viral compartmentalization comparing HIV isolates from the CSF and plasma. 61-65 Although so far, very little information on intraparenchymal penetration rate of drugs is available, it has been well established that the brain tissue harboured unique sequences compared to the whole body.13,66-68 Moreover, drug-resistant mutations in different areas of the brains have also been explored, suggesting compartmentalized evolution of HIV-1 in the brain.69 In addition, the data on compartmentalization of HIV in the CNS support the belief that HIV infection of the brain contributes in part to the occurrence and pathogenesis of HIV associated neurocognitive disorders (HAND). This hypothesis also has been supported by the fact that some patients have poor CSF viral load control but good plasma viral suppression. 70 Further, the compartmentalization of HIV may have regional implications.71
The cellular reservoirs of HIV in the CNS are mainly microglia cells and macrophages. The role of each macrophage population in HIV spread and persistence depends on their turnover rate. Perivascular macrophages are assumed to be responsible for trafficking HIV between the periphery and the CNS, including dissemination of HIV from the peripheral blood to the CNS, commonly referred as the Trojan horse model; and reseeding CNS residing HIV strains back to the periphery.72 In contrast, microglia has relatively longer turnover rate and it might play a more crucial role as HIV CNS reservoirs. Recently, the role of microglia in promoting HIV latency has been linked to the transcription factors, such as CTIP2, which can repress HIV-1 gene expression in microglia.73,74 Moreover, variable levels of HIV-1 infections have been detected in other cell types within the CNS as well, such as: neurons, microvascular endothelial cells (MVEC) and astrocytes.75 Recently, extensive astrocyte infection has been demonstrated in HAD patients and positive correlation degree between its infection frequency and the severity of neuropathological changes has been shown comparable to perivascular macrophages.76 This suggests that astrocytes might play a crucial role as HIV reservoir.
HIV-associated neurocognitive disorders
HIV-associated neurocognitive disorders were recognized by clinicians shortly after the AIDS epidemic in 1981.77 Identification of the retroviral aetiology of AIDS allowed introduction of the hypothesis that HIV-1 itself might affect the CNS and cause neurological disorders, referred to as AIDS dementia complex (ADC) or HIV-associated dementia (HAD).78,79 The terms HIV encephalopathy, or HIV encephalitis, or HIV dementia are also commonly used.
Diagnostic criteria and current nomenclature
A diagnostic guideline was outlined by AIDS Task Force of the American Academy of Neurology (AAN) in 1991 proposing two levels disturbance:80 HAD (Including HAD with motor symptoms, HAD with behavioural or psychosocial symptoms and HAD with both motor and behavioural/psychosocial symptoms), and minor cognitive motor disorder (MCMD). The specific criteria for reaching these diagnoses were provided as well. In 1995, this guideline was expanded by adding the diagnosis of asymptomatic neurocognitive impairment, which described mild neurocognitive deficits that do not substantially interfere with daily function but being recognized increasingly frequently.81 Recently, a refinement of the AAN criteria was established by the HIV Neurobehavioral Research Centre at UCSD with the recommendation from an NIH working group.82 These criteria include three diagnoses: asymptomatic neurocognitive impairment (ANI), HIV-associated mild neurocognitive disorder (MND), and HAD (Supplementary Table 1). According to these criteria, at least five areas of well known HIV affecting neurocognitive functioning need to be assessed to arrive at the diagnosis. Apart from ideal comprehensive neuropsychological evaluation, a HIV dementia scale is used for assessing these domains due to its feasibility. In addition, the presence (or absence) of decline in everyday functioning is very important for the diagnosis of HAND. Unfortunately, to date, there are no widely agreed clinical measures of daily functioning,83 thus the assessments of that mainly depends on self-report, using questionnaires such as Lawton & Brody’s modified Activities of Daily Living scale and the Patient’s Assessment of Own Functioning.84,85
Table 1.
Role of selected chemokines and chemokine receptors in HIV-associated dementia.
| Chemokine | Chemokine receptor | Location of receptor expression in brain | Effects in the brain |
|---|---|---|---|
| CXCL8 (IL-8) | CXCR1 | Microglia, subsets of neurons, astrocytes and oligodendrocytes | Modulation of synaptic transmission and plasticity and inhibition of long-term potentiation in hippocampus* |
| CXCR2 | Microglia, neurons, astrocytes and oligodendrocytes precusrsors | ||
| CXCL10 (IP10) | CXCR3 | Microglia, subsets of neurons and astrocytes | Alteration of synaptic plasticity in hippocampus and induction of leukocyte infiltration |
| CXCL12 (SDFlα,β) | CXCR4 | Microglia, neurons, astrocytes and endothelial cells | Promotion of neuronal migration during cerebella development, microglial chemotaxis and mesenchymal stem-cell migration to site of injury; promotion of survival or apoptosis of hippocampal neurons; regulation of cholinergic and dopaminergic systems; promotion of astrocyte proliferation; and promotion of cytokine and glutamate release |
| CCL2(MCP1) | CCR2 | Human fetal glia and neurons, astrocytes and NT2N cells | Protection of neurons and astrocytes from NMDA- or HIV Tat-induced apoptosis, through release of astrocyte growth factors |
| CCL3 (MlPlα) | CCR1 | Subsets of neurons, astrocytes and oligodendrocyte precursors | Development of CNS; migration of astrocytes and microglia; recruitment of monocytes to brain parenchyma in patients with HAD or other neurological disorders |
| CCR5 | Microlia, neurons and astrocytes | ||
| CCL4 (MIPIβ) | CCR5 | Microglia, neurons and astrocytes | Recruitment of monocytes to brain parenchyma; involvement in migration of macrophages, microglia and astrocytes |
| CCL5 (RANTES) | CCR1 | Microglia, neurons and astrocytes | Recruitment of monocytes to brain parenchyma; involvement in migration of macrophages, microglia and astrocytes |
| CCR3 | |||
| CR5 | |||
| CCL7 (MCP3) | CCR1 | Microglia, neurons and astrocytes | Recruitment of monocytes to brain parenchyma |
| CCR2 | |||
| CCR3 | |||
| CX3CL1 (Fractalkine) | CX3CR1 | Microglia, subsets of neurons, astrocytes and endothelial cells | Recruitment of receptive cells (mainly microglia), when in soluble form; polymorphisms affect the development of AIDS |
*Long-term potentiation is a persistent increase in the size of the synaptic response that is induced by several mechanisms; in the hippocampus, it is thought to be the synaptic basis of learning and memory in vertebrates. CCL, CC-chemokine ligand; CCR, CC-chemokine receptor; CNS, central nervous system; CSF, cerebrospinal fluid; CXCL, CXC-chemokine ligand; CX3CL1, CX3C-chemokine ligand 1; CXCR, CXC-chemokine receptor; CX3CR1, CX3C-chemokine receptor 1; HAD, HIV-associated dementia; IL, interleukin; IP10, interferon-γ-induced protein of 10 kDa; MCP, monocyte-chemotactic protein; MIP1, macrophage inflammatory protein 1; NMDA, N-methyl-D-aspartate; RANTES, regulated upon activation, normally T-cell expressed and presumably secreted; SDF1, stromal-cell-derived factor 1; SHIV, simian-human immunodeficiency virus; SIV, simian immunodeficiency virus; Tat, transcriptional transactivator; TNF, tumour-necrosis factor. Taken from Gonzalez-Scarano et al.107
Asymptomatic neurocognitive impairment and mild neurocognitive disorder
Asymptomatic neurocognitive impairment (ANI) refers to the mild neurocognitive deficits (MND) in two or more cognitive areas without a substantial interference in everyday functioning (Supplementary Table 1). It represents more than 50% of diagnosed HAND cases and 21-30% of the asymptomatic HIV-infected individuals.86 Moreover, it has been reported to be well associated with HIV neuropathological abnormalities.87,88 Thus, it will be particularly important to identify these cases and introduce intervention at this earliest stage of HAND for the best prognosis.
MND is marked by mild to moderate impairment in two or more cognitive areas in addition to mild to moderate decline in daily functioning. Based on the ANI criteria of MCMD, additional everyday functioning decline has been included. The incidence of MND remains high and the prevalence of MND has not changed despite the introduction of HAART.82,89 Moreover, it has become more prevalent form since the severe forms of HAND are now not seen as frequently in the HAART era. The prevalence of MND has been estimated at between 5-14% in individuals with early symptoms and approximately 25% of those with AIDS.81,90 In addition, it has been reported that HAART failed to provide complete protection for MND from developing into HAD based on over more than 10 years of observations,82,91-94 although other studies have shown that HARRT can temporarily deduce the incidence rate of MND in high risk populations.95
HIV-associated dementia
HAD is the most severe form of HAND in terms of its functional impact. It requires moderate-to-severe cognitive impairment in more than two areas with remarkable daily function declines and together with an additional abnormality of either motor function or specified neuropsychiatric/psychosocial functions, which cannot be explained by co-morbid conditions. In addition, sufficient consciousness must be retained for cognitive abilities assessment. Although the incidence of HAD has decreased dramatically after the introduction of HAART, antiretroviral drugs still fail to completely protect HIV-infected patients from developing HAD. Recently, the concerns about HAD are not only limited to its cause but the consequence as well, since HAD in the HAART era can signal patients’ death.96,97
Epidemiology of HIV-associated dementia before and in the Era of highly-active antiretroviral therapy
HAD is one of the end-stage complications of HIV infection, which is not suppressed completely by HAART, although the incidence rate of HAD has declined dramatically. Before the HAART era, HAD affected almost 50-70% individuals with AIDS and became the most frequent neurological disorder at that time.98 Since the introduction of HAART, the incidence rate of HAD dropped by almost 50% compared to early 1990s (Figure 1A).99 However, the prevalence of HAD has stayed stable and even appears to be rising (Figure 1B) due to the longevity of HIV-infected patients on HAART. In addition, HAART has started to show its own neurological toxicity, which possibly also affects neurocognitive functions.100-102
Figure 1.
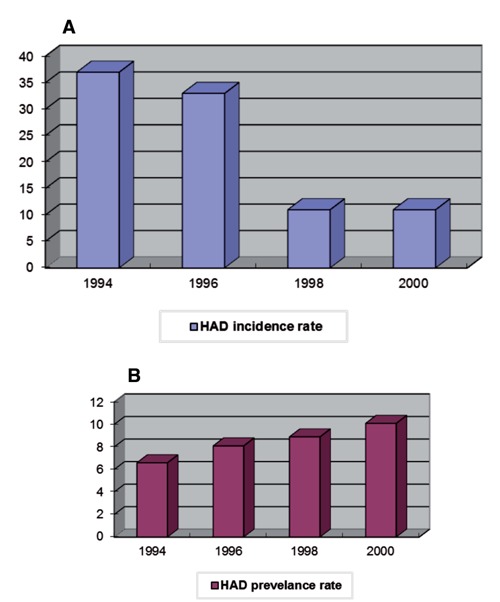
Incidence rate (A) and prevalence rate (B) of HAD in the Johns Hopkins HIV clinic. The x-axis corresponds to the calendar year. The y-axis corresponds to the incidence rate/prevalence rate per 1000 person years.
Neuropathology before and in the era of highly-active antiretroviral therapy
Before HAART, the most significant of HIV associated neuropathology were HIV encephalitis (HIVE), opportunistic infections and/or primary CNS lymphomas.103 HIVE is characterized by perivascular macrophage infiltration, multinucleated giant cells, activated microglias/microglia nodules, pronounced reactive astrocytosis, myelin pallor on microscopic sections and neuron loss.78,104-108 Although the presence of HIVE correlates to HAD to some degree, the best correlates are macrophage infiltration, activated microglia and reduced synaptic/dendritic density and selective neuronal loss.87,109
After the introduction of HAART, the neuropathology of HIV infection and HAD has shifted.87,94,110 Due to controlled plasma viral load levels and restored immune competencies after HAART, the opportunistic infections and primary CNS lymphomas declined dramatically. 111 So, there is a dramatic decrease in cerebral toxoplasmosis and cytomegalovirus (CMV) encephalitis, and more burn-out forms of HIVE are found, possibly part of the benefits from HAART.112 However, the neuro-inflammation does not improve significantly and the extent of microglial activation is still comparable to pre-HAART era.110 In addition, following HAART, a reversible HIV-associated amyotrophic lateral sclerosis (ALS)-like disorder has been observed.113 Moreover, HAART influences HIV neuropathology by changing the predominant sites of involvement. In post-HAART era, the pronounced inflammation was found in the hippocampus and adjacent parts of entorhinal and temporal cortex, while basal ganglia is the most involved in pre-HAART era.93,94,110 Furthermore, HAART causes immune reconstitution, which may lead to increased lymphocytic infiltration into the brain.112,114 Several cases of immune reconstitution-related neuropathology, also called immune reconstitution inflammatory syndrome (IRIS), have been reported; characterized by massive lymphocytosis, extensive demyelination and white matter damage.93,110 Peripheral neuropathies due to drug neurotoxicity are frequent.115
However, it is important to bear in mind that it is more difficult to organize the systematic neurological studies based on autopsies due to the longevity of HIV-infected patients after HAART. Hence, recent findings might not fully reflect the difference between pre-HAART and post-HAART, and also cannot represent those who survive from HAART but only HIV seropositive individuals who died following the failure of HAART. Besides, after HAART, a new chronic subtype of dementia has emerged among HIV+ patients together with the involvement of additional cognitive domains in previous phenotype,116,117 partly due to the longevity of HIV seropositive patients after the introduction of HAART.
Possible pathogenesis of HIV-associated dementia
Viral factors
It has been well documented that productive HIV infection of the CNS is detectable on macrophage/microglia and astrocytes,104,118 although so far infection of astrocytes is only limited to the transcriptional level or early expressed proteins (TAT, NEF and REV).75,119-121 In addition, HIV proteins can be released from these HIV-infected cells and then exhibit their neuronal toxicity directly or indirectly, although neurons are not infected.122-124 Three HIV proteins have been reported to cause direct neuronal injury or death: the virus’s glycoprotein (gp120), trans-activator of transcription (TAT), and the viral protein R (VPR).
Gp120 is essential for HIV infectivity and has been shown to induce neuron apoptotic death using the CXCR4 receptor with and without the presence of glial cells in a dose-dependent manner, 125-128 and gp120 over-expression in transgenic mice can cause neuropathological similarity to that of HAD.129 In addition, gp120 can also cause dysfunction of nigrostriatal dopaminergic system and injection to rat striatum result in neuronal apoptosis in the substantia nigra,130 which might explain why dopaminergic neurons are more susceptible to gp120 neurotoxicity. 131 Neuronal injury or death induced by gp120 can be via interaction with N-Methyl-D Aspartate (NMDA) receptors,127,132,133 which can influence the influx of Ca2+, and therefore trigger further neuronal injury by harmful enzymes, as well as free radicals and additional glutamate.67 Moreover, gp120 can also cause neuronal injury or death by interacting with apoptosis regulator, such as p38 mitogen-activated protein kinase,134 or influencing the expression of pro-apoptotic transcription factor, such as E2F1.135
Another neurotoxic HIV protein is TAT, which is mainly active in nucleus and essential for HIV replication. In vivo, it has been shown to cause direct tissue loss when injected to the striatum of adult rats.51 In vitro, TAT can cause dendritic loss and neuronal death at lower concentrations than those needed for viral replication.136 The neurotoxicity of TAT has been supported by many other studies.137-146 Interestingly, TAT neurotoxicity has regional preference, and some brain regions are more susceptible compared to others, such as the striatum, hippocampal dentate gyrus and the CA3 region of the hippocampus.137,140,147 TAT can dysregulate neuronal microRNAs (miR),148 which were found functioning in neurodevelopment and can mediate regulations of local synaptic and dendritic translation.149 TAT can alter the tight junction protein expression and BBB function, which can promote brain infiltration. In addition, its neurotoxicity can also be through mediating mitochondrial energy metabolism failure, and therefore influencing normal synaptic communication;150 activating p53 pathway and involving multiple intracellular-signalling pathways.151-155
Another HIV accessory protein is VPR, which is important for HIV initial infection and replication, and plays a role in HIV neurotoxicity as well. VPR can be detected from CSF of HIV seropositive patients, and may be involved in pro-apoptotic activity in AIDS-associated dementia.156,157 Moreover, it has been shown that both intracellular and extracellular VPR is capable of inducing apoptosis in both rat and human neuronal cells, including the NT2 cell line, and mature and differentiated neurons by direct activation of the initiator caspase-8.158-161 VPR neurotoxicity is possibly through several mechanisms: inducing cell cycle arrest at G2/M phase;162 altering mitochondrial permeability; 163 regulating some apoptotic related proteins; 164,165 facilitating transporting of pre-integration complex, and promoting transcription. 166-169
Other HIV proteins, such as NEF, REV and GP41, have also been reported to induce neurotoxicity. NEF is a known virulence factor or progression factor to AIDS,170 which can manipulate infection, survival and replication of HIV.171 In addition, NEF shares significant sequence homology with scorpion neurotoxins, which can inactivate potassium channels. 172-174 Consistently, NEF is lethal to neuron in vitro and abundantly expressed in astrocytes of HIV seropositive patients with pathological neuronal damage/dementia.119,120,175,176
REV protein accumulates in the nucleus and exports unspliced RNA from nucleus to the cytoplasm.177 It has been shown that REV has neurotoxicity in vitro and in vivo.178 It has also been shown that HIV seropositive patients, with NEF and REV expressed in astrocytes, progressed most rapidly to severe dementia.119 GP41 is also elevated in HAD patients, but in vitro studies showed it can only mediate neuronal injury in the presence of glial cells rather than directly.179
Role of mononuclear phagocytes
Apart from the direct neurotoxicity of HIV proteins, mononuclear phagocytes, including perivascular macrophages, resident microglia, etc. play a significant role in the development of HAD. First of all, as discussed above, macrophages have been proposed to traffic HIV into the brain and then infect/activate other macrophages or other cell types.180 Actually, HIV proteins are predominantly released from infected macrophages since they are the major cell types supporting productive infection in the brain. Other than these, infected or activated macrophages can also release long list of soluble factors, such as cytokines, chemokines (see Cytokine and Chemokine sections), which have been implicated in the pathogenesis of HAD. Some studies have shown a better correlation between neurocognitive deficits and activated microglial cells than infection itself,181 although we have recently shown that the direct and productive infection of the CNS macrophages is vital for HAD manifestation.71 These disparate results might be due to the different sample sets, but the contribution of different disease progression pace appears more likely the cause. As a matter of fact, all human in vivo studies are based on autopsy brain samples, rather than in vivo tissue. Therefore, it is difficult to make any sole correlation between disease stage and possible pathological factors (such as HIV productive infection, or degree of cellular activation), because some patients progress too fast to show all HIV-related neurological stages before their death. So, disease progression pace would be a better parameter with which to correlate. We have found very extensive HIV productive infection in rapid progressors with relatively low cellular infiltration compared to those who progress more slowly. However, it is very hard to distinguish the role of HIV, activated systemic macrophages and activated CNS residential macrophages individually due to: i) HIV infection persists in the CNS, latently if not productively, after its initial entry at the very early stages of HIV infection; ii) microphage/microglia is the major cell type supporting productive CNS infection; iii) currently, there is no reliable marker available to separate perivascular macrophages and microglia.
Cytokines
Apart from direct HIV proteins neurotoxicity discussed above, soluble factors (such as cytokines, chemokines and their receptors) also play significant roles in the pathogenesis of HAD. Many neuronal injuries are mediated by cytokines and chemokines, which are secreted by HIV-infected or activated macrophage/microglia or astrocytes. It has been reported that HIV-infected or activated macrophage/microglia can increase several pro-inflammatory cytokines expression, including TNF-α, IL-6, GM-CSF and IL-1β,182-185 which can enhance CNS inflammation further. In addition, these cytokines have also been reported to upregulate in the CNS or CSF of HAD patients.186,187
Among them, TNF-α plays dual roles in HAD pathogenesis. It is a pro-inflammatory cytokine and also characterized as an oxidative stress mediator. It can stimulate reactive oxidative intermediates (ROIs) production in T cells,188,189 and in turn cause T cell apoptosis, which has been proposed as one of the mechanisms of AIDS.190,191 In addition, it can also cause apoptosis in human neuronal cells via similar mechanism and accelerate neurodegenerative disease pathology.192 In contrast, some studies have also shown its neuro-protective role due to its capabilities to enhance anti-apoptotic and anti-oxidative protein expression.193-197 These dual actions might be because of different inflammatory time course,198 or different TNF receptors to which it binds.199
Tumor Necrosis Factor-Related Apoptosis-Inducing Ligand (TRAIL), a member of the TNF superfamily, has been shown to cause rapid apoptosis of different cells.200-204 It increases in human monocyte-derived macrophages after HIV-1 infection and immune activation.204-206 Moreover, it has been demonstrated that TRAIL-expressing macrophages are in close association with neurons undergoing apoptosis in HIV-1 encephalitis.207 Interestingly, antibodies against TRAIL can dramatically prevent neuronal injury in both in vitro experiments and in an animal model of HIV-1 infection in the brain.204,207 Worthy to note, different from TNF-α and IL-1β, TRAIL is preferentially expressed in HIV-infected macrophage/microglia.207 All these results indicate that it contributes to macrophage-mediated neuronal loss in HAD.
Apart from TNF-α, all the other cytokines mentioned above have shown only indirect neurotoxicity. Interestingly, all of them can be related to TNF-α. M-CSF is majorly induced by TNF-α.208 It can act on proliferation of cells of macrophage lineage, differentiation and survival and its antagonists have been shown to inhibit HIV replication in macrophages.209,210 Therefore, it might contribute to cellular reservoir of HIV infection. IL-1β can upregulate TNF-α expression and IL-6 in mononuclear phagocytes, and also it can contribute to neuronal injury by promoting the expression of nitric oxide (NO);211-213 IL-6 has to synergize with TNF-α to induce HIV expression at the transcriptional level, but not alone.214
Chemokines
HIV-infected or activated macrophages also secrete chemokines, which are a family of small chemotactic cytokines and can combine with their receptors and play important roles in immune surveillance and inflammatory process. Chemokines are essential components for normal neuronal physiology in the CNS. However, the over-expression of some chemokines can lead to excess activated leukocytes infiltration into the CNS and consequently to neuronal injury, while others exhibit neuroprotective function. So, the balance between their neuro-protection and neuro-degeneration roles are crucial to HAD pathogenesis. Not surprisingly, altered expression of chemokines and chemokine receptors has been found in HAD brain,215-217 indicating their involvement in HAD pathogenesis. However, their contribution still remains unclear. So far, CC chemokines have not shown any neurotoxicity, but only neuroprotective roles in vitro, such as RANTES and MIP-1β,128,218,219 while CXC chemokines have been found to be neurotoxic, such as IP10 and SDF-1.220-222 Table 1 shows the detailed effects of selected chemokines and chemokine receptors in relation to HAD.
Other soluble factors
HIV-infected or activated macrophages also secrete other soluble factors which have been reported to be neurotoxic, including L-cysteine, 223 quinolinic aicd,224 neurotoxic amine NTox,225 NO,226 and eicosanoids.184 Their neurotoxicity are all mediated or related to glutamate. The neurotoxic effects of L-cysteine, quinolinic aicd and NTox are all NMDA receptor directed.227-229 Some eicosanoids expression altered upon HIV infection as well, such as arachidonic acid and PAF,230 a metabolite of arachidonate.184,231 PAF can be rapidly produced by HIV-infected mononuclear phagocytes and it has been reported to mediate the release and activation of glutamate.232,233 Of NO, although the most of the neurotoxic actions are mediated by peroxynitrite (ONOO-), the reaction product from NO and superoxide anions, still partly contribute to glutamate neurotoxicity in primary neuronal cell cultures and in animal models of stroke.234 In addition, it has been proven that glutamate is the predominant neurotoxic factor released from activated macrophage/microglia.235
DNA microarray and its applications to HIV-associated dementia
It is well known that host genetics plays a role in the aetiology of neurodegenerative disease, including HAD. Thus, it is necessary to study host genetics in order to define hostviral interaction and understand the genetic basis of disease. First of all, the gene expression products are critical for the normal development, function and adaptive response of the nervous system,236 and minor fluctuations or alterations in gene expression can influence the susceptibility of the host to neurological disorders, including attention deficit disorder, and schizophrenia,237 PD and AD,238 as well as HAD.239 Therefore, genetic studies will offer a direct clinical impact on HAD in terms of diagnosis and pre-symptomatic testing. Consequently, it will shed light on broader genomic aspect of pathogenesis of HAD, which is not limited on individual genes or genetic forms, but to provide potential target pathways for therapy. In addition, these studies have the potential to contribute to the development of effective animal model to study HAD and other neurodegenerative diseases.
DNA microarray has become the most popular tool for global gene expression study. It is characterized by high-density arrays of DNA oligonucleotides bonding to a structural support, which differ with types of arrays (e.g. a solid surface, such as glass, plastic or silicon biochip or coded beads). The core principle behind microarrays is hybridization between target samples and probes, then based on different labeled target (e.g. fluorophore-, silver-, or chemiluminescence-labeled), these hybridizations can be detected and quantified by relative abundance of nucleic acid sequences in the target. It can be used to detect either DNA or RNA (most commonly as cDNA) that may or may not be translated into proteins. One of the greatest advantages of these methods is that they allow the analysis of thousands genes in relation to specific disease in one experiment. Gene expression profiling is one of the applications of DNA microarray to identify genes whose expression is changed in response to pathogens or other stimulating factors.
Since the first microarray-based study in gene expression of host cells in relation to HIV infection,240 a variety of different types and generations of microarrays have been applied to HIV viral-host interaction studies, and to HAD pathogenesis as well. However, most of them have been done on glial cells because of the difficulties of analysing multiple cell types from brain rather than clonal expansion of a single-cell type, and accessing the relevant tissues during the lifetime of the patient.241 In addition, because astrocytes constitute 50-60% of brain cell volume,242 they have been chosen as a target cell type by many researchers. Although only a very small astrocytic population can be infected in vivo by HIV and even in vitro,243-245 the infection is passive and not cytolytic,246,247 astrocytic function can be altered by binding HIV-1 or envelope protein gp120, which is consistent with changes in gene expression.248 Among those studies, several are comprehensive gene expression profiling of astrocytes exposed to HIV-1 or viral proteins using high-flux microarray platforms for parallel detection of multiple differentially expressed genes.249-251 The details are listed in Table 2.
Table 2.
Gene profiling studies in HIV-1 infected astrocytes and HIV-1 or SIV infected brains.
| Sample source | Microarray | Experiment design | Conclusion | Ref. |
|---|---|---|---|---|
| Astrocytes | ||||
| Primary human astroyctes | NIA immuno and neuroarray | HIV vs non-HIV HIV-gp120 vs non-HIV-gp120 | Differential effect of HIV-1 and gpl20 in astrocytes. Gp120 has more profound effect but chemokine and cytokine induction occurs predominantly by HIV infection | 249 |
| Primary human astroyctes | AffymetrixU133 A/B | VSV-HIV vs non-VSV-HIV | Up-regulation of IFN antiviral responses, intercellular contacts, cell adhesion, and signalling. Down-regulation of cell cycle, DNA replication, and cell proliferation | 250 |
| Astrocytoma | BD bioscience clontech | Native Nef vs non-myristoylated Nef | Up-regulation of small GTPase signalling, regulation of apoptosis, lipid metabolism, JAK/STAT and MAPK signalling pathways | 251 |
| Brain tissue | ||||
| Macaque-basal ganglia | Clontech chemokine and cytokine array | SIVE vs non-SIVE | Upregulation in SIVE of genes involved in promoting macrophage infiltration, activation and virus replication. Down regulation of genes regulating neurotrophic functions | 255 |
| Macaque-frontal lobe | Affymetrix U95Av2 | SIVE vs ni | Up-regulation in SIVE of genes implicated in monocyte entry to the brain, inflammation, IFN response, antigen presentation, production of neurotoxic effects, transcription factors and others Up-regulation in acute S1V infection of genes involved in IFN and IL-6 pathways. Many of these genes also up-regulated in long-term infection and SIVE | 256 257 |
| Macaque-cortical brain | Clontech cytokine array | SHIV vs ni | Up-regulated genes, including Cripto-1 and genes implicated in inflammatory, neuroprotective, cognitive, and stress responses | 258 |
| Human-frontal cortex | Affymetrix U95Av2 | HIVE vs non-HIVE | Up-regulated pathways included neuroimmune and antiviral response, transcription factors, and cytoskeletal components | 253 |
| Human brain cortex (middle frontal gyrus) | Affymetrix HG-U133 | HIVE vs non-HIVE | The analysis focused on ionic conductance carriers that control membrane excitation. They found six ionic channel genes overexpressed in HAD brains compared to control while seven downregulated. Conclude the relevance between channelopathy and subcoritcal dementias. | 252 |
| Human-frontal cortex | Affymetrix human genome U95A | HIV-1 infected and 4 HIV-1 negative control subjects | Focusing on analytic approaches | 254 |
Modified from Sui et al.255
Apart from cells, several microarray studies have been carried out in different brain regions of macaques with and without SIV encephalitis (Table 2). Moreover, limited genomic studies (partial human genome) on human brain tissues from patients with and without HIV-associated CNS disorders have also been done.252-254 The details are listed in the Table 2.255-258 Interestingly, there are considerable number of consistently dysregulated genes in human astrocytes and in macaque and human brain, which might suggest the important role that HIV-infected or activated astrocytes play in HAD pathogenesis. The common genes are mostly implicated in immune responses, and neurological function/diseases. Although many in vitro and in vivo studies have been done, so far the whole genome microarray fingerprint profiling using autopsy human brain tissue is still lacking.
References
- 1.Weiss RA.How does HIV cause AIDS? Science. 1993; 260:1273-9 [DOI] [PubMed] [Google Scholar]
- 2.Gilbert PB, McKeague IW, Eisen G, et al. Comparison of HIV-1 and HIV-2 infectivity from a prospective cohort study in Senegal. Stat Med. 2003; 22:573-93 [DOI] [PubMed] [Google Scholar]
- 3.Holmes KK, Corey L, Collier AC, Handsfield HH, AIDS dx/rx. New York: McGraw-Hill; 1990 [Google Scholar]
- 4.Kuiken C, Foley B, Marx P, et al. HIV Sequence Compendium 2008. Available from:http://www.hiv.lanl.gov/content/sequence/HIV/COMPENDIUM/2008/frontmatter. pdf [Google Scholar]
- 5.Doms RW, Trono D.The plasma membrane as a combat zone in the HIV battlefield. Genes Dev. 2000; 14:2677-88 [DOI] [PubMed] [Google Scholar]
- 6.Anderson J, Akkina R.Complete knockdown of CCR5 by lentiviral vectorexpressed siRNAs and protection of transgenic macrophages against HIV-1 infection. Gene Ther. 2007; 14:1287-97 [DOI] [PubMed] [Google Scholar]
- 7.Liu R, Paxton WA, Choe S, et al. Homozygous defect in HIV-1 coreceptor accounts for resistance of some multiplyexposed individuals to HIV-1 infection. Cell. 1996; 86:367-77 [DOI] [PubMed] [Google Scholar]
- 8.Martinson JJ, Chapman NH, Rees DC, et al. Global distribution of the CCR5 gene 32-basepair deletion. Nat Genet. 1997;16: 100-3 [DOI] [PubMed] [Google Scholar]
- 9.Naif HM, Cunningham AL, Alali M, et al. A human immunodeficiency virus type 1 isolate from an infected person homozygous for CCR5Delta32 exhibits dual tropism by infecting macrophages and MT2 cells via CXCR4. J Virol. 2002; 76:3114-24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gray L, Roche M, Churchill MJ, et al. Tissue-specific sequence alterations in the human immunodeficiency virus type 1 envelope favoring CCR5 usage contribute to persistence of dual-tropic virus in the brain. J Virol. 2009; 83:5430-41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Masliah E, DeTeresa RM, Mallory ME, Hansen LA.Changes in pathological findings at autopsy in AIDS cases for the last 15 years. Aids. 2000; 14:69-74 [DOI] [PubMed] [Google Scholar]
- 12.Kaul M, Garden GA, Lipton SA.Pathways to neuronal injury and apoptosis in HIVassociated dementia. Nature. 2001;410: 988-94 [DOI] [PubMed] [Google Scholar]
- 13.Chen H, Wood C, Petito CK.Comparisons of HIV-1 viral sequences in brain, choroid plexus and spleen: potential role of choroid plexus in the pathogenesis of HIV encephalitis. J Neurovirol. 2000;6: 498-506 [DOI] [PubMed] [Google Scholar]
- 14.Harouse JM, Wroblewska Z, Laughlin MA, et al. Human choroid plexus cells can be latently infected with human immunodeficiency virus. Ann Neurol. 1989; 25:406-11 [DOI] [PubMed] [Google Scholar]
- 15.Haase AT.Pathogenesis of lentivirus infections. Nature. 1986; 322:130-6 [DOI] [PubMed] [Google Scholar]
- 16.Peluso R, Haase A, Stowring L, et al. A Trojan horse mechanism for the spread of visna virus in monocytes. Virology. 1985; 147:231-6 [DOI] [PubMed] [Google Scholar]
- 17.Bobardt MD, Salmon P, Wang L, et al. Contribution of proteoglycans to human immunodeficiency virus type 1 brain invasion. J Virol. 2004; 78:6567-84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu NQ, Lossinsky AS, Popik W, et al. Human immunodeficiency virus type 1 enters brain microvascular endothelia by macropinocytosis dependent on lipid rafts and the mitogen-activated protein kinase signaling pathway. J Virol. 2002; 76:6689-700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Falangola MF, Hanly A, Galvao-Castro B, Petito CK.HIV infection of human choroid plexus: a possible mechanism of viral entry into the CNS. J Neuropathol Exp Neurol. 1995; 54:497-503 [DOI] [PubMed] [Google Scholar]
- 20.Petito CK, Chen H, Mastri AR, et al. HIV infection of choroid plexus in AIDS and asymptomatic HIV-infected patients suggests that the choroid plexus may be a reservoir of productive infection. J Neurovirol. 1999; 5:670-7 [DOI] [PubMed] [Google Scholar]
- 21.Zhou L, Ng T, Yuksel A, et al. Short communication: absence of HIV infection in the choroid plexus of two patients who died rapidly with HIV-associated dementia. AIDS Res Hum Retroviruses. 2008; 24:839-43 [DOI] [PubMed] [Google Scholar]
- 22.Lackner AA, Smith MO, Munn RJ, et al. Localization of simian immunodeficiency virus in the central nervous system of rhesus monkeys. Am J Pathol. 1991;139: 609-21 [PMC free article] [PubMed] [Google Scholar]
- 23.Albright AV, Soldan SS, Gonzalez-Scarano F.Pathogenesis of human immunodeficiency virus-induced neurological disease. J Neurovirol. 2003; 9:222-7 [DOI] [PubMed] [Google Scholar]
- 24.Kolson DL, Lavi E, Gonzalez-Scarano F.The effects of human immunodeficiency virus in the central nervous system. Adv Virus Res. 1998; 50:1-47 [DOI] [PubMed] [Google Scholar]
- 25.Gabuzda D, Wang J.Chemokine receptors and virus entry in the central nervous system. J Neurovirol. 1999; 5:643-58 [DOI] [PubMed] [Google Scholar]
- 26.Mahad D, Callahan MK, Williams KA, et al. Modulating CCR2 and CCL2 at the blood-brain barrier: relevance for multiple sclerosis pathogenesis. Brain. 2006; 129:212-23 [DOI] [PubMed] [Google Scholar]
- 27.Jinno A, Shimizu N, Soda Y, et al. Identification of the chemokine receptor TER1/CCR8 expressed in brain-derived cells and T cells as a new coreceptor for HIV-1 infection. Biochem Biophys Res Commun. 1998; 243:497-502 [DOI] [PubMed] [Google Scholar]
- 28.Fuhrmann M, Bittner T, Jung CK, et al. Microglial Cx3cr1 knockout prevents neuron loss in a mouse model of Alzheimer’s disease. Nat Neurosci. 2010; 13:411-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Edinger AL, Hoffman TL, Sharron M, et al. An orphan seven-transmembrane domain receptor expressed widely in the brain functions as a coreceptor for human immunodeficiency virus type 1 and simian immunodeficiency virus. J Virol. 1998; 72:7934-40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhou N, Zhang X, Fan X, et al. The N-terminal domain of APJ, a CNS-based coreceptor for HIV-1, is essential for its receptor function and coreceptor activity. Virology. 2003; 317:84-94 [DOI] [PubMed] [Google Scholar]
- 31.Balakrishnan J, Becker PS, Kumar AJ, et al. Acquired immunodeficiency syndrome: correlation of radiologic and pathologic findings in the brain. Radiographics. 1990; 10:201-15 [DOI] [PubMed] [Google Scholar]
- 32.Petito CK, Cash KS.Blood-brain barrier abnormalities in the acquired immunodeficiency syndrome: immunohistochemical localization of serum proteins in postmortem brain. Ann Neurol. 1992;32: 658-66 [DOI] [PubMed] [Google Scholar]
- 33.Buttner A, Mehraein P, Weis S.Vascular changes in the cerebral cortex in HIV-1 infection. II. An immunohistochemical and lectinhistochemical investigation. Acta Neuropathol (Berl). 1996; 92:35-41 [DOI] [PubMed] [Google Scholar]
- 34.Weis S, Haug H, Budka H.Vascular changes in the cerebral cortex in HIV-1 infection: I. A morphometric investigation by light and electron microscopy. Clin Neuropathol. 1996; 15:361-6 [PubMed] [Google Scholar]
- 35.Gendelman HE, Zheng J, Coulter CL, et al. Suppression of inflammatory neurotoxins by highly active antiretroviral therapy in human immunodeficiency virus-associated dementia. J Infect Dis. 1998; 178:1000-7 [DOI] [PubMed] [Google Scholar]
- 36.McArthur JC, Nance-Sproson TE, Griffin DE, et al. The diagnostic utility of elevation in cerebrospinal fluid beta 2-microglobulin in HIV-1 dementia. Multicenter AIDS Cohort Study. Neurology. 1992; 42:1707-12 [DOI] [PubMed] [Google Scholar]
- 37.Sporer B, Paul R, Koedel U, et al. Presence of matrix metalloproteinase-9 activity in the cerebrospinal fluid of human immunodeficiency virus-infected patients. J Infect Dis. 1998; 178:854-7 [DOI] [PubMed] [Google Scholar]
- 38.Giovannoni G, Miller RF, Heales SJ, et al. Elevated cerebrospinal fluid and serum nitrate and nitrite levels in patients with central nervous system complications of HIV-1 infection: a correlation with bloodbrain-barrier dysfunction. J Neurol Sci. 1998; 156:53-8 [DOI] [PubMed] [Google Scholar]
- 39.Persidsky Y, Zheng J, Miller D, Gendelman HE.Mononuclear phagocytes mediate blood-brain barrier compromise and neuronal injury during HIV-1-associated dementia. J Leukoc Biol. 2000;68: 413-22 [PubMed] [Google Scholar]
- 40.Chaudhuri A, Duan F, Morsey B, et al. HIV-1 activates proinflammatory and interferon-inducible genes in human brain microvascular endothelial cells: putative mechanisms of blood-brain barrier dysfunction. J Cereb Blood Flow Metab. 2008; 28:697-711 [DOI] [PubMed] [Google Scholar]
- 41.Ricardo-Dukelow M, Kadiu I, Rozek W, et al. HIV-1 infected monocyte-derived macrophages affect the human brain microvascular endothelial cell proteome: new insights into blood-brain barrier dysfunction for HIV-1-associated dementia. J Neuroimmunol. 2007; 185:37-46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Douek D.HIV disease progression: immune activation, microbes, and a leaky gut. Top HIV Med. 2007; 15:114-7 [PubMed] [Google Scholar]
- 43.Blum MS, Toninelli E, Anderson JM, et al. Cytoskeletal rearrangement mediates human microvascular endothelial tight junction modulation by cytokines. Am J Physiol. 1997;273:H286-94 [DOI] [PubMed] [Google Scholar]
- 44.Brett FM, Mizisin AP, Powell HC, Campbell IL.Evolution of neuropathologic abnormalities associated with blood-brain barrier breakdown in transgenic mice expressing interleukin-6 in astrocytes. J Neuropathol Exp Neurol. 1995; 54:766-75 [DOI] [PubMed] [Google Scholar]
- 45.Annunziata P.Blood-brain barrier changes during invasion of the central nervous system by HIV-1. Old and new insights into the mechanism. J Neurol. 2003; 250:901-6 [DOI] [PubMed] [Google Scholar]
- 46.Cioni C, Annunziata P.Circulating gp120 alters the blood-brain barrier permeability in HIV-1 gp120 transgenic mice. Neurosci Lett. 2002; 330:299-301 [DOI] [PubMed] [Google Scholar]
- 47.Hurwitz AA, Berman JW, Lyman WD.The role of the blood-brain barrier in HIV infection of the central nervous system. Adv Neuroimmunol. 1994; 4:249-56 [DOI] [PubMed] [Google Scholar]
- 48.Ridet JL, Malhotra SK, Privat A, Gage FH.Reactive astrocytes: cellular and molecular cues to biological function. Trends Neurosci 1997;20):570-7 [DOI] [PubMed] [Google Scholar]
- 49.Price TO, Ercal N, Nakaoke R, Banks WA.HIV-1 viral proteins gp120 and Tat induce oxidative stress in brain endothelial cells. Brain Res. 2005; 1045:57-63 [DOI] [PubMed] [Google Scholar]
- 50.Toborek M, Lee YW, Flora G, et al. Mechanisms of the blood-brain barrier disruption in HIV-1 infection. Cell Mol Neurobiol. 2005; 25:181-99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Avison MJ, Nath A, Greene-Avison R, et al. Neuroimaging correlates of HIV-associated BBB compromise. J Neuroimmu-nol. 2004; 157:140-6 [DOI] [PubMed] [Google Scholar]
- 52.Laverda AM, Gallo P, De Rossi A, et al. Cerebrospinal fluid analysis in HIV-1-infected children: immunological and virological findings before and after AZT therapy. Acta Paediatr. 1994; 83:1038-42 [DOI] [PubMed] [Google Scholar]
- 53.Perrella O, Carrieri PB, Guarnaccia D, Soscia M.Cerebrospinal fluid cytokines in AIDS dementia complex. J Neurol. 1992; 239:387-8 [DOI] [PubMed] [Google Scholar]
- 54.Poluektova L, Moran T, Zelivyanskaya M, et al. The regulation of alpha chemokines during HIV-1 infection and leukocyte activation: relevance for HIV-1-associated dementia. J Neuroimmunol. 2001; 120:112-28 [DOI] [PubMed] [Google Scholar]
- 55.Schrager LK, D’Souza MP.Cellular and anatomical reservoirs of HIV-1 in patients receiving potent antiretroviral combination therapy. JAMA. 1998; 280:67-71 [DOI] [PubMed] [Google Scholar]
- 56.van der Sandt IC, Vos CM, Nabulsi L, et al. Assessment of active transport of HIV protease inhibitors in various cell lines and the in vitro blood-brain barrier. AIDS. 2001; 15:483-91 [DOI] [PubMed] [Google Scholar]
- 57.Staprans S, Marlowe N, Glidden D, et al. Time course of cerebrospinal fluid responses to antiretroviral therapy: evidence for variable compartmentalization of infection. AIDS. 1999; 13:1051-61 [DOI] [PubMed] [Google Scholar]
- 58.Price RW, Paxinos EE, Grant RM, et al. Cerebrospinal fluid response to structured treatment interruption after virological failure. AIDS. 2001; 15:1251-9 [DOI] [PubMed] [Google Scholar]
- 59.Ellis RJ, Gamst AC, Capparelli E, et al. Cerebrospinal fluid HIV RNA originates from both local CNS and systemic sources. Neurology. 2000; 54:927-36 [DOI] [PubMed] [Google Scholar]
- 60.Cinque P, Vago L, Ceresa D, et al. Cerebrospinal fluid HIV-1 RNA levels: correlation with HIV encephalitis. AIDS. 1998; 12:389-94 [DOI] [PubMed] [Google Scholar]
- 61.Di Stefano M, Monno L, Fiore JR, et al. Neurological disorders during HIV-1 infection correlate with viral load in cerebrospinal fluid but not with virus phenotype. AIDS. 1998; 12:737-43 [DOI] [PubMed] [Google Scholar]
- 62.Pratt RD, Nichols S, McKinney N, et al. Virologic markers of human immunodeficiency virus type 1 in cerebrospinal fluid of infected children. J Infect Dis. 1996; 174:288-93 [DOI] [PubMed] [Google Scholar]
- 63.Wong JK, Ignacio CC, Torriani F, et al. In vivo compartmentalization of human immunodeficiency virus: evidence from the examination of pol sequences from autopsy tissues. J Virol. 1997; 71:2059-71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Stingele K, Haas J, Zimmermann T, et al. Independent HIV replication in paired CSF and blood viral isolates during antiretroviral therapy. Neurology. 2001;56: 355-61 [DOI] [PubMed] [Google Scholar]
- 65.Tashima KT, Flanigan TP, Kurpewski J, et al. Discordant human immunodeficiency virus Type 1 drug resistance mutations, including K103N, observed in cerebrospinal fluid and plasma. Clin Infect Dis. 2002; 35:82-3 [DOI] [PubMed] [Google Scholar]
- 66.Peters PJ, Bhattacharya J, Hibbitts S, et al. Biological analysis of human immunodeficiency virus type 1 R5 envelopes amplified from brain and lymph node tissues of AIDS patients with neuropathology reveals two distinct tropism phenotypes and identifies envelopes in the brain that confer an enhanced tropism and fusigenicity for macrophages. J Virol. 2004; 78:6915-26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Haughey NJ, Mattson MP.Calcium dysregulation and neuronal apoptosis by the HIV-1 proteins Tat and gp120. J Acquir Immune Defic Syndr 2002;31 Suppl 2:S55-61 [DOI] [PubMed] [Google Scholar]
- 68.Chang J, Jozwiak R, Wang B, et al. Unique HIV type 1 V3 region sequences derived from six different regions of brain: regionspecific evolution within host-determined quasispecies. AIDS Res Hum Retroviruses. 1998; 14:25-30 [DOI] [PubMed] [Google Scholar]
- 69.Smit TK, Brew BJ, Tourtellotte W, et al. Independent evolution of human immunodeficiency virus (HIV) drug resistance mutations in diverse areas of the brain in HIV-infected patients, with and without dementia, on antiretroviral treatment. J Virol. 2004; 78:10133-48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Baeuerle M, Schmitt-Haendle M, Taubald A, et al. Severe HIV-1 encephalitis and development of cerebral non-Hodgkin lymphoma in a patient with persistent strong HIV-1 replication in the brain despite potent HAART—case report and review of the literature. Eur J Med Res. 2005; 10:309-16 [PubMed] [Google Scholar]
- 71.Zhou L, Rua R, Ng T, et al. Evidence for predilection of macrophage infiltration patterns in the deeper midline and mesial temporal structures of the brain uniquely in patients with HIV-associated dementia. BMC Infect Dis. 2009;9:192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Alexaki A, Liu Y, Wigdahl B.Cellular reservoirs of HIV-1 and their role in viral persistence. Curr HIV Res. 2008; 6:388-400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rohr O, Lecestre D, Chasserot-Golaz S, et al. Recruitment of Tat to heterochromatin protein HP1 via interaction with CTIP2 inhibits human immunodeficiency virus type 1 replication in microglial cells. J Virol. 2003; 77:5415-27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Marban C, Redel L, Suzanne S, et al. COUP-TF interacting protein 2 represses the initial phase of HIV-1 gene transcription in human microglial cells. Nucleic Acids Res. 2005; 33:2318-31 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 75.Bagasra O, Lavi E, Bobroski L, et al. Cellular reservoirs of HIV-1 in the central nervous system of infected individuals: identification by the combination of in situ polymerase chain reaction and immunohistochemistry. AIDS. 1996;10: 573-85 [DOI] [PubMed] [Google Scholar]
- 76.Churchill MJ, Wesselingh SL, Cowley D, et al. Extensive astrocyte infection is prominent in human immunodeficiency virusassociated dementia. Ann Neurol. 2009; 66:253-8 [DOI] [PubMed] [Google Scholar]
- 77.Snider WD, Simpson DM, Nielsen S, et al. Neurological complications of acquired immune deficiency syndrome: analysis of 50 patients. Ann Neurol. 1983; 14:403-18 [DOI] [PubMed] [Google Scholar]
- 78.Navia BA, Cho ES, Petito CK, Price RW.The AIDS dementia complex: II. neuropathology. Ann Neurol. 1986; 19:525-35 [DOI] [PubMed] [Google Scholar]
- 79.Navia BA, Jordan BD, Price RW.The AIDS dementia complex: I. clinical features. Ann Neurol. 1986; 19:517-24 [DOI] [PubMed] [Google Scholar]
- 80.[No authors listed] Nomenclature and research case definitions for neurologic manifestations of human immunodeficiency virus-type 1 (HIV-1) infection. Report of a Working Group of the American Academy of Neurology AIDS Task Force. Neurology. 1991; 41:778-85 [DOI] [PubMed] [Google Scholar]
- 81.Grant I, Atkinson JH.Psychiatric aspects of acquired immune deficiency syndrome. Kaplan H, Sadock BJ, Baltimore: Williams and Wilkins; 1995 [Google Scholar]
- 82.Antinori A, Arendt G, Becker JT, et al. Updated research nosology for HIV-associated neurocognitive disorders. Neuro-logy. 2007; 69:1789-99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Morgan EE, Heaton RK.The neuropsychological approach to predicting everyday functioning. 3rd ed Grant IAK, New York: Oxford; 2009 [Google Scholar]
- 84.Heaton RK, Marcotte TD, Mindt MR, et al. The impact of HIV-associated neuropsychological impairment on everyday functioning. J Int Neuropsychol Soc. 2004; 10:317-31 [DOI] [PubMed] [Google Scholar]
- 85.Chelune GJ, Heaton RK, Lehman RA.Neuropsychological and personality correlates of patients’ complaints of disability. Adv Clin Neuropsychol 1986:3:95-126 [Google Scholar]
- 86.Robertson KR, Smurzynski M, Parsons TD, et al. The prevalence and incidence of neurocognitive impairment in the HAART era. AIDS. 2007; 21:1915-21 [DOI] [PubMed] [Google Scholar]
- 87.Masliah E, Heaton RK, Marcotte TD, et al. Dendritic injury is a pathological substrate for human immunodeficiency virus-related cognitive disorders. HNRC Group. The HIV Neurobehavioral Research Center. Ann Neurol. 1997;42: 963-72 [DOI] [PubMed] [Google Scholar]
- 88.Cherner M, Cysique L, Heaton RK, et al. Neuropathologic confirmation of definitional criteria for human immunodeficiency virus-associated neurocognitive disorders. J Neurovirol. 2007; 13:23-8 [DOI] [PubMed] [Google Scholar]
- 89.Cysique LA, Maruff P, Brew BJ.Prevalence and pattern of neuropsychological impairment in human immunodeficiency virusinfected/acquired immunodeficiency syndrome (HIV/AIDS) patients across preand post-highly active antiretroviral therapy eras: a combined study of two cohorts. J Neurovirol. 2004; 10:350-7 [DOI] [PubMed] [Google Scholar]
- 90.Goodkin K, Wilkie FL, Concha M, et al. Aging and neuro-AIDS conditions and the changing spectrum of HIV-1-associated morbidity and mortality. J Clin Epidemiol 2001;54 Suppl 1:S35-43 [DOI] [PubMed] [Google Scholar]
- 91.Ellis RJ, Hsia K, Spector SA, et al. Cerebrospinal fluid human immunodeficiency virus type 1 RNA levels are elevated in neurocognitively impaired individuals with acquired immunodeficiency syndrome. HIV Neurobehavioral Research Center Group. Ann Neurol. 1997; 42:679-88 [DOI] [PubMed] [Google Scholar]
- 92.Liner KJ, 2nd, Hall CD, Robertson KR.Effects of antiretroviral therapy on cognitive impairment. Curr HIV/AIDS Rep. 2008; 5:64-71 [DOI] [PubMed] [Google Scholar]
- 93.Boisse L, Gill MJ, Power C.HIV infection of the central nervous system: clinical features and neuropathogenesis. Neurol Clin. 2008; 26:799-819 [DOI] [PubMed] [Google Scholar]
- 94.Brew BJ, Crowe SM, Landay A, et al. Neurodegeneration and ageing in the HAART era. J Neuroimmune Pharmacol. 2009; 4:163-74 [DOI] [PubMed] [Google Scholar]
- 95.Deutsch R, Ellis RJ, McCutchan JA, et al. AIDS-associated mild neurocognitive impairment is delayed in the era of highly active antiretroviral therapy. AIDS. 2001; 15:1898-9 [DOI] [PubMed] [Google Scholar]
- 96.Sacktor N, McDermott MP, Marder K, et al. HIV-associated cognitive impairment before and after the advent of combination therapy. J Neurovirol. 2002; 8:136-42 [DOI] [PubMed] [Google Scholar]
- 97.Tozzi V, Balestra P, Serraino D, et al. Neurocognitive impairment and survival in a cohort of HIV-infected patients treated with HAART. AIDS Res Hum Retroviruses. 2005; 21:706-13 [DOI] [PubMed] [Google Scholar]
- 98.Price RW, Brew B, Sidtis J, et al. The brain in AIDS: central nervous system HIV-1 infection and AIDS dementia complex. Science. 1988; 239:586-92 [DOI] [PubMed] [Google Scholar]
- 99.Mocroft A, Katlama C, Johnson AM, et al. AIDS across Europe, 1994-98: the EuroSIDA study. Lancet. 2000; 356:291-6 [DOI] [PubMed] [Google Scholar]
- 100.McArthur JC.HIV dementia: an evolving disease. J Neuroimmunol. 2004; 157:3-10 [DOI] [PubMed] [Google Scholar]
- 101.Michael NL, Vahey M, Burke DS, Redfield RR.Viral DNA and mRNA expression correlate with the stage of human immunodeficiency virus (HIV) type 1 infection in humans: evidence for viral replication in all stages of HIV disease. J Virol. 1992; 66:310-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Finzi D, Hermankova M, Pierson T, et al. Identification of a reservoir for HIV-1 in patients on highly active antiretroviral therapy. Science. 1997; 278:1295-300 [DOI] [PubMed] [Google Scholar]
- 103.Budka H.Multinucleated giant cells in brain: a hallmark of the acquired immune deficiency syndrome (AIDS). Acta Neuropathol (Berl). 1986; 69:253-8 [DOI] [PubMed] [Google Scholar]
- 104.Sharer LR.Pathology of HIV-1 infection of the central nervous system. A review. J Neuropathol Exp Neurol. 1992; 51:3-11 [DOI] [PubMed] [Google Scholar]
- 105.Anders KH, Vinters HV.Neuropathology of AIDS. Boca Raton: CRC Press; 1990 [Google Scholar]
- 106.Lawrence DM, Major EO.HIV-1 and the brain: connections between HIV-1-associated dementia, neuropathology and neuroimmunology. Microbes Infect. 2002; 4:301-8 [DOI] [PubMed] [Google Scholar]
- 107.Gonzalez-Scarano F, Martin-Garcia J.The neuropathogenesis of AIDS. Nat Rev Immunol. 2005; 5:69-81 [DOI] [PubMed] [Google Scholar]
- 108.Gendelman HE, Lipton SA, Tardieu M, et al. The neuropathogenesis of HIV-1 infection. J Leukoc Biol. 1994; 56:389-98 [DOI] [PubMed] [Google Scholar]
- 109.Glass JD, Fedor H, Wesselingh SL, McArthur JC.Immunocytochemical quantitation of human immunodeficiency virus in the brain: correlations with dementia. Ann Neurol. 1995; 38:755-62 [DOI] [PubMed] [Google Scholar]
- 110.Anthony IC, Bell JE.The Neuropathology of HIV/AIDS. Int Rev Psychiatry. 2008; 20:15-24 [DOI] [PubMed] [Google Scholar]
- 111.Mamidi A, DeSimone JA, Pomerantz RJ.Central nervous system infections in individuals with HIV-1 infection. J Neurovirol. 2002; 8:158-67 [DOI] [PubMed] [Google Scholar]
- 112.Gray F, Chretien F, Vallat-Decouvelaere AV, Scaravilli F.The changing pattern of HIV neuropathology in the HAART era. J Neuropathol Exp Neurol. 2003; 62:429-40 [DOI] [PubMed] [Google Scholar]
- 113.Moulignier A, Moulonguet A, Pialoux G, Rozenbaum W.Reversible ALS-like disorder in HIV infection. Neurology. 2001;57: 995-1001 [DOI] [PubMed] [Google Scholar]
- 114.Langford TD, Letendre SL, Marcotte TD, et al. Severe, demyelinating leukoencephalopathy in AIDS patients on antiretroviral therapy. AIDS. 2002; 16:1019-29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Authier FJ, Gheradi RK.Peripheral neuropathies in HIV-infected patients in the era of HAART. Brain Pathol. 2003; 13:223-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Brew BJ.Evidence for a change in AIDS dementia complex in the era of highly active antiretroviral therapy and the possibility of new forms of AIDS dementia complex. AIDS 2004;18 Suppl 1:S75-8 [PubMed] [Google Scholar]
- 117.McArthur JC, Haughey N, Gartner S, et al. Human immunodeficiency virus-associated dementia: an evolving disease. J Neurovirol. 2003; 9:205-21 [DOI] [PubMed] [Google Scholar]
- 118.Wiley CA, Schrier RD, Nelson JA, et al. Cellular localization of human immunodeficiency virus infection within the brains of acquired immune deficiency syndrome patients. Proc Natl Acad Sci USA. 1986; 83:7089-93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ranki A, Nyberg M, Ovod V, et al. Abundant expression of HIV Nef and Rev proteins in brain astrocytes in vivo is associated with dementia. AIDS. 1995;9: 1001-8 [DOI] [PubMed] [Google Scholar]
- 120.Saito Y, Sharer LR, Epstein LG, et al. Overexpression of nef as a marker for restricted HIV-1 infection of astrocytes in postmortem pediatric central nervous tissues. Neurology. 1994; 44:474-81 [DOI] [PubMed] [Google Scholar]
- 121.Tornatore C, Chandra R, Berger JR, Major EO.HIV-1 infection of subcortical astrocytes in the pediatric central nervous system. Neurology. 1994; 44:481-7 [DOI] [PubMed] [Google Scholar]
- 122.Chang HC, Samaniego F, Nair BC, et al. HIV-1 Tat protein exits from cells via a leaderless secretory pathway and binds to extracellular matrix-associated heparan sulfate proteoglycans through its basic region. AIDS. 1997; 11:1421-31 [DOI] [PubMed] [Google Scholar]
- 123.Ensoli B, Buonaguro L, Barillari G, et al. Release, uptake, and effects of extracellular human immunodeficiency virus type 1 Tat protein on cell growth and viral transactivation. J Virol. 1993; 67:277-87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Tardieu M, Hery C, Peudenier S, et al. Human immunodeficiency virus type 1-infected monocytic cells can destroy human neural cells after cell-to-cell adhesion. Ann Neurol. 1992; 32:11-7 [DOI] [PubMed] [Google Scholar]
- 125.Hesselgesser J, Taub D, Baskar P, et al. Neuronal apoptosis induced by HIV-1 gp120 and the chemokine SDF-1 alpha is mediated by the chemokine receptor CXCR4. Curr Biol. 1998; 8:595-8 [DOI] [PubMed] [Google Scholar]
- 126.Meucci O, Miller RJ.gp120-induced neurotoxicity in hippocampal pyramidal neuron cultures: protective action of TGFbeta1. J Neurosci. 1996; 16:4080-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Dreyer EB, Kaiser PK, Offermann JT, Lipton SA.HIV-1 coat protein neurotoxicity prevented by calcium channel antagonists. Science. 1990; 248:364-7 [DOI] [PubMed] [Google Scholar]
- 128.Kaul M, Lipton SA.Chemokines and activated macrophages in HIV gp120-induced neuronal apoptosis. Proc Natl Acad Sci USA. 1999; 96:8212-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Geeraerts T, Deiva K, M’Sika I, et al. Effects of SDF-1alpha and gp120IIIB on apoptotic pathways in SK-N-SH neuroblastoma cells. Neurosci Lett. 2006;399: 115-20 [DOI] [PubMed] [Google Scholar]
- 130.Nosheny RL, Bachis A, Aden SA, et al. Intrastriatal administration of human immunodeficiency virus-1 glycoprotein 120 reduces glial cell-line derived neurotrophic factor levels and causes apoptosis in the substantia nigra. J Neurobiol. 2006; 66:1311-21 [DOI] [PubMed] [Google Scholar]
- 131.Bennett BA, Rusyniak DE, Hollingsworth CK.HIV-1 gp120-induced neurotoxicity to midbrain dopamine cultures. Brain Res. 1995; 705:168-76 [DOI] [PubMed] [Google Scholar]
- 132.Barks JD, Liu XH, Sun R, Silverstein FS.gp120, a human immunodeficiency virus-1 coat protein, augments excitotoxic hippocampal injury in perinatal rats. Neuroscience. 1997; 76:397-409 [DOI] [PubMed] [Google Scholar]
- 133.Lipton SA, Sucher NJ, Kaiser PK, Dreyer EB.Synergistic effects of HIV coat protein and NMDA receptor-mediated neurotoxicity. Neuron. 1991; 7:111-8 [DOI] [PubMed] [Google Scholar]
- 134.Singh IN, El-Hage N, Campbell ME, et al. Differential involvement of p38 and JNK MAP kinases in HIV-1 Tat and gp120-induced apoptosis and neurite degeneration in striatal neurons. Neuroscience. 2005; 135:781-90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Shimizu S, Khan MZ, Hippensteel RL, et al. Role of the transcription factor E2F1 in CXCR4-mediated neurotoxicity and HIV neuropathology. Neurobiol Dis. 2007; 25:17-26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Chauhan A, Turchan J, Pocernich C, et al. Intracellular human immunodeficiency virus Tat expression in astrocytes promotes astrocyte survival but induces potent neurotoxicity at distant sites via axonal transport. J Biol Chem. 2003;278: 13512-9 [DOI] [PubMed] [Google Scholar]
- 137.Hayman M, Arbuthnott G, Harkiss G, et al. Neurotoxicity of peptide analogues of the transactivating protein tat from Maedi-Visna virus and human immunodeficiency virus. Neuroscience. 1993; 53:1-6 [DOI] [PubMed] [Google Scholar]
- 138.Jones M, Olafson K, Del Bigio MR, et al. Intraventricular injection of human immunodeficiency virus type 1 (HIV-1) tat protein causes inflammation, gliosis, apoptosis, and ventricular enlargement. J Neuropathol Exp Neurol. 1998; 57:563-70 [DOI] [PubMed] [Google Scholar]
- 139.Magnuson DS, Knudsen BE, Geiger JD, et al. Human immunodeficiency virus type 1 tat activates non-N-methyl-D-aspartate excitatory amino acid receptors and causes neurotoxicity. Ann Neurol. 1995; 37:373-80 [DOI] [PubMed] [Google Scholar]
- 140.Maragos WF, Tillman P, Jones M, et al. Neuronal injury in hippocampus with human immunodeficiency virus transactivating protein, Tat. Neuroscience. 2003; 117:43-53 [DOI] [PubMed] [Google Scholar]
- 141.Liu Y, Jones M, Hingtgen CM, et al. Uptake of HIV-1 tat protein mediated by low-density lipoprotein receptor-related protein disrupts the neuronal metabolic balance of the receptor ligands. Nat Med. 2000; 6:1380-7 [DOI] [PubMed] [Google Scholar]
- 142.Zauli G, Milani D, Mirandola P, et al. HIV-1 Tat protein down-regulates CREB transcription factor expression in PC12 neuronal cells through a phosphatidylinositol 3-kinase/AKT/cyclic nucleoside phosphodiesterase pathway. Faseb J. 2001; 15:483-91 [DOI] [PubMed] [Google Scholar]
- 143.Ramirez SH, Sanchez JF, Dimitri CA, et al. Neurotrophins prevent HIV Tat-induced neuronal apoptosis via a nuclear factorkappaB (NF-kappaB)-dependent mechanism. J Neurochem. 2001; 78:874-89 [DOI] [PubMed] [Google Scholar]
- 144.New DR, Ma M, Epstein LG, et al. Human immunodeficiency virus type 1 Tat protein induces death by apoptosis in primary human neuron cultures. J Neurovirol. 1997; 3:168-73 [DOI] [PubMed] [Google Scholar]
- 145.Kruman II, Nath A, Mattson MP.HIV-1 protein Tat induces apoptosis of hippocampal neurons by a mechanism involving caspase activation, calcium overload, and oxidative stress. Exp Neurol. 1998; 154:276-88 [DOI] [PubMed] [Google Scholar]
- 146.Kruman II, Nath A, Maragos WF, et al. Evidence that Par-4 participates in the pathogenesis of HIV encephalitis. Am J Pathol. 1999; 155:39-46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Everall I, Barnes H, Spargo E, Lantos P.Assessment of neuronal density in the putamen in human immunodeficiency virus (HIV) infection. Application of stereology and spatial analysis of quadrats. J Neurovirol. 1995; 1:126-9 [DOI] [PubMed] [Google Scholar]
- 148.Eletto D, Russo G, Passiatore G, et al. Inhibition of SNAP25 expression by HIV-1 Tat involves the activity of mir-128a. J Cell Physiol. 2008; 216:764-70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Kosik KS.The neuronal microRNA system. Nat Rev Neurosci. 2006; 7:911-20 [DOI] [PubMed] [Google Scholar]
- 150.Norman JP, Perry SW, Reynolds HM, et al. HIV-1 Tat activates neuronal ryanodine receptors with rapid induction of the unfolded protein response and mitochondrial hyperpolarization. PLoS One. 2008;3:e3731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Mukerjee R, Deshmane SL, Fan S, et al. Involvement of the p53 and p73 transcription factors in neuroAIDS. Cell Cycle. 2008; 7:2682-90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Andras IE, Pu H, Deli MA, et al. HIV-1 Tat protein alters tight junction protein expression and distribution in cultured brain endothelial cells. J Neurosci Res. 2003; 74:255-65 [DOI] [PubMed] [Google Scholar]
- 153.McManus CM, Weidenheim K, Woodman SE, et al. Chemokine and chemokinereceptor expression in human glial elements: induction by the HIV protein, Tat, and chemokine autoregulation. Am J Pathol. 2000; 156:1441-53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Park IW, Wang JF, Groopman JE.HIV-1 Tat promotes monocyte chemoattractant protein-1 secretion followed by transmigration of monocytes. Blood. 2001; 97:352-8 [DOI] [PubMed] [Google Scholar]
- 155.Song L, Nath A, Geiger JD, et al. Human immunodeficiency virus type 1 Tat protein directly activates neuronal N-methyl-Daspartate receptors at an allosteric zincsensitive site. J Neurovirol. 2003;9: 399-403 [DOI] [PubMed] [Google Scholar]
- 156.Levy DN, Refaeli Y, Weiner DB.Extracellular Vpr protein increases cellular permissiveness to human immunodeficiency virus replication and reactivates virus from latency. J Virol. 1995; 69:1243-52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Levy DN, Refaeli Y, MacGregor RR, Weiner DB.Serum Vpr regulates productive infection and latency of human immunodeficiency virus type 1. Proc Natl Acad Sci USA. 1994; 91:10873-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Patel CA, Mukhtar M, Pomerantz RJ.Human immunodeficiency virus type 1 Vpr induces apoptosis in human neuronal cells. J Virol. 2000; 74:9717-26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Patel CA, Mukhtar M, Harley S, et al. Lentiviral expression of HIV-1 Vpr induces apoptosis in human neurons. J Neurovirol. 2002; 8:86-99 [DOI] [PubMed] [Google Scholar]
- 160.Piller SC, Ewart GD, Jans DA, et al. The amino-terminal region of Vpr from human immunodeficiency virus type 1 forms ion channels and kills neurons. J Virol. 1999; 73:4230-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Huang MB, Weeks O, Zhao LJ, et al. Effects of extracellular human immunodeficiency virus type 1 vpr protein in primary rat cortical cell cultures. J Neurovirol. 2000; 6:202-20 [DOI] [PubMed] [Google Scholar]
- 162.Stewart SA, Poon B, Song JY, Chen IS.Human immunodeficiency virus type 1 vpr induces apoptosis through caspase activation. J Virol. 2000; 74:3105-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Jacotot E, Ravagnan L, Loeffler M, et al. The HIV-1 viral protein R induces apoptosis via a direct effect on the mitochondrial permeability transition pore. J Exp Med. 2000; 191:33-46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Andersen JL, Zimmerman ES, DeHart JL, et al. ATR and GADD45alpha mediate HIV-1 Vpr-induced apoptosis. Cell Death Differ. 2005; 12:326-34 [DOI] [PubMed] [Google Scholar]
- 165.Yedavalli VS, Shih HM, Chiang YP, et al. Human immunodeficiency virus type 1 Vpr interacts with antiapoptotic mitochondrial protein HAX-1. J Virol. 2005; 79:13735-46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Sherman MP, Schubert U, Williams SA, et al. HIV-1 Vpr displays natural proteintransducing properties: implications for viral pathogenesis. Virology 2002;302 :95-105 [DOI] [PubMed] [Google Scholar]
- 167.Tungaturthi PK, Sawaya BE, Singh SP, et al. Role of HIV-1 Vpr in AIDS pathogenesis: relevance and implications of intravirion, intracellular and free Vpr. Biomed Pharmacother. 2003; 57:20-4 [DOI] [PubMed] [Google Scholar]
- 168.Zhang S, Feng Y, Narayan O, Zhao LJ.Cytoplasmic retention of HIV-1 regulatory protein Vpr by protein-protein interaction with a novel human cytoplasmic protein VprBP. Gene. 2001; 263:131-40 [DOI] [PubMed] [Google Scholar]
- 169.Yao XJ, Lemay J, Rougeau N, et al. Genetic selection of peptide inhibitors of human immunodeficiency virus type 1 Vpr. J Biol Chem. 2002; 277:48816-26 [DOI] [PubMed] [Google Scholar]
- 170.Greene WC, Peterlin BM.Charting HIV’s remarkable voyage through the cell: Basic science as a passport to future therapy. Nat Med. 2002; 8:673-80 [DOI] [PubMed] [Google Scholar]
- 171.Das SR, Jameel S.Biology of the HIV Nef protein. Indian J Med Res. 2005;121: 315-32 [PubMed] [Google Scholar]
- 172.Garry RF, Kort JJ, Koch-Nolte F, Koch G.Similarities of viral proteins to toxins that interact with monovalent cation channels. AIDS. 1991; 5:1381-4 [DOI] [PubMed] [Google Scholar]
- 173.Kort JJ, Jalonen TO.The nef protein of the human immunodeficiency virus type 1 (HIV-1) inhibits a large-conductance potassium channel in human glial cells. Neurosci Lett. 1998; 251:1-4 [DOI] [PubMed] [Google Scholar]
- 174.Werner T, Ferroni S, Saermark T, et al. HIV-1 Nef protein exhibits structural and functional similarity to scorpion peptides interacting with K+ channels. AIDS. 1991; 5:1301-8 [DOI] [PubMed] [Google Scholar]
- 175.Nath A, Conant K, Chen P, et al. Transient exposure to HIV-1 Tat protein results in cytokine production in macrophages and astrocytes. A hit and run phenomenon. J Biol Chem. 1999;274: 17098-102 [DOI] [PubMed] [Google Scholar]
- 176.Kutsch O, Oh J, Nath A, Benveniste EN.Induction of the chemokines interleukin-8 and IP-10 by human immunodeficiency virus type 1 tat in astrocytes. J Virol. 2000; 74:9214-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Pollard VW, Malim MH.The HIV-1 Rev protein. Ann Rev Microbiol. 1998;52: 491-532 [DOI] [PubMed] [Google Scholar]
- 178.Mabrouk K, Van Rietschoten J, Vives E, et al. Lethal neurotoxicity in mice of the basic domains of HIV and SIV Rev proteins. Study of these regions by circular dichroism. FEBS Lett. 1991; 289:13-7 [DOI] [PubMed] [Google Scholar]
- 179.Mattson MP, Haughey NJ, Nath A.Cell death in HIV dementia. Cell Death Differ 2005;12 Suppl 1:893-904 [DOI] [PubMed] [Google Scholar]
- 180.Meltzer MS, Skillman DR, Gomatos PJ, et al. Role of mononuclear phagocytes in the pathogenesis of human immunodeficiency virus infection. Annu Rev Immunol. 1990; 8:169-94 [DOI] [PubMed] [Google Scholar]
- 181.Adle-Biassette H, Chretien F, Wingertsmann L, et al. Neuronal apoptosis does not correlate with dementia in HIV infection but is related to microglial activation and axonal damage. Neuropathol Appl Neurobiol. 1999; 25:123-33 [DOI] [PubMed] [Google Scholar]
- 182.Rappaport J, Joseph J, Croul S, et al. Molecular pathway involved in HIV-1-induced CNS pathology: role of viral regulatory protein, Tat. J Leukoc Biol. 1999; 65:458-65 [DOI] [PubMed] [Google Scholar]
- 183.Sundar SK, Cierpial MA, Kamaraju LS, et al. Human immunodeficiency virus glycoprotein (gp120) infused into rat brain induces interleukin 1 to elevate pituitary-adrenal activity and decrease peripheral cellular immune responses. Proc Natl Acad Sci USA. 1991; 88:11246-50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Genis P, Jett M, Bernton EW, et al. Cytokines and arachidonic metabolites produced during human immunodeficiency virus (HIV)-infected macrophageastroglia interactions: implications for the neuropathogenesis of HIV disease. J Exp Med. 1992; 176:1703-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Ehrenreich H, Rieckmann P, Sinowatz F, et al. Potent stimulation of monocytic endothelin-1 production by HIV-1 glycoprotein 120. J Immunol. 1993; 150:4601-9 [PubMed] [Google Scholar]
- 186.Perrella O, Guerriero M, Izzo E, et al. Interleukin-6 and granulocyte macrophage-CSF in the cerebrospinal fluid from HIV infected subjects with involvement of the central nervous system. Arq Neuropsiquiatr. 1992; 50:180-2 [DOI] [PubMed] [Google Scholar]
- 187.Wesselingh SL, Power C, Glass JD, et al. Intracerebral cytokine messenger RNA expression in acquired immunodeficiency syndrome dementia. Ann Neurol. 1993; 33:576-82 [DOI] [PubMed] [Google Scholar]
- 188.Matsuda M, Masutani H, Nakamura H, et al. Protective activity of adult T cell leukemia-derived factor (ADF) against tumor necrosis factor-dependent cytotoxicity on U937 cells. J Immunol. 1991; 147:3837-41 [PubMed] [Google Scholar]
- 189.Chang DJ, Ringold GM, Heller RA.Cell killing and induction of manganous superoxide dismutase by tumor necrosis factoralpha is mediated by lipoxygenase metabolites of arachidonic acid. Biochem Biophys Res Commun. 1992; 188:538-46 [DOI] [PubMed] [Google Scholar]
- 190.Ameisen JC, Capron A.Cell dysfunction and depletion in AIDS: the programmed cell death hypothesis. Immunol Today. 1991; 12:102-5 [DOI] [PubMed] [Google Scholar]
- 191.Gougeon ML, Montagnier L.Apoptosis in AIDS. Science. 1993; 260:1269-70 [DOI] [PubMed] [Google Scholar]
- 192.Talley AK, Dewhurst S, Perry SW, et al. Tumor necrosis factor alpha-induced apoptosis in human neuronal cells: protection by the antioxidant N-acetylcysteine and the genes bcl-2 and crmA. Mol Cell Biol. 1995; 15:2359-66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Suzuki T, Hide I, Ido K, et al. Production and release of neuroprotective tumor necrosis factor by P2X7 receptor-activated microglia. J Neurosci. 2004; 24:1-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Glazner GW, Mattson MP.Differential effects of BDNF, ADNF9, and TNFalpha on levels of NMDA receptor subunits, calcium homeostasis, and neuronal vulnerability to excitotoxicity. Exp Neurol. 2000; 161:442-52 [DOI] [PubMed] [Google Scholar]
- 195.Toku K, Tanaka J, Yano H, et al. Microglial cells prevent nitric oxide-induced neuronal apoptosis in vitro. J Neurosci Res. 1998; 53:415-25 [DOI] [PubMed] [Google Scholar]
- 196.Barger SW, Horster D, Furukawa K, et al. Tumor necrosis factors alpha and beta protect neurons against amyloid beta-peptide toxicity: evidence for involvement of a kappa B-binding factor and attenuation of peroxide and Ca2+ accumulation. Proc Natl Acad Sci USA. 1995; 92:9328-32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Cheng B, Christakos S, Mattson MP.Tumor necrosis factors protect neurons against metabolic-excitotoxic insults and promote maintenance of calcium homeostasis. Neuron. 1994; 12:139-53 [DOI] [PubMed] [Google Scholar]
- 198.Scherbel U, Raghupathi R, Nakamura M, et al. Differential acute and chronic responses of tumor necrosis factor-deficient mice to experimental brain injury. Proc Natl Acad Sci USA. 1999; 96:8721-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Yang L, Lindholm K, Konishi Y, et al. Target depletion of distinct tumor necrosis factor receptor subtypes reveals hippocampal neuron death and survival through different signal transduction pathways. J Neurosci. 2002; 22:3025-32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Sedger LM, Shows DM, Blanton RA, et al. IFN-gamma mediates a novel antiviral activity through dynamic modulation of TRAIL and TRAIL receptor expression. J Immunol. 1999; 163:920-6 [PubMed] [Google Scholar]
- 201.Lum JJ, Pilon AA, Sanchez-Dardon J, et al. Induction of cell death in human immunodeficiency virus-infected macrophages and resting memory CD4 T cells by TRAIL/Apo2l. J Virol. 2001; 75:11128-36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Sato K, Hida S, Takayanagi H, et al. Antiviral response by natural killer cells through TRAIL gene induction by IFNalpha/beta. Eur J Immunol. 2001;31: 3138-46 [DOI] [PubMed] [Google Scholar]
- 203.Nitsch R, Bechmann I, Deisz RA, et al. Human brain-cell death induced by tumour-necrosis-factor-related apoptosisinducing ligand (TRAIL). Lancet. 2000; 356:827-8 [DOI] [PubMed] [Google Scholar]
- 204.Ryan LA, Zheng J, Brester M, et al. Plasma levels of soluble CD14 and tumor necrosis factor-alpha type II receptor correlate with cognitive dysfunction during human immunodeficiency virus type 1 infection. J Infect Dis. 2001; 184:699-706 [DOI] [PubMed] [Google Scholar]
- 205.Griffith TS, Wiley SR, Kubin MZ, et al. Monocyte-mediated tumoricidal activity via the tumor necrosis factor-related cytokine, TRAIL. J Exp Med. 1999;189: 1343-54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Halaas O, Vik R, Ashkenazi A, Espevik T.Lipopolysaccharide induces expression of APO2 ligand/TRAIL in human monocytes and macrophages. Scand J Immunol. 2000; 51:244-50 [DOI] [PubMed] [Google Scholar]
- 207.Miura Y, Misawa N, Kawano Y, et al. Tumor necrosis factor-related apoptosis-inducing ligand induces neuronal death in a murine model of HIV central nervous system infection. Proc Natl Acad Sci USA. 2003; 100:2777-82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Oster W, Lindemann A, Horn S, et al. Tumor necrosis factor (TNF)-alpha but not TNF-beta induces secretion of colony stimulating factor for macrophages (CSF-1) by human monocytes. Blood. 1987; 70:1700-3 [PubMed] [Google Scholar]
- 209.Pixley FJ, Stanley ER.CSF-1 regulation of the wandering macrophage: complexity in action. Trends Cell Biol. 2004;14: 628-38 [DOI] [PubMed] [Google Scholar]
- 210.Kutza J, Crim L, Feldman S, et al. Macrophage colony-stimulating factor antagonists inhibit replication of HIV-1 in human macrophages. J Immunol. 2000; 164:4955-60 [DOI] [PubMed] [Google Scholar]
- 211.Bose M, Farnia P.Proinflammatory cytokines can significantly induce human mononuclear phagocytes to produce nitric oxide by a cell maturation-dependent process. Immunol Lett. 1995; 48:59-64 [DOI] [PubMed] [Google Scholar]
- 212.Granowitz EV, Clark BD, Vannier E, et al. Effect of interleukin-1 (IL-1) blockade on cytokine synthesis: I. IL-1 receptor antagonist inhibits IL-1-induced cytokine synthesis and blocks the binding of IL-1 to its type II receptor on human monocytes. Blood. 1992; 79:2356-63 [PubMed] [Google Scholar]
- 213.Tosato G, Jones KD.Interleukin-1 induces interleukin-6 production in peripheral blood monocytes. Blood. 1990; 75:1305-10 [PubMed] [Google Scholar]
- 214.Poli G, Bressler P, Kinter A, et al. Interleukin 6 induces human immunodeficiency virus expression in infected monocytic cells alone and in synergy with tumor necrosis factor alpha by transcriptional and post-transcriptional mechanisms. J Exp Med. 1990; 172:151-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Lavi E, Kolson DL, Ulrich AM, et al. Chemokine receptors in the human brain and their relationship to HIV infection. J Neurovirol. 1998; 4:301-11 [DOI] [PubMed] [Google Scholar]
- 216.Zheng J, Thylin MR, Ghorpade A, et al. Intracellular CXCR4 signaling, neuronal apoptosis and neuropathogenic mechanisms of HIV-1-associated dementia. J Neuroimmunol. 1999; 98:185-200 [DOI] [PubMed] [Google Scholar]
- 217.Schmidtmayerova H, Nottet HS, Nuovo G, et al. Human immunodeficiency virus type 1 infection alters chemokine beta peptide expression in human monocytes: implications for recruitment of leukocytes into brain and lymph nodes. Proc Natl Acad Sci USA. 1996; 93:700-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Meucci O, Fatatis A, Simen AA, Miller RJ.Expression of CX3CR1 chemokine receptors on neurons and their role in neuronal survival. Proc Natl Acad Sci USA. 2000; 97:8075-80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Meucci O, Fatatis A, Simen AA, et al. Chemokines regulate hippocampal neuronal signaling and gp120 neurotoxicity. Proc Natl Acad Sci USA. 1998; 95:14500-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Zhang K, McQuibban GA, Silva C, et al. HIV-induced metalloproteinase processing of the chemokine stromal cell derived factor-1 causes neurodegeneration. Nat Neurosci. 2003; 6:1064-71 [DOI] [PubMed] [Google Scholar]
- 221.McQuibban GA, Butler GS, Gong JH, et al. Matrix metalloproteinase activity inactivates the CXC chemokine stromal cellderived factor-1. J Biol Chem. 2001;276: 43503-8 [DOI] [PubMed] [Google Scholar]
- 222.Kolb SA, Sporer B, Lahrtz F, et al. Identification of a T cell chemotactic factor in the cerebrospinal fluid of HIV-1-infected individuals as interferon-gamma inducible protein 10. J Neuroimmunol. 1999; 93:172-81 [DOI] [PubMed] [Google Scholar]
- 223.Yeh MW, Kaul M, Zheng J, et al. Cytokinestimulated, but not HIV-infected, human monocyte-derived macrophages produce neurotoxic levels of l -cysteine. J Immunol 200015;164:4265-70 [DOI] [PubMed] [Google Scholar]
- 224.Guillemin GJ, Kerr SJ, Brew BJ.Involvement of quinolinic acid in AIDS dementia complex. Neurotox Res. 2005;7: 103-23 [DOI] [PubMed] [Google Scholar]
- 225.Giulian D, Vaca K, Noonan CA.Secretion of neurotoxins by mononuclear phagocytes infected with HIV-1. Science. 1990; 250:1593-6 [DOI] [PubMed] [Google Scholar]
- 226.Bukrinsky MI, Nottet HS, Schmidtmayerova H, et al. Regulation of nitric oxide synthase activity in human immunodeficiency virus type 1 (HIV-1)-infected monocytes: implications for HIV-associated neurological disease. J Exp Med. 1995; 181:735-45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Olney JW, Zorumski C, Price MT, Labruyere J.L-cysteine, a bicarbonatesensitive endogenous excitotoxin. Science. 1990; 248:596-9 [DOI] [PubMed] [Google Scholar]
- 228.Levivier M, Przedborski S.Quinolinic acidinduced lesions of the rat striatum: quantitative autoradiographic binding assessment. Neurol Res. 1998; 20:46-56 [DOI] [PubMed] [Google Scholar]
- 229.Giulian D, Yu J, Li X, et al. Study of receptor-mediated neurotoxins released by HIV-1-infected mononuclear phagocytes found in human brain. J Neurosci. 1996; 16:3139-53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Barbour B, Szatkowski M, Ingledew N, Attwell D.Arachidonic acid induces a prolonged inhibition of glutamate uptake into glial cells. Nature. 1989; 342:918-20 [DOI] [PubMed] [Google Scholar]
- 231.Heyes MP, Rubinow D, Lane C, Markey SP.Cerebrospinal fluid quinolinic acid concentrations are increased in acquired immune deficiency syndrome. Ann Neurol. 1989; 26:275-7 [DOI] [PubMed] [Google Scholar]
- 232.Gelbard HA, Nottet HS, Swindells S, et al. Platelet-activating factor: a candidate human immunodeficiency virus type 1-induced neurotoxin. J Virol. 1994;68: 4628-35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Aihara M, Ishii S, Kume K, Shimizu T.Interaction between neurone and microglia mediated by platelet-activating factor. Genes Cells. 2000; 5:397-406 [DOI] [PubMed] [Google Scholar]
- 234.Dawson VL, Dawson TM.Nitric oxide neurotoxicity. J Chem Neuroanat. 1996; 10:179-90 [DOI] [PubMed] [Google Scholar]
- 235.Takeuchi H, Mizuno T, Zhang G, et al. Neuritic beading induced by activated microglia is an early feature of neuronal dysfunction toward neuronal death by inhibition of mitochondrial respiration and axonal transport. J Biol Chem. 2005; 280:10444-54 [DOI] [PubMed] [Google Scholar]
- 236.Meaney MJ.Maternal care, gene expression, and the transmission of individual differences in stress reactivity across generations. Annu Rev Neurosci. 2001; 24:1161-92 [DOI] [PubMed] [Google Scholar]
- 237.Cowan WM, Kopnisky KL, Hyman SE.The human genome project and its impact on psychiatry. Annu Rev Neurosci. 2002; 25:1-50 [DOI] [PubMed] [Google Scholar]
- 238.Tsuji T, Shiozaki A, Kohno R, et al. Proteomic profiling and neurodegeneration in Alzheimer’s disease. Neurochem Res. 2002; 27:1245-53 [DOI] [PubMed] [Google Scholar]
- 239.Singh KK, Barroga CF, Hughes MD, et al. Genetic influence of CCR5, CCR2, and SDF1 variants on human immunodeficiency virus 1 (HIV-1)-related disease progression and neurological impairment, in children with symptomatic HIV-1 infection. J Infect Dis. 2003; 188:1461-72 [DOI] [PubMed] [Google Scholar]
- 240.Geiss GK, Bumgarner RE, An MC, et al. Large-scale monitoring of host cell gene expression during HIV-1 infection using cDNA microarrays. Virology. 2000; 266:8-16 [DOI] [PubMed] [Google Scholar]
- 241.Glanzer JG, Haydon PG, Eberwine JH.Expression profile analysis of neurodegenerative disease: advances in specificity and resolution. Neurochem Res. 2004; 29:1161-8 [DOI] [PubMed] [Google Scholar]
- 242.Schubert D.Developmental biology of cultured nerve, muscle, and glia. New York: Wiley; 1984 [Google Scholar]
- 243.Nuovo GJ, Gallery F, MacConnell P, Braun A.In situ detection of polymerase chain reaction-amplified HIV-1 nucleic acids and tumor necrosis factor-alpha RNA in the central nervous system. Am J Pathol. 1994; 144:659-66 [PMC free article] [PubMed] [Google Scholar]
- 244.Takahashi K, Wesselingh SL, Griffin DE, et al. Localization of HIV-1 in human brain using polymerase chain reaction/in situ hybridization and immunocytochemistry. Ann Neurol. 1996; 39:705-11 [DOI] [PubMed] [Google Scholar]
- 245.Trillo-Pazos G, Diamanturos A, Rislove L, et al. Detection of HIV-1 DNA in microglia/macrophages, astrocytes and neurons isolated from brain tissue with HIV-1 encephalitis by laser capture microdissection. Brain Pathol. 2003;13: 144-54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Tornatore C, Nath A, Amemiya K, Major EO.Persistent human immunodeficiency virus type 1 infection in human fetal glial cells reactivated by T-cell factor(s) or by the cytokines tumor necrosis factor alpha and interleukin-1 beta. J Virol. 1991; 65:6094-100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Nath A, Hartloper V, Furer M, Fowke KR.Infection of human fetal astrocytes with HIV-1: viral tropism and the role of cell to cell contact in viral transmission. J Neuropathol Exp Neurol. 1995; 54:320-30 [DOI] [PubMed] [Google Scholar]
- 248.Wang Z, Trillo-Pazos G, Kim SY, et al. Effects of human immunodeficiency virus type 1 on astrocyte gene expression and function: potential role in neuropathogenesis. J Neurovirol 2004;10 Suppl 1:25-32 [DOI] [PubMed] [Google Scholar]
- 249.Galey D, Becker K, Haughey N, et al. Differential transcriptional regulation by human immunodeficiency virus type 1 and gp120 in human astrocytes. J Neurovirol. 2003; 9:358-71 [DOI] [PubMed] [Google Scholar]
- 250.Kim SY, Li J, Bentsman G, et al. Microarray analysis of changes in cellular gene expression induced by productive infection of primary human astrocytes: implications for HAD. J Neuroimmunol. 2004; 157:17-26 [DOI] [PubMed] [Google Scholar]
- 251.Kramer-Hammerle S, Hahn A, Brack-Werner R, Werner T.Elucidating effects of long-term expression of HIV-1 Nef on astrocytes by microarray, promoter, and literature analyses. Gene. 2005; 358:31-8 [DOI] [PubMed] [Google Scholar]
- 252.Gelman BB, Soukup VM, Schuenke KW, et al. Acquired neuronal channelopathies in HIV-associated dementia. J Neuroimmunol. 2004; 157:111-9 [DOI] [PubMed] [Google Scholar]
- 253.Masliah E, Roberts ES, Langford D, et al. Patterns of gene dysregulation in the frontal cortex of patients with HIV encephalitis. J Neuroimmunol. 2004;157: 163-75 [DOI] [PubMed] [Google Scholar]
- 254.Shapshak P, Duncan R, Torres-Munoz JE, et al. Analytic approaches to differential gene expression in AIDS versus control brains. Front Biosci. 2004; 9:2935-46 [DOI] [PubMed] [Google Scholar]
- 255.Sui Y, Potula R, Pinson D, et al. Microarray analysis of cytokine and chemokine genes in the brains of macaques with SHIVencephalitis. J Med Primatol. 2003; 32:229-39 [DOI] [PubMed] [Google Scholar]
- 256.Roberts ES, Zandonatti MA, Watry DD, et al. Induction of pathogenic sets of genes in macrophages and neurons in NeuroAIDS. Am J Pathol. 2003;162: 2041-57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Roberts ES, Burudi EM, Flynn C, et al. Acute SIV infection of the brain leads to upregulation of IL6 and interferon-regulated genes: expression patterns throughout disease progression and impact on neuroAIDS. J Neuroimmunol. 2004; 157:81-92 [DOI] [PubMed] [Google Scholar]
- 258.Stephens EB, Jackson M, Cui L, et al. Early dysregulation of cripto-1 and immunomodulatory genes in the cerebral cortex in a macaque model of neuroAIDS. Neurosci Lett. 2006; 410:94-9 [DOI] [PubMed] [Google Scholar]


