Abstract
Introduction: There is a pressing need for research leading to the development of new effective drugs with lower side effects and more efficacy for treating inflammatory bowel disease (IBD). The analgesic and anti-inflammatory properties of 5-Hydroxytryptamine (5-HT)-3 receptor antagonists have been shown in in vivo and in vitro studies. The present study was designed to investigate the effects of tropisetron, a 5-HT3 receptor antagonist, on an immune-based animal model of IBD. Methods: In the present study, the trinitrobenzenesulfonic acid (TNBS) model of colitis in the rat was used. Two hours after induction of colitis in rats, tropisetron (2 mg/kg), dexamethasone (1 mg/kg), meta-chlorophenylbiguanide (mCPBG, 5 mg/kg), a 5-HT3 receptor agonist, or tropisetron + mCPBG were intraperitoneally (i.p.) administrated for 6 days. Animals were then sacrificed; macroscopic, histological, biochemical (myeloperoxidase [MPO]) assessments and ELISA test (tumor necrosis factor-alpha, interleukin-6 and interleukin-1 beta) were performed on distal colon samples. Results: Tropisetron or dexamethasone treatment significantly reduced macroscopic and microscopic colonic damages. In addition, a significant reduction in MPO activity and colonic levels of inflammatory cytokines was seen. The beneficial effects of tropisetron were antagonized by concurrent administration of mCPBG. Conclusion: The present study indicates that the protective effects of tropisetron on TNBS-induced colitis can be mediated by 5-HT3 receptors.
Keywords: 5-HT3 receptor, TNBS-induced colitis, Tropisetron, Ulcerative colitis
Introduction
Inflammatory bowel disease (IBD), comprising ulcerative colitis (UC) and Crohn’s disease (CD), is a chronic inflammatory disorder of the gastrointestinal (GI) tract characterized by a relapsing course. The etiology of both diseases remains ambiguous; however, they are likely to result from complex interactions of susceptibility genes, environment, and immune system.1,2 Both entities have a broad spectrum of clinical presentations. IBD refers to massive cellular infiltrates and pertains to immunological abnormalities indicating increasing number of CD4+ T lymphocytes, mast cells, neutrophils, and eosinophils.3 Patients with IBD frequently suffer from fatigue, inflammation, ulceration, edema, diarrhea along with blood and/or mucus, fever and gastric dysmotility.4 Many medical therapies have been proposed for IBD such as 5-aminosalisylate derivatives, glucocorticoids, and immunosuppressives;5 nonetheless, available medicines are not universally effective and result in marked deleterious effects. Therefore, the medical management of IBD remains challenging and investigations on novel treatments are required.6
5-hydroxytryptamine (serotonin) is an important gastrointestinal (GI) signaling molecule involving in motor, secretory and sensory functions.7 It is also found in the immune-inflammatory axis and modulates the immune response in several autoimmune conditions.8,9 Intestinal inflammation may arise from a change in 5-HT-producing enterochromaffin (EC) cells and an increase in 5-HT content associated with the pattern of IBD.10 These actions are mediated by a large family of serotonin receptors located within the neural circuitry and on other cell types in the gut. Of the 5-HT receptors expressed in intestines, the 5-HT3 receptor has been one of the most widely studied receptor in GI function.11 To illustrate, 5-HT3 receptors are widely expressed in cells of the immune system including primary human monocytes and T cells.12 Serotonin can modulate T-cell activation and proliferation through activation of their 5-HT3 receptors. In addition, experimental studies revealed that 5-HT3 receptor antagonists including tropisetron and ondansetron possess both analgesic and anti-inflammatory effects.13 Nevertheless, the mechanisms for these impacts have not been understood yet. Fiebich et al reported that lipopolysaccharide-stimulated secretion of tumor necrosis factor-α (TNF-α) and interleukin-1b (IL-1b) was dose-dependently inhibited by tropisetron in human monocytes.13 Moreover, tropisetron inhibits both IL-2 gene transcription and IL-2 synthesis in stimulated T cell.14 Preliminary data have shown that the intra-articular administration of tropisetron exerts a beneficial effect upon patients with osteoarthritis, rheumatoid arthritis and scleroderma.15 Furthermore, Musavizadeh et al16 demonstrated the anti-inflammatory effect of tropisetron on acid acetic induced colitis, although the authors reported that this beneficial effect is likely to be independent of 5-HT3 receptors.
Recent progress in our understanding about mucosal immunity and pathophysiology of IBD has mostly been achieved by the development in new experimental animal models of intestinal inflammation. Large intestine inflammation induced by rectal administration of 2,4,6- trinitrobenzenesulfonic acid (TNBS) is among the most common models of colitis described in the literature.17 This model can exhibit the inflammation related to cytokines secretion,18 and it is efficiently able to mimic both acute and chronic colitis resembling the human UC.19
With regard to the probable association between inflammation and 5-HT, it can be inferred that the anti-inflammatory property of tropisetron in experimental colitis, may be at least partly mediated through its effect on 5-HT3 receptor pathways. The aim of this investigation was to assess the anti-inflammatory property of tropisetron (through its effect on 5-HT3 receptors) upon colonic inflammation markers, to compare the effects of tropisetron with dexamethasone as a reference drug, and to explore the probable involvement of 5-HT3 receptors in producing anti-inflammatory effect of tropisetron against colitis in immune-based animal model of colitis.
Materials and methods
Animals
Twelve-week-old male Wistar rats weighing approximately 250 ± 20 g bred in animal house of School of Pharmacy, Isfahan University of Medical Sciences were used. All studies were performed under approval of Animal Care Committee of the Isfahan University of Medical Sciences (Tehran Protocol no. 302386; February 20, 2005) and were in agreement with the guidelines for the proper use of animals in biomedical research. The rats were housed in groups of 6 in temperature-controlled rooms with a 12-h light/dark cycle and fed standard pelleted chow and water ad libitum. Rats were ultimately sacrificed by inhalation of ether.
Chemicals
Dexamethasone was obtained from Iran Hormone Pharmaceutical Co. (Tehran, Iran). TNBS, metachlorophenylbiguanide (mPBG), tropisetron hydrochloride, hexadecyltrimethyl-ammonium bromide (HTAB), aprotinin A, bovine serum albumin, phenylmethylsulfonyl fluoride, benzethonium chloride, ethylene diamine tetra-acetic acid (EDTA), and Tween 20 were all purchased from Sigma Chemical Company (St. Louis, MO, USA). The amount of colonic rat TNF-α, IL-1β and IL-6 were quantified by commercially available enzyme-linked immunosorbent assay (ELISA) kits (ALPCO, USA).
Induction of colitis
All rats were fasted for 36 h prior to the induction of colitis, with free access to water. Colitis was induced by means of method of Morris et al.20 They were slightly anaesthetized with ether and given 10 mg (50 mg/kg) of TNBS dissolved in 0.25 mL of 50% ethanol (v/v) by means of a polyethylene catheter inserted 8 cm proximal to the anus. Following instillation of TNBS, rats were maintained in a supine Trendelenburg position for 2-3 min in order to prevent anal leakage of TNBS. Thereafter, they returned to their cages with free access to food and water. Normal group received enema of 0.25 ml of normal saline.
Grouping
Animals were randomly assigned to six groups (n = 6). TNBS-control group received normal saline, 2 h subsequent to induction of colitis. In normal group, normal saline (0.25 ml/rat) was administrated intrarectally instead of TNBS. Dexamethasone (1 mg/kg)21 and tropisetron (2 mg/kg)16 were administered to dexamethasone and tropisetron groups, respectively. Meta chlorophenylbiguanide (mCPBG) group received mCPBG (5 mg/kg), a selective 5-HT3 agonist22 and finally in tropisetron + mCPBG group, tropisetron and mCPBG were administrated concurrently. All treatments were carried out intraperitoneally (i.p.), 2 h after induction of colitis by TNBS, and continued daily for six days.20
Assessment of body weight changes and diarrheal status
Animal body weights and occurrence of diarrhea were recorded daily over the experiments. The Percent of body weight loss was thereafter measured. Using arbitrary criteria (1. Formed stools, 2. Loosed stools, 3. Diarrhea), fecal output was assessed every day for six days.
Macroscopic assessment
Once the animals were sacrificed by means of ether inhalation, the distal colon was removed and opened longitudinally. Afterwards, the colonic segment was cleaned of fecal content, fat and mesentery and processed for assessment by macroscopic, histological scores and biochemical markers. Each specimen (under a constant load of 8 cm from the anus) was weighed (mg), and weight/ length ratio (mg/cm) was measured. Each colon was scored for macroscopically visible damage on a 0–15 scale by an observer unaware of the treatment, according to criteria previously defined by Ballester et al (some modifications have been undertaken as shown in Table 1).23 The colon samples were subsequently sectioned in 3-4 longitudinal fragments and immediately frozen in liquid nitrogen for biochemical assessment.
Table 1 . Scoring criteria for assessment of macroscopic rat colonic injuries .
| Adhesions | 0 No adhesions |
| 1 Difficult dissection | |
| 2 Visible adhesions | |
| 3 ‘Wrapped’ intestine | |
| Obstruction | 0 No obstruction |
| 1 Need for gentle manual cleaning | |
| 2 Fecal impaction | |
| Thickening | 0 Similar to uninflamed intestine |
| 1 Thicker than normal ( ~1–2mm) | |
| 2 Much thicker than normal (>2mm) | |
| Hyperemia | 0 Similar to uninflamed intestine |
| 1 Mild and generalized or intense but localized hyperemia | |
| 2 Intense and localized hyperemia | |
| 3 Frank hemorrhage | |
| Necrosis | 0 No signs of necrosis |
| 1 Small areas of necrosis | |
| 2 Patchy necrosis | |
| 3 Focal necrosis <0.8 cm | |
| 4 Focal necrosis >0.8 cm | |
| 5 Extended necrotic lesion |
After taking photos from distal colons, ulcer area and percent of necrosis were determined according to method of measurement of ulcer area and percent of necrosis mentioned in our previous study.24
Histological assessment
For histological examination, a sample of colonic tissue was fixed in 10% formalin, dehydrated, paraffin embedded, processed, sliced into 4 µm-thick sections and stained with haematoxylin and eosin (H&E). The histological damage was evaluated by a pathologist coworker who was blinded to the experimental groups, according to previously described criteria.25,26 Total colitis index was then derived by summing three sub-scores (inflammation severity, inflammation extent, crypt damage) on H&E-stained and coded sections.
Measurement of myeloperoxidase (MPO) Activity
MPO which is an enzyme found predominantly in the azurophilic granules of neutrophils was measured as a quantitative index of inflammation in intestine, using the modified method of Bradley et al.27 Each segment was weighed and chopped in 1 ml of 50 mM potassium phosphate buffer involving 0.5% HTAB. Having chopped, we placed the tissue in a homogenizing tube. The container was then rinsed with 2×1 ml HTAB in buffer solution. Afterwards, we added more buffer in order to provide a concentration equivalent to 5 ml per 0.1 g of colon tissue and homogenized (15000 rpm) for 4×45 s at 1 min intervals. The homogenate was placed in a sample tube, sonicated in an ice bath for 10 s, subjected to 3 cycles of freezing and thawing, and sonicated again for 10 s. The suspensions were thereafter centrifuged (15000 rpm for 15 min at 4°C) and the supernatant was decanted for assessment. The MPO activity was analyzed spectrophotometrically as follows: 0.1 ml of the supernatant was added to 2.9 ml of 50 mM K3PO4 buffer (pH = 6.0) involving O-dianisidine dihydrochloride (0.167 mg/ml) and 0.005 % hydrogen peroxide. The absorbance of the reaction mixture was recorded at a wave length of 450 nm by means of a UV–Vis spectrophotometer. The data were reported as the change in absorbance/min/mg colonic wet weight.
Measurement of cytokine in the rat colon
The levels of TNF-α, IL-1b and IL-6 in the colon samples were measured using a commercially available enzyme-linked immunosorbent assay (ELISA) kit (ALPCO, USA) as described previously.28 The colon tissue segments were weighed and processed in order to determine IL-1β, IL-6 and TNF-α content. Thereafter, colon samples were homogenized in phosphate buffered saline (PBS; pH = 7.4) containing 0.4 M NaCl, 0.05% Tween-20, 0.5% bovine serum albumin, 0.1 mM phenylmethylsulfonyl fluoride, 0.1 mM benzethonium chloride, aprotinin A 20 KI, and 10 mM EDTA. They were then centrifuged at 12000×g for 30 min at 4°C, and ELISA was performed in order to assess the levels of IL-1β and TNF-α in the supernatants.
Statistical analysis
Data analysis was performed using the SPSS statistical package (Version 17.0). Comparison between groups was made using one-way analysis of variance (ANOVA) with Tukey post hoc test. Clinical activity score of colitis and macroscopic and histological scores were statistically analyzed using the Mann-Whitney U test. Results were reported as mean ± standard error of mean (SEM). A P- value < 0.05 was considered significant.
Results
Change in animals’ body weight and diarrheal status
TNBS-treated rats experienced a body weight loss after 6 days (P < 0.001). Percentage of body weight loss in tropisetron and dexamethasone-treated groups was significantly lower than TNBS-control group after 6 days (Table 2); however, these groups showed a significant body weight loss in comparison with normal group.
Table 2 . Effect of tropisetron (2 mg/kg, daily) on macroscopic and histopathological parameters of the colon 6 days after induction of colitis with TNBS (50 mg/kg).
| Group |
Colonic weight/length ratio (mg/cm) |
Body weight loss after 6 days (%) |
Ulcer severity
(0-15) |
Ulcer area
(cm 2 ) |
Necrosis
(%) |
Total colitis index (0-10) |
| Normal | 64.8 ± 2.3 | -1.9 ± 0.6 | 0.0 | 0.0 | 0.0 | 0.0 |
| TNBS-control | 256.3 ± 9.5 | 7.7 ± 0.7 | 12.2 ± 0.6 | 6.2 ± 0.1 | 51.5 ± 2.8 | 9.9 ± 0.1 |
| Trop. (2mg/kg, i.p.) | 158.3 ± 23.6 ** | 3.3 ± 0.8 ** | 5.6 ± 1.1 ** | 4.1 ± 0.5 * | 28.1 ± 6.8** | 5.5 ± 0.7 ** |
| mCPBG (5mg/kg,i.p.) | 252.1 ± 8.2 | 8.1 ± 0.8 | 11.5 ± 0.6 | 6.1 ± 0.2 | 51.3 ± 3.2 | 9.7 ± 0.1 |
| mCPBG+ Trop. | 245.2 ± 27.6 | 7.7 ± 0.9 | 11.7 ± 0.5 | 5.7 ± 0.2 | 51.3 ± 4.5 | 9.3 ± 0.3 |
| Dex. (1mg/kg, i.p.) | 161.9 ± 25.9 ** | 3.9 ± 0.3 * | 5.33 ± 1.4 ** | 4.0 ± 0.7 * | 27.0 ± 7.9** | 4.8 ± 0.8 ** |
1 TNBS,2,4,6-trinitrobenzenesulfonic acid; Trop., tropisetron; mCPG, meta-chlorophenylbiguanide; Dex., dexamethasone; i.p., intraperitoneal
2 Values are means ± SEM; n =6.
3**P < 0.01 and *P < 0 .05: Significant difference compared to TNBS-control group.
As can be noted in Fig.1, diarrheal status of TNBS-control group was significantly higher than that of normal group, over the experiment (P < 0.01). During the first four days after induction of colitis, dexamethasone and tropisetron-treated rats showed a significant increase in the diarrhea index, in comparison with normal group (P values are shown in Fig.1). However, diarrhea index reduced gradually in these groups during this time period, and after fourth day, there was no significant difference in diarrheal status of aforementioned groups compared to that of normal group. In addition, no significant difference was observed in the daily diarrheal status and percentage of body weight loss between the tropisetron-treated and dexamethasone-treated groups during the experiment. The mCPBG, a serotonin agonist, could not change the diarrheal status of TNBS-treated rat; however, it antagonized the beneficial effects of tropisetron.
Fi. 1 .
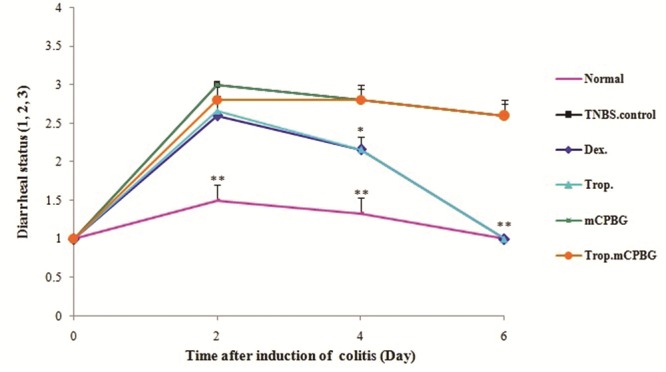
Changes in diarrheal status before (Day 0) and during 6 days of treatment after induction of colitis (TNBS, 50 mg/kg) in rats. Values are means ± SEM (n=6). **P < 0.01 and *P < 0.05 compared with TNBS-control group. TNBS, 2,4,6-trinitrobenzenesulfonic acid. Trop., tropisetron; mCPG, meta-chlorophenylbiguanide; Dex., dexamethasone. Stool consistency daily were checked in rats (1. Formed stools, 2. Loosed stools and 3. Diarrhea).
Effect of tropisetron on macroscopic features
Six days after induction of colitis, the distal colon of the TNBS-control group showed massive injuries. The mucosa was hyperemic, inflamed, ulcerated and hemorrhagic. Necrosis and grossly visible thickness of the colon wall were observed, while the normal macroscopic features were evident in the colons of the normal group (Table 2). The animals treated with tropisetron or dexamethasone experienced a significant decrease in ulcer severity and weight/length ratio, compared with the TNBS-control group (P < 0.01). No significant difference was observed in these variables between the tropisetron and dexamethasone-treated rats. Moreover, ulcer severity and weight/length ratio in the tropisetron + mCPBG-treated rats were significantly higher than in the tropisetron-treated group (P < 0.01).
Distal colon of the animals treated with dexamethasone and tropisetron showed a significant decrease in ulcer area and percent of necrosis compared with that of the TNBS-control group (Table 2). These macroscopic features were found more worsened in tropisetron + mCPBG-treated group, compared with the tropisetron-treated rats (P < 0.05 for ulcer area and P < 0.01 for percent of necrosis). The mCPBG, itself, did not influence macroscopic features of colitis.
Effect of tropisetron on histopathological features
The histological features of tissues were examined 6 days (24 h after the last treatment) after the administration of TNBS (50 mg/kg) showed tissue damage characterized by extensive transmural inflammation and/or diffuse necrosis, inflammatory granulomas and submucosal neutrophils’ infiltration. Normal group showed a normal architecture with intact epithelium in colonic mucosa. Treatment with dexamethasone and tropisetron for six days significantly decreased total colitis index (inflammation severity, inflammation extent and crypt damage) in injurious colons, compared with that of the TNBS-control group (Table 2). Furthermore, these groups experienced re-epithelization of the mucosal layer and reduced inflammatory cell infiltration in lamina propria. Concomitant administration of mCPBG and tropisetron significantly worsened histopathological aspects, in comparison with tropisetron administration alone (p < 0.01). In mCPBG-treated animals, microscopic features did not differ from those of TNBS-control group (Fig. 2).
Fig. 2 .
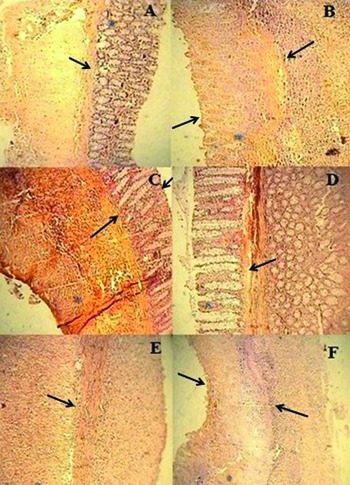
Microscopic presentation of TNBS-induced colitis in rats (hematoxylin and eosin staining; original magnification 10×). (A) Normal group: mucus layer and crypts are normal; (B) TNBS-control group: epithelial distortion, crypt damage and inflammatory cell infiltrates; (C & D) Dexamethasone and tropisetron groups respectively: mild to moderate mucosal and submucosal inflammation and mucosal inflammatory cell infiltrates; (E & F): mCPBG and mCPBG + tropisetron, respectively: infiltration of neutrophils and destruction of mucosal architecture.
Effects of tropisetron on MPO activity in inflamed colonic tissues
As shown in Table 3, MPO activity significantly increased in the TNBS-control group compared with normal group. This finding was in agreement with the histological assessment, which showed increased leucocyte infiltration in the TNBS control group. In the groups treated with dexamethasone and tropisetron, the MPO activity of colonic tissues was lower than that in the TNBS-control group (P < 0.05). A concurrent treatment with mCPBG and tropisetron significantly resulted in a high MPO activity, in contrast to tropisetron administration alone (P < 0.05). Data from mCPBG group did not differ from TNBS-control group.
Table 3 . Biochemical and inflammatory parameters of the rat colon 6 days after induction of colitis with TNBS (50 mg/kg).
| Group | MPO activity
(Unit/100 mg wet tissue) |
TNF-α
(pg/g wet tissue) |
IL-6
(pg/g wet tissue) |
IL-1β
(pg/g wet tissue) |
| Normal | 0.6 ± 0.1 | 141.0 ± 13.8 | 3663.3 ± 240.2 | 1647.5 ± 452.4 |
| TNBS-control | 3.4 ± 0.4 | 249.9 ± 26.6 | 5210.3 ± 457.9 | 15296.0 ± 1579.7 |
| Trop. (2mg/kg, i.p.) | 1.9 ± 0.2 * | 163.7 ± 8.6 * | 3800.5 ± 164.8 * | 8770.8 ±1047.3 ** |
| mCPBG (5mg/kg, i.p.) | 3.5 ± 0.3 | 250.0 ± 23.1 | 5152.0 ± 385.3 | 15317.9 ± 1315.7 |
| mCPBG+ Trop. | 3.4 ± 0.3 | 251.8 ±18.5 | 5249.0 ± 312.5 | 15580.9 ± 1486.3 |
| Dex. (1mg/kg, i.p.) | 1.9 ± 0.4 * | 160.6 ± 12.6 * | 3821.7 ± 270.7 * | 8641.2 ± 985.2 ** |
1 TNBS,2,4,6-trinitrobenzenesulfonic acid; Trop., tropisetron; mCPG, meta-chlorophenylbiguanide; Dex., dexamethasone; i.p., intraperitoneal.
2 Values are means ± SEM; n =6.
3**P < 0.01 and *P < 0 .05: Significant difference compared to TNBS-control group.
Effect of tropisetron on cytokines production
As can be noted in Table 3, TNF-α, IL-6 and IL-1β contents increased in the TNBS-control group, compared with those of normal rats. These parameters reduced significantly in rats treated with either tropisetron or dexamethasone. No significant difference was observed in the content of TNF-a, IL-1b and IL-6 between tropisetron and dexamethasone-treated rats. In the group concurrently treated with tropisetron and mCPBG, the levels of aforementioned cytokines were significantly higher than those treated with tropisetron alone (P < 0.05 for TNF-α and IL-6, P < 0.01 for IL-1b). There was no significant difference in inflammatory cytokins content between mCPBG-treated and TNBS-control groups.
Discussion
In the present study, the administration of tropisetron markedly attenuated the inflammatory response to TNBS-induced colitis in rat. This is evidenced by improved signs, decreased percentage of body weight loss and colonic weight/length ratio, reduced colonic macroscopic and microscopic damage scores, inhibited MPO activity and abated inflamatory cytokine levels. As these effects were antagonized by concurrent administration of mCPBG (a selective 5-HT3 agonist), a possible explanation is that the protection conferred by tropisetron is at least partly mediated by 5-HT3 receptors.
Tropisetron as a 5-HT3 receptor antagonist is a potent antiemetic;29 besides, recent studies have also revealed new potential applications for this drug.30 The anti-inflammatory property of tropisetron has been demonstrated by in vivo and in vitro studies. Fiebich et al.13 reported that lipopolysaccharide-stimulated secretion of TNF-α and IL-1β was dose-dependently inhibited by tropisetron in human monocytes. Additionally, preliminary studies showed clinical efficacy of tropisetron as an analgesic and anti-inflammatory agent in patients with chronic inflammatory joint diseases and soft tissue rheumatism.31 Musavizadeh et al.16 reported the salutary effects of single dose of tropisetron administration in experimental colitis. In spite of the fact that the authors did not investigate probable involvement of 5-HT3 receptors in protection provided by tropisetron against acetic acid-induced colitis in rats, they ultimately suggested that the anti-inflammatory effect of tropisetron may be mediated by pathways other than 5-HT3 receptors. The present study set out to determine whether the 5-HT3 receptors are involved in producing anti-inflammatory effect of tropisetron on an immune-based animal model of IBD (TNBS model) in a 6-day treatment. Our study produced results which corroborate the findings of Mousavizadeh et al. showing protective role of tropisetron against TNBS-induced colitis. Nevertheless, we demonstrated that the anti-inflammatory effect of tropisetron is at least partly mediated through its effect on 5-HT3 receptor pathways.
One of the most widely used models for human IBD is colitis induced by haptinizing agent. TNBS is a simple and reproducible process and can mimic efficiently the pattern of inflammation similar to human UC.32 As increasing 5-HT availability has been demonstrated in TNBS-induced colitis, this model is likely to be ideal for assessment of drugs which influence serotonin pathway in intestinal inflammation.33 In addition, TNBS model is associated with activated T helper (h)1 and Th2 cells.34
As mentioned above, TNBS model can mimic both acute and chronic phases of colitis predicated upon experiment period. Present study was designed as a six-day experiment to evaluate the effect of administration of tropisetron on TNBS-induced colitis. This period is corresponding with the previous studies carried out on models of TNBS-induced chronic inflammation in the rat.20,24
An alteration in EC cell numbers and in amount of 5-HT was observed in intestinal mucosal inflammation such as ulcerative colitis.10 Intestinal inflammation is associated with mucosal recruitment of macrophages which are a major source of proinflammatory cytokines IL-1β, IL-6, and TNF-α. Serotoninergic receptors were found in immune cells including macrophages.12 Due to the strategic location of EC, it is likely that 5-HT may serve an important role in infiltration and activation of macrophages in the gut inflammation. More to the point, intestinal immunocytes can release cytokines, which bring about activation of adjacent EC cells to liberate 5-HT. Through a positive feedback mechanism, increased 5-HT secretion can activate more 5-HT3 receptors on EC cells.35,36 Immune cells also liberate serotonin and this exogenously added serotonin can increase T-cell proliferation.12 It was demonstrated that the T-cells play a crucial role in pathogenesis of IBD. Activation of Th1 cell gives rise to liberation of proinflammatory cytokines. Furthermore, it stimulates tissue macrophages in order to release additional proinflammatory cytokines (e.g. TNF-α, IL-1β, IL-6, IL-8, and IL-12), nitric oxide and reactive oxygen species.37
The Proinflammatory cytokines (e.g., TNF-α, IL-1β, and IL-6) are elevated in the most inflammatory states. Many studies have shown that the levels of these cytokines liberated from macrophages, neutrophils and endothelial cells are increased in TNBS-induced colitis38 and in human IBD.39 They can liberate other cytokines, arachidonic acid metabolites and lytic enzymes, and thus result in edema, fibrosis and necrosis.40 TNF-α and IL-1β are the main mediators pertaining to neutrophil activation and mobilization, fibroblasts’ proliferation and cytotoxic initiation.41 In the present study, the extent of these proinflammatory cytokines increased subsequent to TNBS instillation. Additionally, the colonic profile of these mediators was reduced by administration of tropisetron. This result is possibly due to inhibition of synthesis and/or release of these mediators.
MPO activity, a quantitative index of neutrophil influx into inflamed intestinal tissue, is reportedly augmented in both experimental and human IBD.42 In the present study, the increase in MPO activity in TNBS-treated animals reduced after administration of tropisetron. This finding was consistent with attenuation of macroscopic and microscopic damage scores. This suggests its protective effect against colonic injury.
Neurogenic inflammation can pertain to colitis.43 Local liberation of neuropeptides, for instance, substance P (SP) and calcitonin gene-related peptide (CGRP), from enteric and sensory afferent neurons mediates this type of inflammation. In fact, these neuropeptides and their receptors influence initiation and modulation of GI inflammation. However, Inhibition of proinflammatory neuropeptides’ release from enteric and/or sensory afferent nerves can abate colonic inflammation. Moreover, SP-induced neurogenic inflammation was shown to be associated with 5-HT through acting at 5-HT3 receptors on capsaicin-sensitive fibers.43,44
It is plausible that some useful influences of tropisetron on experimental rat colitis can be at least explained by its proclivity to block 5-HT-induced inflammatory neuropeptide release.
According to our finding, mCPBG, a potent and selective 5-HT3 receptor agonist, negated the salutary effect of tropisetron on TNB-induced colitis. Animals treated with mCPBG experienced colitis comparable to that of TNBS-control group. This might result from the severity of colitis reached its climax at the day of evaluation of colon damage and thus no more severity could be achieved by mCPBG. Furthermore, it can be deduced that there is the maximum level of involvement of serotonin pathway in TNBS-induced colitis and the administration of mCPBG as a potent 5-HT3 agonist, cannot further aggravate colitis through activation of this pathway.
Conclusion
In conclusion, the data from the present study reveal that treatment with tropisetron is able to alleviate intestinal inflammation in the TNBS model of colitis in rats. Tropisetron reduced significantly neutrophil infiltration and colonic levels of inflammatory cytokines. In addition, clinical efficacy of tropisetron as an analgesic agent in patients with chronic inflammatory joint diseases and soft tissue rheumatism has been documented. According to our finding, it is likely that anti-inflammatory effects of tropisetron on TNBS-induced colitis, at least partly arise from the ability of this drug to block 5-HT3 receptors. Considering the low incidence of side-effects of tropisetron, it is contemplated that tropisetron may be useful in the therapy of patients with IBD. However, further studies are required to predict the efficacy and effectiveness of treatment with tropisetron in IBD.
Competing interests
The authors declared no competing interests.
References
- Fiocchi C. Inflammatory bowel disease: Etiology and pathogenesis. Gastroenterology . 1998;115:182–205. doi: 10.1016/s0016-5085(98)70381-6. [DOI] [PubMed] [Google Scholar]
- Podolsky DK. Inflammatory bowel disease. N Engl J Med . 2002;347:417–29. doi: 10.1056/NEJMra020831. [DOI] [PubMed] [Google Scholar]
- Forbes E, Murase T, Yang M, Matthaei KI, Lee J, Lee AN. et al. Immunopathogenesis of experimental ulcerative colitis is mediated by eosinophil peroxidase. J Immunol . 2004;172:5664–75. doi: 10.4049/jimmunol.172.9.5664. [DOI] [PubMed] [Google Scholar]
- Langan RC, Gotsch PB, Krafczyk MA, Skillinge DD. Ulcerative colitis: Diagnosis and treatment. Am Fam Physician . 2007;76:1323–30. [PubMed] [Google Scholar]
- Langmead L, Rampton DS. Review article: Complementary and alternative therapies for inflammatory bowel disease. Aliment Pharmacol Ther . 2006;23:341–9. doi: 10.1111/j.1365-2036.2006.02761.x. [DOI] [PubMed] [Google Scholar]
- Van Assche G, Vermeire S, Rutgeerts P. Medical treatment of inflammatory bowel diseases. Curr Opin Gastroen . 2005;21:443–7. [PubMed] [Google Scholar]
- Gershon MD. Serotonin receptors and transporters - roles in normal and abnormal gastrointestinal motility. Aliment Pharmacol Ther . 2004;7:3–14. doi: 10.1111/j.1365-2036.2004.02180.x. [DOI] [PubMed] [Google Scholar]
- Kim DY, Camilleri M. Serotonin: A mediator of the brain-gut connection. Am J Gastroenterol . 2000;95:2668–709. doi: 10.1111/j.1572-0241.2000.03177.x. [DOI] [PubMed] [Google Scholar]
- Cloez-Tayarani I, Petit-Bertron AF, Venters HD, Cavaillon JM. Differential effect of serotonin on cytokine production in lipopolysaccharide-stimulated human peripheral blood mononuclear cells: Involvement of 5-hydroxytryptamine 2A receptors. Int Immunol . 2003;15:233–40. doi: 10.1093/intimm/dxg027. [DOI] [PubMed] [Google Scholar]
- Coates MD, Mahoney CR, Linden DR, Sampson JE, Chen J, Blaszyk H. Molecular defects in mucosal serotonin content and decreased serotonin reuptake transporter in ulcerative colitis and irritable bowel syndrome. Gastroenterology . 2004;126:1657–64. doi: 10.1053/j.gastro.2004.03.013. [DOI] [PubMed] [Google Scholar]
- Costedio MM, Hyman N, Mawe GM. Serotonin and its role in colonic function and in gastrointestinal disorders. Dis Colon Rectum . 2006;50:376–88. doi: 10.1007/s10350-006-0763-3. [DOI] [PubMed] [Google Scholar]
- Fiebich BL, Akundi RS, Seidel M, Geyer V, Haus U, Müller W. et al. Expression of 5-HT 3A receptors in cells of the immune system. Scand J Rheumatol Suppl . 2004;119:9–11. [PubMed] [Google Scholar]
- Fiebich BL, Akundi RS, Lieb K, Candelario-Jalil E, Gmeiner D, Haus U. et al. Anti-inflammatory effects of 5-HT3 receptor antagonists in lipopolysaccharide-stimulated primary human monocytes. Scand J Rheumatol Suppl . 2004;119:28–32. [PubMed] [Google Scholar]
- Vega Lde L, Muñoz E, Calzado MA, Lieb K, Candelario-Jalil E, Gschaidmeir H. et al. The 5-HT3 receptor antagonist tropisetron inhibits T cell activation by targeting the calcineurin pathway. Biochem Pharmacol . 2005;70:369–80. doi: 10.1016/j.bcp.2005.04.031. [DOI] [PubMed] [Google Scholar]
- Hrycaj P. Serotonin type 3 receptor antagonist tropisetron in the treatment of chronic inflammatory rheumatic conditions; preliminary clinical experience. Scand J Rheumatol . 2004;33:55–8. doi: 10.1080/03009740410007069. [DOI] [PubMed] [Google Scholar]
- Mousavizadeh K, Rahimian R, Fakhfouri G, Aslani FS, Ghafourifar P. Anti-inflammatory effects of 5-HT3 receptor antagonist, tropisetron on experimental colitis in rats. Eur J Clin Invest . 2009;39:375–83. doi: 10.1111/j.1365-2362.2009.02102.x. [DOI] [PubMed] [Google Scholar]
- Celiński K, Dworzański T, Korolczuk A, Słomka M, Radej S, Piasecki R. et al. Comparison of main models of experimental colitis essential for studies on novel therapies of inflammatory bowel disease. Gastroenterologia Polska . 2010;17:195–202. [Google Scholar]
- Wirtz S, Neurath MF. Mouse models of inflammatory bowel disease. Adv Drug Deliver Rev . 2007;59:1073–83. doi: 10.1016/j.addr.2007.07.003. [DOI] [PubMed] [Google Scholar]
- Ajuebor MN, Hogaboam CM, Kunkel SL, Proudfoot AEI, Wallace JL. The chemokine RANTES is a crucial mediator of the progression from acute to chronic colitis in the rat. J Immunol . 2001;166:552–8. doi: 10.4049/jimmunol.166.1.552. [DOI] [PubMed] [Google Scholar]
- Morris GP, Beck PL, Herridge MS, Depew WT, Szewczuk MR, Wallace JL. Hapten-induced model of chronic inflammation and ulceration in the rat colon. Gastroenterology . 1989; 96:795–803. [PubMed] [Google Scholar]
- Minaiyan M, Ghannadi AR, Etemad M, Mahzouni P. A study of the effects of Cydonia oblonga Miller (Quince) on TNBS-induced ulcerative colitis in rats. Res Pharm Sci . 2012;7:103–110. [PMC free article] [PubMed] [Google Scholar]
- Callahan PM, Cunningham KA. Involvement of 5-HT2C receptors in mediating the discriminative stimulus properties of m-chlorophenylpiperazine (mCPP) Eur J Pharmacol . 1994;257:27–38. doi: 10.1016/0014-2999(94)90690-4. [DOI] [PubMed] [Google Scholar]
- Ballester I, Daddaoua A, Posadas RL, Nieto A, Suárez MD, Zarzuelo A. et al. The bisphosphonate alendronate improves the damage associated with trinitrobenzenesul-fonic acid-induced colitis in rats. Brit J Pharmacol . 2007;151:206–15. doi: 10.1038/sj.bjp.0707227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motavallian-Naeini A, Minaiyan M, Rabbani M, Mahzuni P. Anti-inflammatory effect of ondansetron through 5HT3 receptors on TNBS-induced coloitis in rat. Ecxli J . 2012;11:30–44. [PMC free article] [PubMed] [Google Scholar]
- Cooper H, Murthy S, Shah R, Sedergran D. Clinicopathologic study of dextran sulfate sodium experimental murine colitis. Lab Invest . 1993;69:238–49. [PubMed] [Google Scholar]
- Dieleman L, Palmen M, Akol H, Bloemena E, Pena A, Meuwissen S. Chronic experimental colitis induced by dextran sulfate sodium (DSS) is characterized by Th1 and Th2 cytokines. Clin Exp Immunol . 1998;114:385–391. doi: 10.1046/j.1365-2249.1998.00728.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley PP, Priebat DA, Christensen RD, Rothstein G. Measurement of cutaneous inflammation: Estimation of neutrophil content with an enzyme marker. J Invest Dermatol . 1982;78:206–9. doi: 10.1111/1523-1747.ep12506462. [DOI] [PubMed] [Google Scholar]
- Nacife VP, Soeiro MN, Gomes RN, D’Avila H, Castro-Faria Neto HC, Meirelles MN. Morphological and biochemical characterization of macrophages activated by carrageenan and lipopolysaccharide in vivo. Cell Struct Funct . 2004;29:27–34. doi: 10.1247/csf.29.27. [DOI] [PubMed] [Google Scholar]
- Wolf H. Preclinical and clinical pharmacology of the 5-HT3 receptor antagonists. Scand J Rheumatol Suppl . 2000;113:37–45. doi: 10.1080/030097400446625. [DOI] [PubMed] [Google Scholar]
- Muller W, Fiebich BL, Stratz T. New treatment options using 5-HT3 receptor antagonists in rheumatic diseases. Curr Top Med Chem . 2006;6:2035–42. doi: 10.2174/156802606778522122. [DOI] [PubMed] [Google Scholar]
- Mousavizadeh K, Stratz T, Mueller W, Fiebich BL. 5-HT3 receptor antagonist for the treatment of tendinopathy. Nat Clin Pract Rheumatol . 2008;4:E4. doi: 10.1038/ncprheum0835. [DOI] [PubMed] [Google Scholar]
- Motavallian-Naeini A, Andalib S, Rabbani M, Mahzouni P, Afsharipour M, Minaiyan M. Validation and optimization of experimental colitis induction in rats using 2, 4, 6-trinitrobenzene sulfonic acid. Res Pharm Sci . 2012;7:159–69. [PMC free article] [PubMed] [Google Scholar]
- Linden DR, Chen JX, Gershon MD, Sharkey KA, Mawe GM. Serotonin availability is increased in mucosa of guinea pigs with TNBS-induced colitis. Am J Physiol Gastrointest Liver Physiol . 2003;285:G207–G216. doi: 10.1152/ajpgi.00488.2002. [DOI] [PubMed] [Google Scholar]
- Dohi T, Fujihashi K. Type 1 and 2 T Helper Cell-mediated Colitis. Curr Opin Gastroenterol . 2006;22:651–7. doi: 10.1097/01.mog.0000245545.80160.0f. [DOI] [PubMed] [Google Scholar]
- Gebauer A, Merger M, Kilbinger H. Modulation of 5-HT 3 and 5-HT4 receptors of the release of 5-hydroxytryptamine from the guinea-pig small intestine. Naunyn-Schmiede-bergs Arch Pharmacol . 1993;347:137–40. doi: 10.1007/BF00169258. [DOI] [PubMed] [Google Scholar]
- Fakhfouri G, Rahimian R, Daneshmand A, Bahremand A, Rasouli MR, Dehpour AR. et al. Granisetron ameliorates acetic acid-induced colitis in rats. Human and Experimental Toxicology . 2010;29:321–8. doi: 10.1177/0960327110362702. [DOI] [PubMed] [Google Scholar]
- Cloez-Tayarani I, Changeux JP. Nicotine and serotonin in immune regulation and inflammatory processes: A perspective. J Leukoc Biol . 2007;81:599–606. doi: 10.1189/jlb.0906544. [DOI] [PubMed] [Google Scholar]
- Hosseini-Tabatabaei A, Esmaily H, Rahimian R, Khorasani R, Baeeri M, Barazesh-Morgani A. Benefit of nicorandil using an immunologic murine model of experimental colitis. Centr Eur J Biol . 2009;4:74–85. [Google Scholar]
- Rahimi R, Nikfar S, Abdollahi M. Meta-analysis technique confirms the effectiveness of anti-TNF-alpha in the management of active ulcerative colitis when administered in combination with corticosteroids. Med Sci Monit . 2007;13:PI13–8. [PubMed] [Google Scholar]
- Maunder R. Mediators of stress effects in inflammatory bowel disease: not the usual suspects. J Psychosomatic Res . 2000;48:569–77. doi: 10.1016/s0022-3999(00)00098-2. [DOI] [PubMed] [Google Scholar]
- Yang XL, Guo TK, Wang YH, Liu X, Wang XX, Li W. et al. Ginsenoside Rd attenuates the inflammatory response via modulating p38 and JNK signaling pathways in rats with TNBS-induced relapsing colitis. Int Immunopharmacology . 2012;12:408–14. doi: 10.1016/j.intimp.2011.12.014. [DOI] [PubMed] [Google Scholar]
- Esmaily H, Hosseini-Tabatabaei A, Rahimian R, Khorasani R, Baeeri M, Barazesh-Morgani A. et al. On the benefits of silymarin in murine colitis by improving balance of destructive cytokines and reduction of toxic stress in the bowel cells. Centr Eur J Biol . 2009;4:204–13. [Google Scholar]
- Hassani H, Lucas G, Rozell B, Ernfors P. Attenuation of acute experimental colitis by preventing NPY Y1 receptor signaling. Am J Physiol Gastrointestinal Liver Physiol . 2005;288:G550–G556. doi: 10.1152/ajpgi.00182.2004. [DOI] [PubMed] [Google Scholar]
- Faerber L, drechsler S, Ladenburger S, Gschaidmeier H, Fischer W. The neuronal 5-HT3 receptor network after 20 years of research–evolving concepts in management of pain and inflammation. Eur J Pharmacol . 2007;560:1–8. doi: 10.1016/j.ejphar.2007.01.028. [DOI] [PubMed] [Google Scholar]


