Abstract
Sand flies include a group of insects that are of medical importance and that vary in geographic distribution, ecology, and pathogen transmission. Approximately 163 species of sand flies have been reported in Colombia. Surveillance of the presence of sand fly species and the actualization of species distribution are important for predicting risks for and monitoring the expansion of diseases which sand flies can transmit. Currently, the identification of phlebotomine sand flies is based on morphological characters. However, morphological identification requires considerable skills and taxonomic expertise. In addition, significant morphological similarity between some species, especially among females, may cause difficulties during the identification process. DNA-based approaches have become increasingly useful and promising tools for estimating sand fly diversity and for ensuring the rapid and accurate identification of species. A partial sequence of the mitochondrial cytochrome oxidase gene subunit I (COI) is currently being used to differentiate species in different animal taxa, including insects, and it is referred as a barcoding sequence. The present study explored the utility of the DNA barcode approach for the identification of phlebotomine sand flies in Colombia. We sequenced 700 bp of the COI gene from 36 species collected from different geographic localities. The COI barcode sequence divergence within a single species was <2% in most cases, whereas this divergence ranged from 9% to 26.6% among different species. These results indicated that the barcoding gene correctly discriminated among the previously morphologically identified species with an efficacy of nearly 100%. Analyses of the generated sequences indicated that the observed species groupings were consistent with the morphological identifications. In conclusion, the barcoding gene was useful for species discrimination in sand flies from Colombia.
Introduction
The subfamily Phlebotominae Rondani, 1840, is represented in America by more than 500 species distributed among the genera Lutzomyia França, 1924, Brumptomyia França and Parrot, 1921, and Warileya Hertig, 1948 [1]–[3]. The Lutzomyia (L.) species are known for their role as vectors of medically important pathogens, such as the kinetoplastida protozoan of the genus Leishmania Ross, 1903 [4], [5], the bacterium Bartonella bacilliformis (Strong et al. 1907), and viruses of the families Bunyaviridae, Reoviridae, and Rhabdoviridae [6]–[8].
Although phlebotomine sand flies have been studied more than most other groups of insects because of their role as vectors of pathogens, the taxonomic knowledge of these insects is far from complete. Three major reviews [1], [2], [9] and the electronic documentation produced by the Walter Reed Biosystematics Unit (WRBU, http://wrbu.si.edu/) are essential tools for studying the systematics, taxonomy, and geographic distribution of this group of medically important insects in the Americas.
To date, in Colombia, 163 species of sand flies (153 Lutzomyia, 8 Brumptomyia, and 2 Warileya species) have been recorded. This list was obtained from taxonomic studies conducted in Colombia in different natural and endemic environments, with a primary focus on areas of leishmaniasis transmission [10]–[19].
There are approximately 13 species in the genus Lutzomyia that have been incriminated as vectors of Leishmania spp. [20] in Colombia. Since various vector species often coexist in areas of disease transmission, specific identification of the species is useful for monitoring and subsequent intervention by officials from the Vector-Borne Diseases (ETV) units [21].
Currently, the identification of species within the subfamily Phlebotominae is based on morphological recognition and morphometric analysis of a large number of structures, primarily in the head and genitalia [1], [2]. However, species identification is complicated because it requires a considerable degree of skill and taxonomic expertise. In addition, morphological identification may be limited by deterioration of samples or by improper mounting techniques.
Molecular methodology is now being used to explore taxonomic questions at different hierarchical levels [22], [23]. Ribosomal gene sequences (18SrDNA and the D2 domain in 28SrDNA) were used to reassess the relationships within the family Phlebotominae [24] and within the genus Lutzomyia [25]–[28]. Gene sequences with a faster rate of evolution, mitochondrial genes (e.g., cytochrome b gene and NADH Dehydrogenase 4 (ND4) gene), and nuclear genes (e.g., the complete internal transcribed spacer 2 (ITS2) and nuclear Elongation Factor 1-alpha (EF-1α) gene), have been used to resolve intraspecific and subgeneric relationships [29]–[37].
Following the proposed use of DNA sequences as a support tool for studies of biological diversity and classification [38], [39], Hebert et al. [40], [41] suggested the use and analysis of the 5′ fragment of the mitochondrial gene Cytochrome C Oxidase Subunit I (COI), called a DNA barcode. This region is of potential importance for facilitating inventories of biodiversity and performing species' identifications. The DNA barcode initiative has been well received due to the connectivity and common language of DNA sequences, allowing researchers worldwide to advance in taxonomy and systematic studies of various groups of organisms, including insect disease vectors [42]. The 5′ fragment of COI appears to be an excellent tool for studying insects of medical importance, as such study requires the quick and accurate identification of species that are present in a transmission area [28], [42], [43].
Regarding the DNA barcode, complications associated with use of this region include nuclear sequences of mitochondrial origin (NUMTs) and endosymbionts. Also, standardization of the methodology is necessary for each group studied. Furthermore, the use of a single marker for the taxonomy of a wide range of taxa is controversial [44]–[48].
Despite many demonstrations of the effectiveness of DNA barcoding in other taxa, few studies on phlebotomine sand flies in Colombia have attempted to use this methodology as a valid molecular tool for identifying species of phlebotomine sand flies [28], [49] and for revealing cryptic species [50]–[53].
In the present study, haplotypes of the barcode sequence were assigned to 148 individuals belonging to 36 phlebotomine sand fly species collected in Colombia, some of which are involved in the transmission of leishmaniasis. Specimens were obtained from sites where outbreaks of the disease have occurred. The present study evaluated the usefulness of the barcode sequence in species differentiation compared with previous morphology-based identifications.
Materials and Methods
Ethics Statement
Collection of sand flies was done according to the parameters of Colombian decree number 1376 http://190.147.213.68:8080/HOMEPAGE/ALEGIS_INTER/LEYES_Y_DECRETOS/2013/DECRETO_1376_de_2013.pdf which regulates the permit for collection of specimens of wild species of biological diversity for non-commercial research. No specific permits were required for this study. The sand flies were collected on private property, and permission was received from land owners prior to sampling.
Collection of specimens
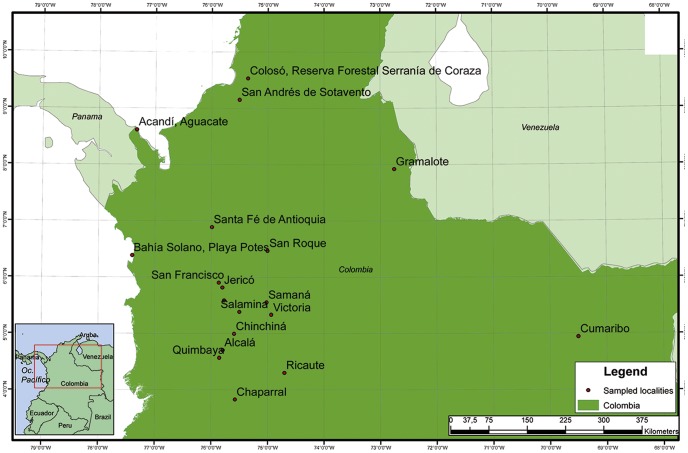
Phlebotomine sand flies were collected using CDC light traps and Shannon traps from different locations in Colombia between 2008 and 2012. Areas selected for the collections were based on previous epidemiological studies of leishmaniasis transmissionmade by the Program for the Study and Control of Tropical Diseases (PECET) (Figure 1 and table S1).The thorax and legs were removed from each specimen for DNA extraction and stored at −20°C, while the head and abdomen were processed for traditional morphological identification [1], [2], [54]. After being assigned an identification code, the remains of each specimen were preserved as a collection of voucher specimens.
Figure 1. Map of the study area showing the sampling sites of sand flies collected in the present study.
Site 1: Jardín; Site 2: Jerico; Site 3: San Francisco; Site 4; San Roque; Site 5: Santa Fé de Antioquia; Site 6: Chinchina; Site 7: Salamina; Site 8: Samaná; Site 9: Victoria, Site 10: Acandí; Site 11: Bahía Solano, Playa Potes; Site 12: San Andrés de Sotavento; Site 13: Ricaute; Site 14: Quimbaya; Site 15: Gramalote; Site 16: Colosó, Serranía de Coraza Forestry Reserve; Site 17: Chaparral; Site 18: Alcalá; and Site 19: Caño Ariba.
DNA extraction and polymerase chain reaction (PCR)
Total DNA (50 µL) was extracted from each sample using the Qiagen® DNeasy Blood & Tissue kit (Qiagen, Chatsworth, CA). To amplify the mitochondrial 5′ COI gene region, PCR was performed using the following thermal profile: an initial denaturation of 5 min (94°C); followed by 35 cycles at 94°C for 1 min (denaturation), 45°C for 1 min (annealing), and 72°C for 1 min (extension); and a final extension at 72°C for 10 min. Each PCR cocktail had a final reaction volume of 50 µl and contained the following: 0.3 µL of Taq DNA polymerase (5 U/µL) (Biolase®), 5 µL of 10× PCR buffer (NH4SO4), 5 µL of 2.5 mM MgCl2, 5 µL of 2 mmol/L dNTP, 2 µl of 10 µmol/L of each oligonucleotide (forward and reverse), 4 µL of the DNA template, and ddH2O to bring the volume to 50 µl. The oligonucleotides used to amplify the 700 bp COI fragment were LCO1490 and HCO2198 [41], [55].
The amplified fragments were run on an agarose gel at 1% in 1× TBE buffer (40 mM Tris-Borate, 1 mM EDTA, pH 8.0) to verify the integrity of the fragments, after which the PCR products were purified using a MultiScreenHTS Vacuum Manifold (Millipore). Subsequently, the PCR products of the amplified fragments were sent for sequencing in both chain directions to Macrogen Inc. (Korea) or to the CDC (Atlanta, GA, USA).
Data analysis
The chromatograms obtained were edited using BioEdit (http://www.mbio.ncsu.edu/bioedit/bioedit.html) [56] to generate a consensus sequence for each specimen. The DNA sequences were aligned using the Clustal W tool incorporated into MEGA v5.0 software [57]. Each sequence was compared with other insect mitochondrial genomes using BLAST (http://blast.ncbi.nlm.nih.gov/Blast.cgi) [58] to confirm the identity of the obtained fragments (the 5′ COI region). The number of haplotypes (h), polymorphic sites (s), and nucleotide diversity were determined using DnaSP v5.10 (http://www.ub.edu/dnasp) [59].
The nucleotide compositions and sequence divergences within and between species were calculated using the distance model Kimura two-parameter (K2P) [60]. A dendrogram of K2P distance Neighbor-Joining (NJ) was generated to provide a graphical representation of the clustering pattern across different species [61].
All of the sequences obtained in the present study have been submitted to GenBank (http://www.ncbi.nlm.nih.gov/genbank/). The collection locations and other information regarding the specimens, such as the GenBank accession numbers, will be available on the BOLD Systems (http://www.boldsystems.org) platform, as the “Phlebotominae Barcoding Initiative of the Americas - PBIN” project.
Results
Specimens collected
A total of 36 phlebotomine species belonging to 3 genera were collected from 11 Colombian departments (Table S1). Some of the collected species had epidemiological histories (Table 1): Lutzomyia columbiana (Ristorcelli and Van Ty, 1941), Lutzomyia evansi (Nuñez-Tovar, 1924), Lutzomyia gomezi (Nitzulescu, 1931), Lutzomyia hartmanni (Fairchild & Hertig, 1957 ), Lutzomyia longiflocosa Osorno, Morales Osorno and Muñoz, 1970, Lutzomyia longipalpis (Lutz & Neiva, 1912), Lutzomyia nuneztovari (Ortiz 1954), Lutzomyia panamensis (Shannon, 1926), Lutzomyia pia (Fairchild & Hertig, 1961), Lutzomyia shannoni (Dyar, 1929), Lutzomyia trapidoi (Fairchild & Hertig, 1952), Lutzomyia youngi Feliciangeli & Murillo, 1987, and Lutzomyia yuilli yuilli Young & Porter, 1972.
Table 1. List of sand fly species and the numbers of individuals with mitochondrial haplotypes.
| Species | Number of specimens | Number of haplotypes | Number of nucleotidic divergences |
| B. beaupertuyi | 1 | 1 | 0 |
| B. hamata | 2 | 2 | 1 |
| B. guimareasi | 3 | 2 | 2 |
| B. mesai | 2 | 1 | 0 |
| L. antunesi | 2 | 2 | 3 |
| L. barretoi majuscula | 3 | 2 | 3 |
| L. bifoliata | 2 | 1 | 0 |
| L. carpenteri | 2 | 1 | 0 |
| L. carrerai thula | 5 | 4 | 16 |
| L. cayennensis cayennensis | 1 | 1 | 0 |
| L. columbiana | 5 | 5 | 7 |
| L. coutinhoi | 3 | 3 | 6 |
| L. evansi | 3 | 3 | 9 |
| L. gomezi | 9 | 9 | 40 |
| L. hartmanni | 4 | 4 | 5 |
| L. lichyi | 1 | 1 | 0 |
| L. longiflocosa | 4 | 2 | 4 |
| L. longipalpis | 4 | 4 | 8 |
| L. (Lutzomyia) sp. | 2 | 1 | 0 |
| L. migonei | 4 | 2 | 1 |
| L. nuneztovari | 2 | 1 | 0 |
| L. panamensis | 7 | 6 | 38 |
| L. pia | 16 | 2 | 14 |
| L. reburra | 3 | 2 | 1 |
| L. scorzai | 9 | 7 | 28 |
| L. (Helcocyrtomyia) sp.1 | 2 | 1 | 0 |
| L. shannoni | 1 | 1 | 0 |
| L. spinicrassa | 4 | 3 | 6 |
| L. sordelli | 2 | 1 | 0 |
| L. trapidoi | 9 | 9 | 32 |
| L. trinidadensis | 10 | 5 | 27 |
| L. triramula | 7 | 6 | 22 |
| L. walkeri | 2 | 1 | 0 |
| L. yuilli yuilli | 8 | 4 | 5 |
| L. youngi | 1 | 1 | 0 |
| W. rotundipennis | 3 | 2 | 1 |
| Total | 148 | 103 |
Sequence analysis
The 5′ fragment of the COI mitochondrial gene was amplified and sequenced for each sample (n = 148), and the total size was 700 bp. The identities of the obtained sequences corresponded with positions 16 to 716 of the COI mitochondrial gene on Aedes aegypti (NC_010241.1), which was used as the reference genome. This segment also corresponded with that proposed by Hebert et al. [41] as a barcode region for species identification. No insertions, deletions, or stop codons were observed indicating the absence of pseudogenes or nuclear copies of mitochondrial origin (NUMTs).The sequences from the 36 species of phlebotomine sand flies that had previously been identified based on morphology were represented by 1 to 16 individuals (Table S1). The identification numbers and GenBank accession numbers for the specimens are summarized in table S1.
During alignment at the nucleotide level, a total of 417 conserved sites and 283 variable sites were observed. The obtained COI sequences contained a large number of A+T pairs (an average of 66.3% for all codons), particularly in the third codon position (87.6%) (Table S2).
Of the 148 sequences obtained, 103 haplotypes were characterized for phlebotomine sand flies, indicating a high degree of diversity. The number of haplotypes within a species ranged from 1 to 9 (Table 1), and variable haplotypes were obtained in most species, except for Brumptomyia mesai, Brumptomyia beaupertuyi, Lutzomyia bifoliata, Lutzomyia carpenteri, Lutzomyia nuneztovari, Lutzomyia sordellii, Lutzomyia walkeri, and Lutzomyia (Helcocyrtomyia) sp. 1, which exhibited unique haplotypes for different specimens. The largest numbers of haplotypes were observed in Lutzomyia gomezi (9 haplotypes), Lutzomyia scorzai (7), Lutzomyia trapidoi (9), Lutzomyia panamensis (6), Lutzomyia trinidadensis (6), Lutzomyia triramula (6), Lutzomyia columbiana (5), and Lutzomyia carrerai thula (5) (Table 1).
Regarding the numbers and types of substitutions observed among different haplotypes of the same species, the results showed between 2 to 40 variable positions (Table 1), and nucleotide changes were primarily synonymous substitutions, such as transitions (purine for purine and pyrimidine for pyrimidine), at the third positions.
Pattern of sequence divergence
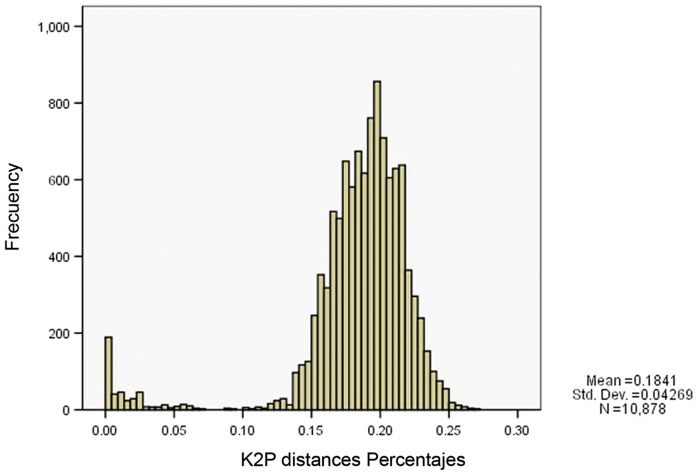
The set of sequences obtained was used to evaluate efficacy of the barcoding method to delineate species. Genetic distances, as determined by the K2P intraspecies values, ranged between 0 and 6% (average intraspecific divergence = 1.6%, standard deviation = 1.38%), and maximum intraspecific divergence was observed in 8 of the 36 species (Table 2). Between species, the genetic distances showed K2P values between 9 and 26.7% (average divergence = 19%, standard deviation = 2.57%).
Table 2. K2P pairwise distances among Colombian sand fly species.
| Species | No. of Specimens | K2P values |
| B. hamata | 2 | 0.001 |
| B. guimareasi | 3 | 0.001–0.003 |
| B. mesai | 2 | 0.000 |
| L. antunesi | 2 | 0.004 |
| L. barretoi majuscula | 3 | 0.000 |
| L. bifoliata | 2 | 0.000 |
| L. carpenteri | 2 | 0.000 |
| L. carrerai thula | 5 | 0.001–0.018 |
| L. columbiana | 5 | 0.001–0.007 |
| L. coutinhoi | 3 | 0.001–0.009 |
| L. evansi | 3 | 0.004–0.012 |
| L. gomezi | 9 | 0.001–0.060 |
| L. hartmanni | 4 | 0.003–0.006 |
| L. longiflocosa | 4 | 0.000–0.006 |
| L. longipalpis | 4 | 0.001–0.012 |
| L. (Lutzomyia) sp. | 2 | 0.000 |
| L. migonei | 4 | 0.001–0.001 |
| L. nuneztovari | 2 | 0.000 |
| L. panamensis | 7 | 0.000–0.046 |
| L. pia | 16 | 0.000–0.021 |
| L. reburra | 3 | 0.000–0.001 |
| L. scorzai | 9 | 0.000–0.026 |
| L. (Helcocyrtomyia) sp.1 | 2 | 0.000 |
| L. spinicrassa | 4 | 0.003–0.007 |
| L. sordelli | 2 | 0.001 |
| L. trapidoi | 9 | 0.000–0.030 |
| L. trinidadensis | 10 | 0.000–0.034 |
| L. triramula | 7 | 0.004–0.024 |
| L. walkeri | 2 | 0.000 |
| L. yuilli yuilli | 8 | 0.001–0.007 |
| W. rotundipennis | 3 | 0.000–0.001 |
Given the presence of the “barcode gap” as a delimiting criterion and an indicator of species differentiation [62], [63], it was determined that the distributions of all intraspecific paired distances and the interspecific distributions of distances were not superimposed. These differences indicated genetic variations between species (Figure 2).
Figure 2. Genetic divergences (K2P distances) between COI sequences for varied taxonomic levels of Colombian sand fly species.
The frequency of pairwise divergences among specimens both within species and among different species.
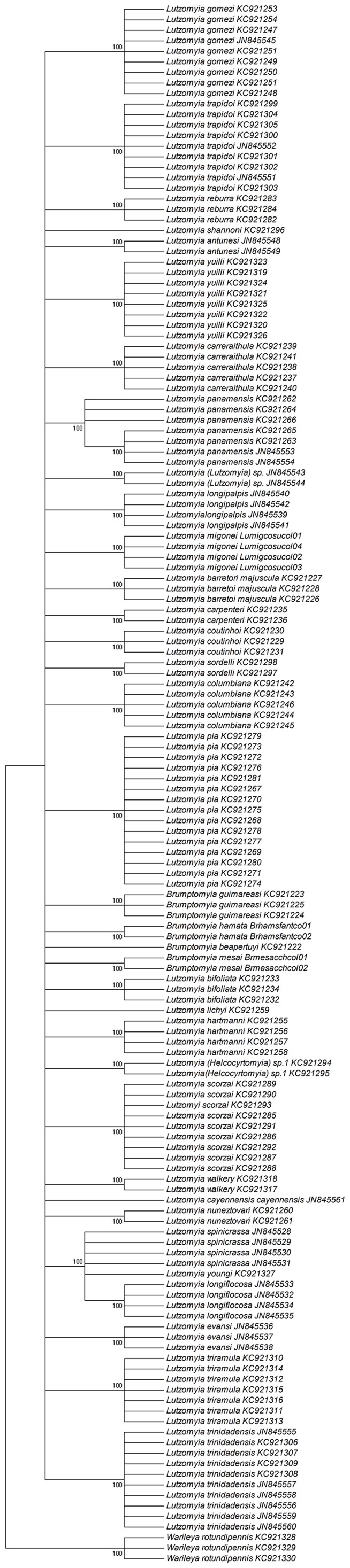
Neighbor-joining dendrogram (NJ)
NJ dendrogram (Figure 3), shows the limits representing different taxonomic groups and differentiation of species. Furthermore, specimens of the same species were always observed to be closely grouped, regardless of the collection site; for the Molecular Operational Taxonomic Units (MOTU), 36 taxonomic groups or units were identified, compared with the 36 species that were recognized by traditional taxonomy based on morphological characters. The nodes connecting the sequences of individuals from the same species were supported by high bootstrap values (99%–100%).
Figure 3. Neighbor-joining analysis of mitochondrial COI sequences of Colombian sand fly species.
Final identification
Using the divergence of K2P distances, NJ dendrogram bootstrapping, and traditional morphological approaches, we found COI sequence always corresponded with species' identifications derived from morphologic characters (Figure 3).
Discussion
DNA barcode is a system designed to provide rapid, accurate, and automatable species-identification based on the standard analysis of short regions of genes to produce species tag [64]. The present study evaluated the efficacy of using COI barcodes for the identification of phlebotomine sand fly species in Colombia and provided a group of sequences associated with the identified species. For most species examined in the present study, a coherent matrix of DNA barcode sequences was obtained, which differentiated each species.
The 5′ region of the mitochondrial COI gene is flanked by highly conserved regions, as noted by Hebert & Gregory [64]. The absence of stop codons was consistent for all of the amplified sequences, suggesting the functionality of the COI mitochondrial sequences. These sequences were 700 bp in length, suggesting that NUMTs were not sequenced [65].
The nucleotide lengths of amplified sequences of the COI gene (700 bp) in the present study were longer than those reported in previous studies performed in Phlebotominae: 549 bp reported by Azpurua et al. [28], 681 bp by Cohnstaedt et al. [50], 689 bp by Kumar et al. [49], and 548 bp reported by Hoyos et al. [51].
Within the DNA barcode sequences in phlebotomine sand flies, high A+T contents were observed, which were within average values (68.1 to 70.7%) reported for species of the order Diptera, including suborders Nematocera and Brachycera [66], [67]. Similar values of A+T contents (66.7%) were observed in Phlebotomus and Sergentomyia in studies examining COI [28], [49]–[51]. There were no significant variations in the percentage of G+C content among the sequences of Phlebotominae from the different localities studied, and no diagnostic correlation for the percentage of G+C content was observed. The G+C content observed for the phlebotomine sand flies is similar to that of other insects and lower categories on the zoological scale [68].
In terms of codon usage, a preference for A or T termination within the triplets was clearly observed. This finding is supported by the fact that the base composition of mitochondrial DNA is highly correlated with codon usage because genes encoding mitochondrial proteins exhibit a preference for the use of codons rich in A+T. This phenomenon appears to be a characteristic of the Insecta mitochondrial genome [69], [70]. These values are similar to those observed other Diptera [66], [70]–[75] and, in particular, for species in the genus Lutzomyia, for which different mitochondrial gene fragments have been analyzed, including Cytochrome b (A+T = 73.09%), tRNAserine (A+T = 81.2%), and ND4 (A+T = 72.5%) [31], [35], [37].
Regarding differences in the numbers and types of substitutions among different haplotypes from the same species, the results revealed 2 to 40 variable positions changes were largely synonymous substitutions of the transitional type (purine by purine and pyrimidine by pyrimidine) at third positions, which do not affect the amino acid composition of the protein. In contrast, between species, nucleotide substitution rates gradually increased in relation to genetic distances. Blouin et al. [76] suggested that nucleotide substitution patterns and rate of divergence between sites provided diagnostic information, thus, indicating the value of DNA sequences as molecular tools for differentiating species.
Aliabadian et al. [77] indicated that the success of barcode identification based on genetic distances ultimately depended on differences between intra- and interspecific divergences; ideally, there is no barcode overlap between the distributions of these two classes of distance. According to Hebert et al. [62], the barcoding gap (i.e., the difference between intra- and interspecific distances) will allow assignment of categories or species taxonomic status to specimens once barcode reference libraries are complete and available. The present study confirms that, among the taxa examined, genetic distances and barcoding gaps within a species are generally much smaller than those between individuals of different species.
The use of DNA barcoding can delineate the boundaries between species and assign taxonomic status to unknown individuals from known species. The analysis of genetic distances is one of the criteria for acquiring this information [62]. The range of intraspecific variation was between 0 and 6%, while the range of interspecific variability was between 9 and 26.7%.
Hebert et al. [41] proposed an interspecific limit of 3% for insects. The high intraspecific variability (>2%) observed between different haplotypes of L. gomezi (6% maximum intraspecific variability), L. panamensis (4.6%), and L. trinidadensis (3.4%) (Table 2) could suggest a population differentiation or the presence of cryptic species [51], [62]. This is a reflection of the large number of mutations observed in relation to the other sequences. Also,the wide intraspecific divergence could be related to rapid variation of the mitochondrial genome [78]; range or distribution of a species, which allows for isolation and genetic differentiation; and presence of ancestral polymorphisms, which may be conserved over time due to the aggregate population distribution characteristic of phlebotomine sand flies [79].
The low intraspecific variability observed may be the effect of sampling [80], [81]. Sample size per species ranged from 1 to 16 specimens, but only two species had more than nine individuals (Table 1). The 6% nucleotide divergence (well above the 3% level often considered an upper limit for intraspecific variation) observed for L. gomezi and this species' extensive geographic distribution (from Central America to South Region of Brazil) suggests the present morphospecies may be comprised of two or more cryptic species.
In the present study, we found a nucleotide divergence range between 9% and 26.7% for differentiation of the 36 species of phlebotomine sand flies identified.
In general, our results revealed that there was no overlap between the levels of intra- and interspecific divergence, the previously mentioned barcode gap, even though higher values of intraspecific divergence were observed. This separation confirms the validity and partial effectiveness of the DNA barcode sequence as a molecular identifier of species [40].
The geographical scale of sampling has a critical impact on the global application of DNA barcoding [81]. The dependency of intraspecific genetic variation on geographical scale of sampling is to be expected based on widely recognized theory and concepts such as distance decay and isolation by distance, as well as from phylogeographic studies. As a general rule, a species sampled throughout its geographical range will reveal greater genetic variation than if the variation was estimated from a single smaller region [44], [45], [63], [78], [80]–[85]. The phlebotomine sand flies species in our study were not sampled across its entire distribution range in Colombia and it will be desirable. However, when our data set was compared with published sequences in genbank, the range of intra and interspecific divergence values (0–6% and 9–26.7%) did not change. This evidence suggests accurate identification of our phlebotomine sand flies. Consequently, data derived from the COI sequence fragment were found to be helpful for species identification, and may be applied in the future for identifying species from different regions.
The NJ method is conceptually related to grouping, but does not involve a phylogenetic tool, the natural history, or the behavior of a molecular clock [86]. Combining a NJ dendrogram with a bootstrap analysis is the most appropriate method for evaluating trees using distance methods [87]. The COI NJ dendrogram confirmed that the species of a genus generally form cohesive groups.
In the present study, all MOTUs, including individuals belonging to the same species, were supported by the high bootstrap value of 100%, thus validating the consistency and usefulness of the COI barcode sequence to assign individuals to groups that corresponded to the species previously defined by specialists based on morphological features. Haplotypes from single species formed barcode groups that were clearly distinguishable from related species. As expected, some species that were represented by single individuals were not grouped with any other species (Figure 3). Including haplotypes from males and females in the analysis (Tables S1 and 1) allowed the formation of associations based on clusters when females of different species were isomorphic, for example, L. Longiflocosa, L. youngi, and L. spinicrassa.
The COI profile obtained for Phlebotominae confirmed the large variation that occurs in the mitochondrial genomes of these insects. Assigning species in this group required a combination of nucleotide divergence values and conventional taxonomic studies.
This study is specific to Colombia and limited to the specimens collected, it allowed for the assignment of haplotypes corresponding to species within the study area. The species included in this study represent 13 of 27 groupings proposed for Young & Duncan [1] for Lutzomyia genus and most of them are present in other South American countries. This is important since the increased availability of sequences for phlebotomine sand fly species will provide a more comprehensive and explicit view regarding the possibility of using the COI sequence as a molecular marker in this region.
Further study on genetic patterns and evolutionary processes will improve the ability to create a barcode-based identification system in phlebotomine sand flies and, consequently, facilitate future studies related to the biology of these insects.
Supporting Information
List of sand fly species, collection sites and number of genotypes per species used in the study.
(DOCX)
Sequence divergence and nucleotide composition for the sand flies genera. The frequencies of nucleotides in sequence are presented as the total average values for all Condon positions and for each condon position separately with the accuracy to tenths of a percent.
(DOCX)
Acknowledgments
We thank Dr. Paula Marcet of the Centers for Disease Control and Prevention (Atlanta, GA, USA) for her collaboration in training and her support in obtaining some of the sequences included in this study.
Funding Statement
This work received financial support from Program of Study and Control of Tropical Diseases (PECET), Molecular Systematics Group and Centers for Disease Control and Prevention (CDC) project Influence of Global Warming on Vector-borne Disease Transmission in Colombia. The funding institutions, PECET, GSM and CDC, had a role in study design, data collection and analysis, decision to publish and preparation of the manuscript.
References
- 1.Young DG, Duncan MA (1994) Guide to the identification and geographic distribution of Lutzomyia sand flies in Mexico, the West Indies, Central and South America (Diptera: Psychodidae). Gainesville, FL: Associated Publishers. 881 p. [Google Scholar]
- 2.Galati EAB (2010) Phlebotominae (Diptera, Psychodidae) Classificação, Morfologia, Terminologia e Identificação de Adultos [Phlebotominae (Diptera, Psychodidae) classification, morphology, terminology and adult identification]. Apostila Disciplina HEP 5752 Bioecologia e Identificação de Phlebotominae Vol. I. São Paulo: Brasil. Press, Departamento de Epidemiologia Faculdade de Saúde Pública Universidade de São Paulo.126 p. [Google Scholar]
- 3. Ready PD (2013) Biology of phlebotomine sand flies as vectors of disease agents. Annu Rev Entomol 58: 227–250 10.1146/annurev-ento-120811-153557 [DOI] [PubMed] [Google Scholar]
- 4. Killick-Kendrick R (1999) The biology and control of phlebotomine sand flies. Clin Dermatol 17: 279–289. [DOI] [PubMed] [Google Scholar]
- 5.Munstermann LE (2004) Phlebotomine sand flies, the Psychodidae. In: Marquardt WC, Black WC, Freier JE, Hagedorn HH, Hemingway J, Higgs S, James AA, Kondratieff B, Moore CG, editors. Biology of disease vectors, 2nd ed. San Diego: Elsevier. pp. 141–151. [Google Scholar]
- 6. Comer J, Tesh R (1991) Phlebotomine sand flies as vectors of vesiculoviruses: A review. Parassitologia 33: 143–150. [PubMed] [Google Scholar]
- 7.Polly R (2007) Orbiviruses. In: Knipe D, Howley P, editors. Fields virology, 5th ed. Philadelphia: Lippincott, Williams and Wilkins. pp. 1975–1997. [Google Scholar]
- 8. Acevedo M, Arrivillaga J (2008) Eco-Epidemiología de flebovirus (Bunyaviridae, Phlebovirus) transmitidos por flebótomos (Psychodidae, Phlebotominae) [Eco-epidemiology of phlebovirus (Bunyaviridae, Phlebovirus) transmitted by phlebotomine (Psychodidae, Phlebotominae)]. Bol Dir Malariol Sanea Amb 48: 2–16. [Google Scholar]
- 9.Martins AV, Williams P, Falcão AL (1978) American sand flies (Diptera: Psychodidae, Phlebotominae). Rio de Janeiro: Academia Brasileira de Ciencias. 195 p. [Google Scholar]
- 10. Osorno-Mesa E, Morales-Alarcón A, Osorno F, Ferro-Vela C (1972) Phlebotominae de Colombia (Diptera, Psychodidae) IX. Distribución geográfica de especies de Brumptomyia França y Parrot, 1921 y Lutzomyia França, 1924 encontradas en Colombia [Phlebotominae of Colombia (Diptera, Psychodidae) IX. Geographical distribution of species Brumptomyia França and Parrot, 1921 and Lutzomyia França, 1924 found in Colombia]. Rev Acad Colomb Cienc 14: 45–68. [Google Scholar]
- 11.Young DG (1979) A review of the bloodsucking psychodid flies of Colombia (Diptera: Phlebotominae and Sycoracinae). Gainesville, FL: Agricultural Experiment Stations, IFAS, University of Florida. [Google Scholar]
- 12.Ferro C, Morales A (1988) Flebótomos de Colombia: Estudios realizados por el laboratorio de entomología 1966–1997 [Phlebotomus of Colombia: Studies by the entomology lab 1966–1997]. In: Toro G, Hernández CA, Raad J, editors. Instituto Nacional de Salud 1917–1997: Una historia, un compromiso. Bogotá: Instituto Nacional de Salud. pp. 219–233. [Google Scholar]
- 13.Montoya-Lerma J, Ferro C (1999) Flebótomos (Diptera: Psychodidae) de Colombia [Phlebotomus (Diptera: Psychodidae) from Colombia]. In: Amat G, Andrade MG, Fernández F, editors. Insectos de Colombia, Volumen II. Colección Jorge Álvarez Lleras, No. 13. Santafé de Bogotá: Academia Colombiana de Ciencias Exactas, Físicas y Naturales, Centro Editorial Javeriano. pp. 211–245. [Google Scholar]
- 14. Bejarano EE (2006) Lista actualizada de los psicódidos (Diptera: Psychodidae) de Colombia [Updated list of moth flies (Diptera: Psychodidae) from Colombia]. Folia Entomol Mex 45: 47–56. [Google Scholar]
- 15.Marín D, Ocampo C, Munstermann L, Ferro C (2008) Actualización de la lista de flebotomíneos reportados para el departamento del Tolima – Colombia [Updated list of phlebotomine reported to the department of Tolima – Colombia]. In: Memorias, XXXV Congreso. Cali: SOCOLEN. pp. 135. [Google Scholar]
- 16. Bejarano EE, Vivero R, Uribe S (2010) Description of Lutzomyia velezi, a new species of phlebotominae sand fly (Diptera: Psychodidae) from the department of Antioquia, Colombia. Mem Inst Oswaldo Cruz 105: 322–325. [DOI] [PubMed] [Google Scholar]
- 17. Vivero R, Muskus C, Torres C (2011) Fauna de flebotomíneos (Diptera: Psychodidae) en Acandí (Chocó, Colombia) [Phlebotomine fauna (Diptera: Psychodidae) in Acandí (Chocó, Colombia)]. Acta Biol Colomb 16: 209–218. [Google Scholar]
- 18. Contreras MA, Vivero RJ, Bejarano EE, Carrillo LM, Vélez ID (2012) Nuevos registros de flebotomíneos (Diptera: Psychodidae) en el área de influencia del río Amoyá en Chaparral, Tolima [New records of sand flies (Diptera: Psychodidae) in the influence area of the river Amoyá in Chaparral, Tolima]. Biomédica 32: 263–268. [DOI] [PubMed] [Google Scholar]
- 19. Carrasquilla MC, Munstermann L, Marín D, Ocampo C, Ferro C (2012) Description of Lutzomyia (Helcocyrtomyia) tolimensis, a new species of phlebotomine sandfly (Diptera: Psychodidae) from Colombia. Mem Inst Oswaldo Cruz 107: 993–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ferro C, Fuya P, Perez S, Lugo L, Gonzales G (2011) Valoración de la ecoepidemiología de la leishmaniasis en Colombia a partir de la distribución espacial y ecológica de los insectos vectores [Assessment of the eco-epidemiology of leishmaniasis in Colombia via the spatial and ecological distributions of vector insects]. Biomédica 31 (suppl 3) 50–59.25284912 [Google Scholar]
- 21. Amóra S, Bevilaqua C, Feijó F, Alves N, Maciel M (2009) Control of phlebotomine (Diptera: Psychodidae) leishmaniasis vectors. Neotrop Entomol 38: 303–310. [DOI] [PubMed] [Google Scholar]
- 22. Hanel R, Sturmbauer C (2000) Multiple recurrent evolution of trophic types in northeastern Atlantic and Mediterranean seabreams (Sparidae, Percoidei). J Mol Evol 50: 276–283. [DOI] [PubMed] [Google Scholar]
- 23. de la Herran R, Rejon C, Rejon M, Garrido-Ramos M (2001) The molecular phylogeny of the Sparidae (Pisces, perciformes) based on two satellite DNA families. Heredity 87: 691–697 10.1046/j.1365-2540.2001.00967.x [DOI] [PubMed] [Google Scholar]
- 24. Aransay AM, Scoulica E, Chaniotis B, Tselentis Y (1999) Typing of sandflies from Greece and Cyprus by DNA polymorphism of 18S rRNA gene. Insect Mol Biol 8: 179–184. [DOI] [PubMed] [Google Scholar]
- 25. Beati L, Cáceres AG, Lee JA, Munstermann LE (2004) Systematic relationships among Lutzomyia sand flies (Diptera: Psychodidae) of Peru and Colombia based on the analysis of 12S and 28S ribosomal DNA sequences. Int J Parasitol 34: 225–234. [DOI] [PubMed] [Google Scholar]
- 26. Kato H, Uezato H, Gomez EA, Terayama Y, Calvopiña M, et al. (2007) Establishment of a mass screening method of sand fly vectors for Leishmania infection by molecular biological methods. Am J Trop Med Hyg 77: 324–329. [PubMed] [Google Scholar]
- 27. Kato H, Cáceres AG, Gomez EA, Mimori T, Uezato H, et al. (2008) Molecular mass screening to incriminate sand fly vectors of Andean-type cutaneous leishmaniasis in Ecuador and Peru. Am J Trop Med Hyg 79: 719–721. [PubMed] [Google Scholar]
- 28. Azpurua J, De La Cruz D, Valderama A, Windsor D (2010) Lutzomyia sand fly diversity and rates of infection by Wolbachia and an exotic Leishmania species on Barro Colorado Island, Panama. PLoS Negl Trop Dis 4: e627 10.1371/journal.pntd.0000627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ishikawa EAY, Ready PD, Souza AA, Day JJ, Rangel EF, et al. (1999) A mitochondrial DNA phylogeny indicates close relationships between populations of Lutzomyia whitmani (Diptera: Psychodidae, Phlebotominae) from the rainforest regions of Amazonian and northeast Brazil. Mem Inst Oswaldo Cruz 94: 339–345. [DOI] [PubMed] [Google Scholar]
- 30. Depaquit J, Ferté H, Léger N, Killick-Kendrick R, Rioux JA, et al. (2000) Molecular systematic of the Phlebotomine sandflies of the subgenus Paraphlebotomus (Diptera, Psychodidae, Phlebotomus) based on ITS2 rDNA sequences. Hypotheses of dispersion and speciation. Insect Mol Biol 9: 293–300. [DOI] [PubMed] [Google Scholar]
- 31. Uribe Soto SI, Lehmann T, Rowton ED, Velez ID, Porter CH (2001) Speciation and population structure in the morphospecies Lutzomyia longipalpis (Lutz and Neiva) as derived from the mitochondrial ND4 gene. Mol Phylogenet Evol 18: 84–93. [DOI] [PubMed] [Google Scholar]
- 32. Testa JM, Montoya-Lerma J, Cadena H, Oviedo M, Ready PD (2002) Molecular identification of vectors of Leishmania in Colombia: Mitochondrial introgression in the Lutzomyia townsendi series. Acta Trop 84: 205–218. [DOI] [PubMed] [Google Scholar]
- 33. Hodgkinson V, Birungi J, Quintana M, Deitze R, Munstermann L (2003) Mitochondrial cytochrome b variation in populations of the visceral leishmaniasis vector Lutzomyia longipalpis across eastern Brazil. Am J Trop Med Hyg 69: 386–392. [PubMed] [Google Scholar]
- 34. Torgerson DG, Lampo M, Velazques Y, Woo PTK (2003) Genetic relationships among some species groups within the genus Lutzomyia (Diptera: Psychodidae). Am J Trop Med Hyg 69: 484–493. [PubMed] [Google Scholar]
- 35. Vivero RJ, Bejarano EE, Contreras MA (2007) Análisis de la estructura primaria y secundaria del ARN de transferencia mitocondrial para serina en siete especies de Lutzomyia [Analysis of the primary and secondary RNA structures regarding mitochondrial transfer of serine in seven species of Lutzomyia]. Biomédica 27: 429–438. [PubMed] [Google Scholar]
- 36. Kuwahara K, Kato H, Gomez EA, Uezato H, Mimori T, et al. (2009) Genetic diversity of ribosomal RNA internal transcribed spacer sequences in Lutzomyia species from areas endemic for New World cutaneous leishmaniasis. Acta Trop 112: 131–136. [DOI] [PubMed] [Google Scholar]
- 37. Vivero RJ, Bejarano EE, Contreras MA (2009) Cambios en el extremo carboxilo terminal de citocromo b como carácter taxonómico en Lutzomyia (Diptera: Psychodidae) [Changes to the extreme terminal carboxyl of cytochrome b as taxonomic character in Lutzomyia (Diptera: Psychodidae)]. Rev Colomb Entomol 35: 83–88. [Google Scholar]
- 38. Tautz D, Arctander P (2002) DNA points the way ahead in taxonomy. Nature 418: 479 10.1038/418479a [DOI] [PubMed] [Google Scholar]
- 39. Tautz D, Arctander P, Minelli A, Thomas RH, Vogler AP (2003) A plea for DNA taxonomy. Trends Ecol Evol 18: 70–74 10.1016/S0169-5347(02)00041-1 [DOI] [Google Scholar]
- 40. Hebert P, Cywinska A, Ball S, deWaard J (2003) Biological identifications through DNA barcodes. Proc R Soc Lond B 270: 313–321 10.1098/rspb.2002.2218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hebert P, Ratnasingham S, deWaard J (2003) Barcoding animal life: cytochrome c oxidase subunit 1 divergence, among closely related species. Proc R Soc Lond B (Suppl) 270: S96–S99 10.1098/rsbl.2003.0025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Besansky N, Severson DW, Ferdig MT (2003) DNA barcoding of parasites and invertebrate disease vectors: What you don't know can hurt you. Trends Parasitol 19: 545–546. [DOI] [PubMed] [Google Scholar]
- 43. Jinbo U, Kato T, Ito M (2011) Current progress in DNA barcoding and future implications for entomology. Entomol Sci 14: 107–124. [Google Scholar]
- 44. Moritz C, Cicero C (2004) DNA barcoding: promise and pitfalls. PLoS Biol 2: e354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Meier R, Shiyang K, Vaidya G, Ng PK (2006) DNA barcoding and taxonomy in diptera: A tale of high intraspecific variability and low identification success. Syst Biol 55: 715–728. [DOI] [PubMed] [Google Scholar]
- 46. Song H, Buhay JE, Whiting MF, Crandall KA (2008) Many species in one: DNA barcoding overestimates the number of species when nuclear mitochondrial pseudogenes are coamplified. Proc Natl Acad Sci USA 105: 13486–13491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Casiraghi M, Labra M, Ferri E, Galimberti M, De Mattia F (2010) DNA barcoding: Theoretical aspects and practical applications. In: Nimis PL, Vignes Lebbe R, editors. Tools for identifying biodiversity: Progress and problems. Trieste: Edizioni Università di Trieste. pp. 269–273. [Google Scholar]
- 48. Leite LA (2012) Mitochondrial pseudogenes in insect DNA barcoding: Differing points of view on the same issue. Biota Neotrop 12: 301–308. [Google Scholar]
- 49. Kumar NP, Srinivasan R, Jambulingam P (2012) DNA barcoding for identification of sand flies (Diptera: Psychodidae) in India. Mol Ecol Resour 12: 414–420 10.1111/j.1755-0998.2012.03117 [DOI] [PubMed] [Google Scholar]
- 50. Cohnstaedt LW, Beati L, Caceres AG, Ferro C, Munstermann LE (2011) Phylogenetics of the phlebotomine sand fly group Verrucarum (Diptera: Psychodidae: Lutzomyia). Am J Trop Med Hyg 84: 913–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Hoyos R, Uribe S, Vélez ID (2012) Tipificación de especímenes colombianos de Lutzomyia longipalpis (Diptera: Psychodidae) mediante “Código de Barras” [Classification of Colombian specimens of Lutzomyia longipalpis (Diptera: Psychodidae) by “Barcode”]. Rev Col Entomol 38: 134–140. [Google Scholar]
- 52. Scarpassa VM, Alencar RB (2012) Lutzomyia umbratilis, the main vector of Leishmania guyanensis, represents a novel species complex? PLoS One 7: e37341 10.1371/journal.pone.0037341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Zapata S, Mejía L, Le Pont F, León R, Pesson B, et al. (2012) A study of a population of Nyssomyia trapidoi (Diptera: Psychodidae) caught on the Pacific coast of Ecuador. Parasit Vectors 5: 144 10.1186/1756-3305-5-144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Ibañez-Bernal S (1999) Phlebotominae de México. I. Brumptomyia França y Parrot; Lutzomyia França, las especies de L. (Lutzomyia) França y del grupo verrucarum [Phlebotominae of Mexico. I. Brumptomyia França and Parrot; Lutzomyia França, the species of L. (Lutzomyia) França and the group verrucarum]. Folia Entomol Mex 107: 61–116. [Google Scholar]
- 55. Folmer O, Black M, Hoeh W, Lutz R, Vrijenhoek R (1994) DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol Mar Biol Biotechnol 3: 294–299. [PubMed] [Google Scholar]
- 56. Hall TA (1999) BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp Ser 41: 95–98. [Google Scholar]
- 57. Tamura K, Peterson D, Peterson N, Stecher G, Nei M, et al. (2011) MEGA 5: Molecular Evolutionary Genetics Analysis using Maximum Likelihood, Evolutionary Distance, and Maximum Parsimony Methods. Mol Biol Evol 28: 2731–2739 10.1093/molbev/msr121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Altschul SF, Madden TL, Schäffer AA, Zhang J, Zhang Z, et al. (1997) Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res 25: 3389–3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Librado P, Rozas J (2009) DnaSP v5: a software for comprehensive analysis of DNA polymorphism data. Bioinformatics 25: 1451–1452 10.1093/bioinformatics/btp187 [DOI] [PubMed] [Google Scholar]
- 60. Kimura M (1980) A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J Mol Evol 15: 111–120. [DOI] [PubMed] [Google Scholar]
- 61. Saitou N, Nei M (1987) The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol 4: 406–425 10.1016/j.ympev.2008.01.019 [DOI] [PubMed] [Google Scholar]
- 62. Hebert PDN, Stoeckle MY, Zemlak TS, Francis CM (2004) Identification of birds through DNA barcodes. PLoS Biol 2: e312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Meyer CP, Paulay G (2005) DNA barcoding: Error rates based on comprehensive sampling. PLoS Biol 3: 2229–2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Hebert PDN, Gregory TR (2005) The promise of DNA barcoding for taxonomy. Syst Biol 54: 852–859 10.1080/10635150500354886 [DOI] [PubMed] [Google Scholar]
- 65. Zhang DX, Hewitt GM (1996) Nuclear integrations: Challenges for mitochondrial DNA markers. Trends Ecol Evol 11: 247–251. [DOI] [PubMed] [Google Scholar]
- 66. Lunt DH, Zhang DX, Szymura JM, Hewitt GM (1996) The insect cytochrome oxidase I gene: Evolutionary patterns and conserved primers for phylogenetic studies. Insect Mol Biol 5: 153–165. [DOI] [PubMed] [Google Scholar]
- 67.Hoy M (2006) Insect molecular genetics: An introduction to principles and applications. San Diego: Academic Press. 544 p. [Google Scholar]
- 68. Clare EL, Kerr KC, von Königslöw TE, Wilson JJ, Hebert PD (2008) Diagnosing mitochondrial DNA diversity: Applications of a sentinel gene approach. J Mol Evol 66: 362–367 10.1007/s00239-008-9088-2 [DOI] [PubMed] [Google Scholar]
- 69. Crozier RH, Crozier YC (1993) The mitochondrial genome of the honeybee Apis mellifera: Complete sequence and genome organization. Genetics 133: 97–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Herbeck JT, Novembre J (2003) Codon usage patterns in cytochrome oxidase I across multiple insect orders. J Mol Evol 56: 691–701. [DOI] [PubMed] [Google Scholar]
- 71. Morlais I, Severson D (2002) Complete mitochondrial DNA sequence and amino acid analysis of the cytochrome c oxidase subunit I (COI) from Aedes aegypti . DNA sequence 13: 123–127. [DOI] [PubMed] [Google Scholar]
- 72. de Oliveira MT, de Azeredo-Espin AM, Lessinger AC (2005) Evolutionary and structural analysis of the cytochrome c oxidase subunit I (COI) gene from Haematobia irritans, Stomoxys calcitrans and Musca domestica (Diptera: Muscidae) mitochondrial DNA. DNA Seq 16: 156–160. [DOI] [PubMed] [Google Scholar]
- 73. Cywinska A, Hunter FF, Hebert PDN (2006) Identifying Canadian mosquito species through DNA barcodes. Med Vet Entomol 20: 413–424. [DOI] [PubMed] [Google Scholar]
- 74. Kumar NP, Rajavel AR, Natarajan R, Jambulingam P (2007) DNA barcodes can distinguish species of Indian mosquitoes (Diptera: Culicidae). J Med Entomol 44: 1–7. [DOI] [PubMed] [Google Scholar]
- 75. Quintero L, Navarro JC (2009) Filogenia intraespecífica y variabilidad genética de Culex quinquefasciatus Say (Diptera: Culicidae) con los genes mitocondriales ND5 y COI [Intraspecific phylogeny and genetic variability of Culex quinquefasciatus Say (Diptera: Culicidae) with ND5 and COI mitochondrial genes]. Bol Mal Salud Amb 52. [Google Scholar]
- 76. Blouin MS, Yowell CA, Courtney CH, Dame JB (1998) Substitution bias, rapid saturation, and use of mtDNA for nematode systematics. Mol Biol Evol 15: 1719–1727. [DOI] [PubMed] [Google Scholar]
- 77. Aliabadian M, Kaboli M, Nijman V, Vences M (2009) Molecular identification of birds: Performance of distance-based DNA barcoding in three genes to delimit parapatric species. PLoS One 4: e4119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Avise JC (2000) Phylogeography: The history and formation of species. Cambridge: Harvard University Press. 464 p. [Google Scholar]
- 79. Memmott J (1991) Sandfly distribution and abundance in a tropical rain forest. Med Vet Entomol 5: 403–411. [DOI] [PubMed] [Google Scholar]
- 80. Zhang A, He LJ, Crozier RH, Muster C, Zhu CD (2010) Estimating sample sizes for DNA barcoding. Mol Phylogenet Evol 54: 1035–1039. [DOI] [PubMed] [Google Scholar]
- 81. Bergsten J, Bilton DT, Fujisawa T, Elliott M, Monaghan M, et al. (2012) The effect of geographical scale of sampling on DNA barcoding. Syst Biol 61: 851–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Desalle R, Egan MG, Siddall M (2005) The unholy trinity: Taxonomy, species delimitation, and DNA barcoding. Philos Trans R Soc Lond B Biol Sci 360: 1905–1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Wiemers M, Fiedler K (2007) Does the DNA barcoding gap exist? A case study in blue butteries (Lepidoptera: Lycaenidae). Front Zool 4: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Virgilio M, Backeljau T, Nevado B, Meyer M (2010) Comparative performances of DNA barcoding across insect orders. BMC Bioinformatics 11: 206 10.1186/1471-2105-11-206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Hendrich L, Pons J, Ribera I, Balke M (2010) Mitochondrial cox1 sequence data reliably uncover patterns of insect diversity but suffer from high lineage idiosyncratic error rates. PLoS One 5: e14448 10.1371/journal.pone.0014448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Moftah M, Abdel Aziz SH, Elramah S, Favereaux A (2011) Classification of sharks in the Egyptian Mediterranean waters using morphological and DNA barcoding approaches. PLoS One 6: e27001 10.1371/journal.pone.0027001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Felsenstein J (1985) Confidence limits on phylogenies: An approach using the bootstrap. Evolution 39: 783–791 10.2307/2408678 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
List of sand fly species, collection sites and number of genotypes per species used in the study.
(DOCX)
Sequence divergence and nucleotide composition for the sand flies genera. The frequencies of nucleotides in sequence are presented as the total average values for all Condon positions and for each condon position separately with the accuracy to tenths of a percent.
(DOCX)