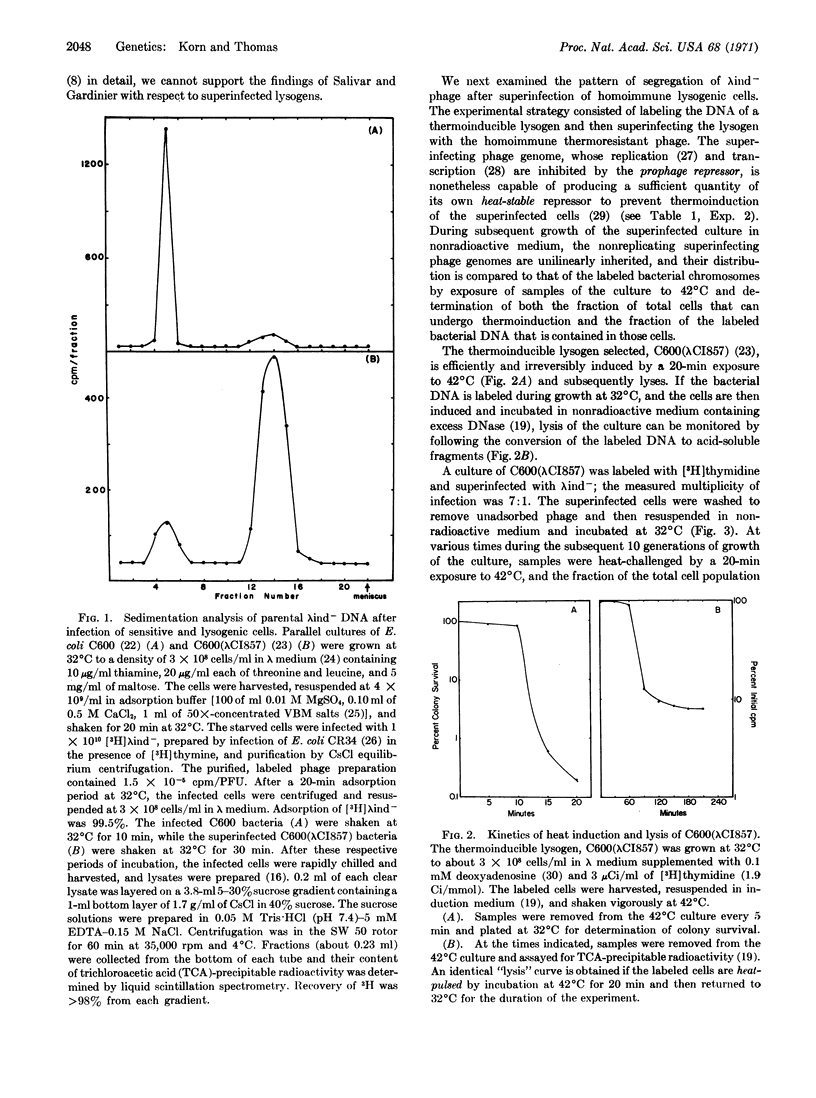
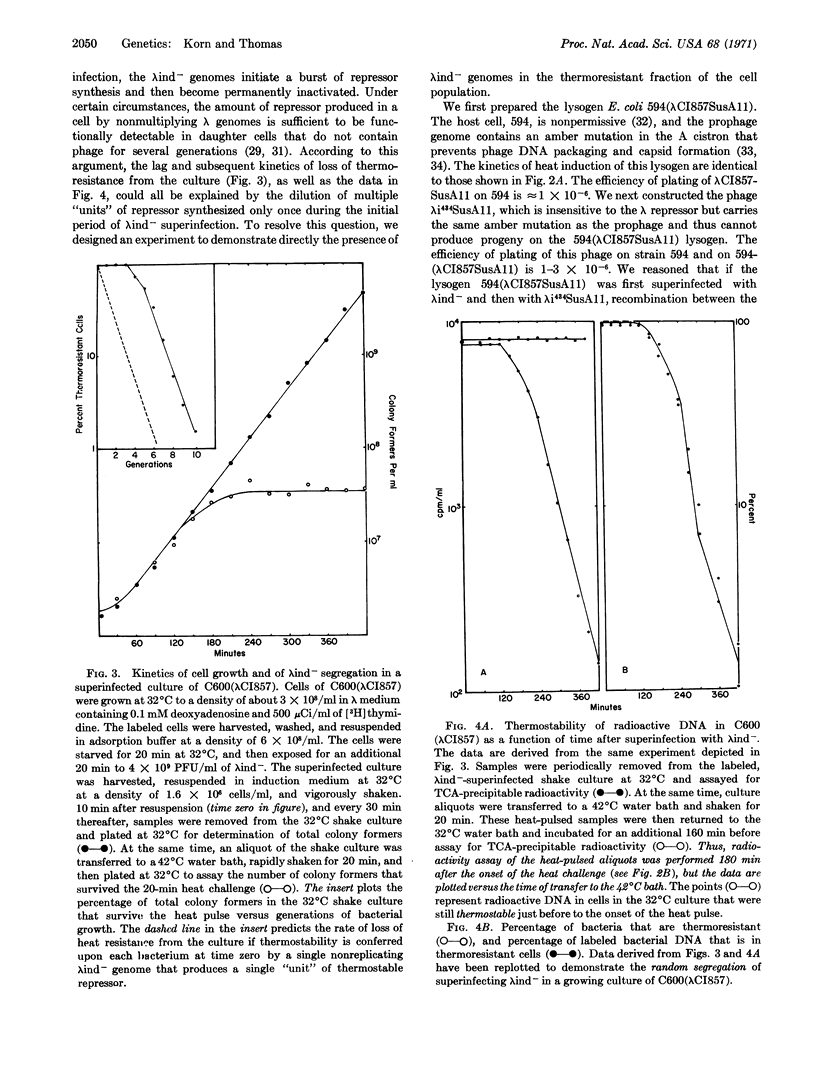
Abstract
The fate of parental λ genomes after superinfection of homoimmune lysogenic cells was studied. The data confirm a previous observation that in the presence of the λ repressor, superinfecting λ DNA does not become associated with replication sites on the bacterial cell membrane. Under these conditions, the nonreplicating, superinfecting phage genomes do not become associated with the bacterial segregation unit. These results support the concept that the attachment of DNA to the bacterial membrane at specific sites is involved in the control of both chromosome replication and segregation, as predicted by the replicon hypothesis.
Keywords: bacteriophage λ, repressor, heat induction, superinfection
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Appleyard R K. Segregation of New Lysogenic Types during Growth of a Doubly Lysogenic Strain Derived from Escherichia Coli K12. Genetics. 1954 Jul;39(4):440–452. doi: 10.1093/genetics/39.4.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botstein D., Levine M. Intermediates in the synthesis of phage P22 DNA. Cold Spring Harb Symp Quant Biol. 1968;33:659–667. doi: 10.1101/sqb.1968.033.01.075. [DOI] [PubMed] [Google Scholar]
- CAMPBELL A. Sensitive mutants of bacteriophage lambda. Virology. 1961 May;14:22–32. doi: 10.1016/0042-6822(61)90128-3. [DOI] [PubMed] [Google Scholar]
- Campbell A. The steric effect in lysogenization by bacteriophage lambda. I. Lysogenization of a partially diploid strain of Escherichia coli K-12. Virology. 1965 Nov;27(3):329–339. doi: 10.1016/0042-6822(65)90112-1. [DOI] [PubMed] [Google Scholar]
- Cuzin F., Jacob F. Existence chez Escherichia coli K12 d'une unité génétique de transmission formée de différents réplicons. Ann Inst Pasteur (Paris) 1967 May;112(5):529–545. [PubMed] [Google Scholar]
- HANAWALT P. C., RAY D. S. ISOLATION OF THE GROWING POINT IN THE BACTERIAL CHROMOSOME. Proc Natl Acad Sci U S A. 1964 Jul;52:125–132. doi: 10.1073/pnas.52.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallick L., Boyce R. P., Echols H. Membrane association by bacteriophage lambda-DNA: possible direct role of regulator gene N. Nature. 1969 Sep 20;223(5212):1239–1242. doi: 10.1038/2231239a0. [DOI] [PubMed] [Google Scholar]
- Hohn B., Korn D. Cosegregation of a sex factor with the Escherichia coli chromosome during curing by acridine orange. J Mol Biol. 1969 Oct 28;45(2):385–395. doi: 10.1016/0022-2836(69)90113-2. [DOI] [PubMed] [Google Scholar]
- JACOB F., CAMPBELL A. Sur le système de répression assurant l'immunité chez les bactéries lysogenes. C R Hebd Seances Acad Sci. 1959 Jun 1;248(22):3219–3221. [PubMed] [Google Scholar]
- KELLENBERGER G., ZICHICHI M. L., WEIGLE J. A mutation affecting the DNA content of bacteriophage lambda and its lysogenizing properties. J Mol Biol. 1961 Aug;3:399–408. doi: 10.1016/s0022-2836(61)80053-3. [DOI] [PubMed] [Google Scholar]
- KORN D., WEISSBACH A. Thymineless induction in Escherichia coli K12 (lambda). Biochim Biophys Acta. 1962 Nov 26;61:775–790. doi: 10.1016/0926-6550(62)90060-9. [DOI] [PubMed] [Google Scholar]
- Knippers R., Sinsheimer R. L. Process of infection with bacteriophage phiX174. XX. Attachment of the parental DNA of bacteriophage phiX174 to a fast-sedimenting cell component. J Mol Biol. 1968 May 28;34(1):17–29. doi: 10.1016/0022-2836(68)90231-3. [DOI] [PubMed] [Google Scholar]
- Knippers R., Strätling W. The DNA replicating capacity of isolated E. coli cell wall-membrane complexes. Nature. 1970 May 23;226(5247):713–717. doi: 10.1038/226713a0. [DOI] [PubMed] [Google Scholar]
- Lieb M. Studies of heat-inducible lambda mutants. II. Production of C-1 product by superinfecting lambda+ in heat-inducible lysogens. Virology. 1966 Jul;29(3):367–376. doi: 10.1016/0042-6822(66)90212-1. [DOI] [PubMed] [Google Scholar]
- OKADA T., YANAGISAWA K., RYAN F. J. Elective production of thymine-less mutants. Nature. 1960 Oct 22;188:340–341. doi: 10.1038/188340a0. [DOI] [PubMed] [Google Scholar]
- Ogawa T., Tomizawa J. Absortive lysogenization of bacteriophage lambda b2 and residual immunity of non-lysogenic segregants. J Mol Biol. 1967 Jan 28;23(2):225–245. doi: 10.1016/s0022-2836(67)80030-5. [DOI] [PubMed] [Google Scholar]
- Ryter A. Association of the nucleus and the membrane of bacteria: a morphological study. Bacteriol Rev. 1968 Mar;32(1):39–54. doi: 10.1128/br.32.1.39-54.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SLY W. S., ECHOLS H., ADLER J. CONTROL OF VIRAL MESSENGER RNA AFTER LAMBDA PHAGE INFECTION AND INDUCTION. Proc Natl Acad Sci U S A. 1965 Feb;53:378–385. doi: 10.1073/pnas.53.2.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SUSSMAN R., JACOB F. [On a thermosensitive repression system in the Escherichia coli lambda bacteriophage]. C R Hebd Seances Acad Sci. 1962 Feb 19;254:1517–1519. [PubMed] [Google Scholar]
- Salivar W. O., Gardinier J. Replication of bacteriophage lambda DNA associated with the host cell membrane. Virology. 1970 May;41(1):38–51. doi: 10.1016/0042-6822(70)90052-8. [DOI] [PubMed] [Google Scholar]
- Salivar W. O., Sinsheimer R. L. Intracellular location and number of replicating parental DNA molecules of bacteriophages lambda and phi-X174. J Mol Biol. 1969 Apr 14;41(1):39–65. doi: 10.1016/0022-2836(69)90124-7. [DOI] [PubMed] [Google Scholar]
- Smith D. W., Hanawalt P. C. Properties of the growing point region in the bacterial chromosome. Biochim Biophys Acta. 1967 Dec 19;149(2):519–531. doi: 10.1016/0005-2787(67)90180-3. [DOI] [PubMed] [Google Scholar]
- Smith D. W., Schaller H. E., Bonhoeffer F. J. DNA synthesis in vitro. Nature. 1970 May 23;226(5247):711–713. doi: 10.1038/226711a0. [DOI] [PubMed] [Google Scholar]
- Sueoka N., Quinn W. G. Membrane attachment of the chromosome replication origin in Bacillus subtilis. Cold Spring Harb Symp Quant Biol. 1968;33:695–705. doi: 10.1101/sqb.1968.033.01.078. [DOI] [PubMed] [Google Scholar]
- THOMAS R., BERTANI L. E. ON THE CONTROL OF THE REPLICATION OF TEMPERATE BACTERIOPHAGES SUPERINFECTING IMMUNE HOSTS. Virology. 1964 Nov;24:241–253. doi: 10.1016/0042-6822(64)90163-1. [DOI] [PubMed] [Google Scholar]
- Tremblay G. Y., Daniels M. J., Schaechter M. Isolation of a cell membrane-DNA-nascent RNA complex from bacteria. J Mol Biol. 1969 Feb 28;40(1):65–76. doi: 10.1016/0022-2836(69)90296-4. [DOI] [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]
- WOLF B., MESELSON M. REPRESSION OF THE REPLICATION OF SUPERINFECTING BACTERIOPHAGE DNA IN IMMUNE CELLS. J Mol Biol. 1963 Dec;7:636–644. doi: 10.1016/s0022-2836(63)80110-2. [DOI] [PubMed] [Google Scholar]
- Weigle J. Assembly of phage lambda in vitro. Proc Natl Acad Sci U S A. 1966 Jun;55(6):1462–1466. doi: 10.1073/pnas.55.6.1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarmolinsky M., Korn D. Evidence for independent segregation of the Escherichia coli chromosome and non-replicating bacteriophage lambda b2. J Mol Biol. 1968 Mar 14;32(2):475–479. doi: 10.1016/0022-2836(68)90025-9. [DOI] [PubMed] [Google Scholar]


