Abstract
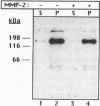
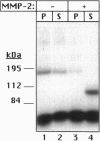
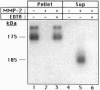
Recent studies have demonstrated the existence of a soluble fibroblast growth factor (FGF) receptor type 1 (FGFR1) extracellular domain in the circulation and in vascular basement membranes. However, the process of FGFR1 ectodomain release from the plasma membrane is not known. Here we report that the 72-kDa gelatinase A (matrix metalloproteinase type 2, MMP2) can hydrolyze the Val368-Met369 peptide bond of the FGFR1 ectodomain, eight amino acids upstream of the transmembrane domain, thus releasing the entire extracellular domain. Similar results were obtained regardless of whether FGF was first bound to the receptor or not. The action of MMP2 abolished binding of FGF to an immobilized recombinant FGFR1 ectodomain fusion protein and to Chinese hamster ovary cells overexpressing FGFR1 The released recombinant FGFR1 ectodomain was able to bind FGF after MMP2 cleavage, suggesting that the cleaved soluble receptor maintained its FGF binding capacity. The activity of MMP2 could not be reproduced by the 92-kDa gelatinase B (MMP9) and was inhibited by tissue inhibitor of metalloproteinase type 2. These studies demonstrate that FGFR1 may be a specific target for MMP2 on the cell surface, yielding a soluble FGF receptor that may modulate the mitogenic and angiogenic activities of FGF.
Full text
PDF

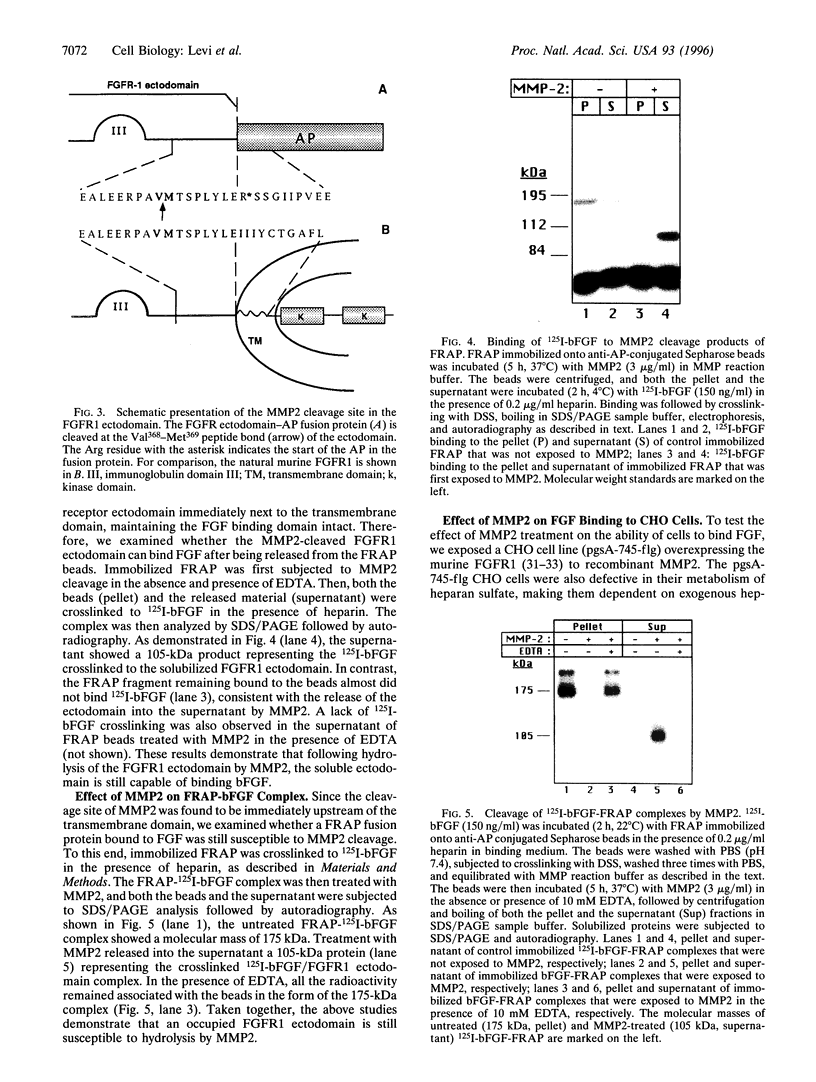
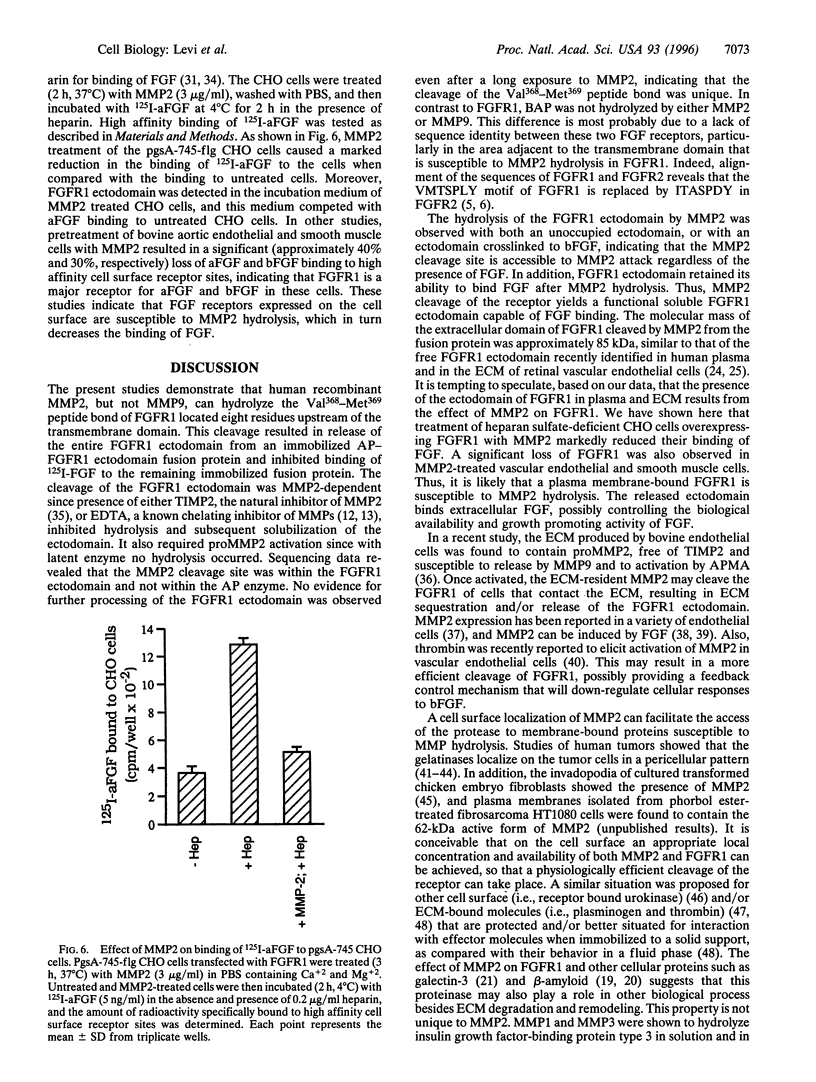

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aviezer D., Levy E., Safran M., Svahn C., Buddecke E., Schmidt A., David G., Vlodavsky I., Yayon A. Differential structural requirements of heparin and heparan sulfate proteoglycans that promote binding of basic fibroblast growth factor to its receptor. J Biol Chem. 1994 Jan 7;269(1):114–121. [PubMed] [Google Scholar]
- Avivi A., Yayon A., Givol D. A novel form of FGF receptor-3 using an alternative exon in the immunoglobulin domain III. FEBS Lett. 1993 Sep 20;330(3):249–252. doi: 10.1016/0014-5793(93)80882-u. [DOI] [PubMed] [Google Scholar]
- Basilico C., Moscatelli D. The FGF family of growth factors and oncogenes. Adv Cancer Res. 1992;59:115–165. doi: 10.1016/s0065-230x(08)60305-x. [DOI] [PubMed] [Google Scholar]
- Benezra M., Vlodavsky I., Yayon A., Bar-Shavit R., Regan J., Chang M., Ben-Sasson S. Reversal of basic fibroblast growth factor-mediated autocrine cell transformation by aromatic anionic compounds. Cancer Res. 1992 Oct 15;52(20):5656–5662. [PubMed] [Google Scholar]
- Burgess W. H., Maciag T. The heparin-binding (fibroblast) growth factor family of proteins. Annu Rev Biochem. 1989;58:575–606. doi: 10.1146/annurev.bi.58.070189.003043. [DOI] [PubMed] [Google Scholar]
- Eisemann A., Ahn J. A., Graziani G., Tronick S. R., Ron D. Alternative splicing generates at least five different isoforms of the human basic-FGF receptor. Oncogene. 1991 Jul;6(7):1195–1202. [PubMed] [Google Scholar]
- Ellis V., Behrendt N., Danø K. Plasminogen activation by receptor-bound urokinase. A kinetic study with both cell-associated and isolated receptor. J Biol Chem. 1991 Jul 5;266(19):12752–12758. [PubMed] [Google Scholar]
- Esko J. D. Genetic analysis of proteoglycan structure, function and metabolism. Curr Opin Cell Biol. 1991 Oct;3(5):805–816. doi: 10.1016/0955-0674(91)90054-3. [DOI] [PubMed] [Google Scholar]
- Flanagan J. G., Leder P. The kit ligand: a cell surface molecule altered in steel mutant fibroblasts. Cell. 1990 Oct 5;63(1):185–194. doi: 10.1016/0092-8674(90)90299-t. [DOI] [PubMed] [Google Scholar]
- Folkman J., Shing Y. Angiogenesis. J Biol Chem. 1992 Jun 5;267(16):10931–10934. [PubMed] [Google Scholar]
- Fowlkes J. L., Enghild J. J., Suzuki K., Nagase H. Matrix metalloproteinases degrade insulin-like growth factor-binding protein-3 in dermal fibroblast cultures. J Biol Chem. 1994 Oct 14;269(41):25742–25746. [PubMed] [Google Scholar]
- Fridman R., Bird R. E., Hoyhtya M., Oelkuct M., Komarek D., Liang C. M., Berman M. L., Liotta L. A., Stetler-Stevenson W. G., Fuerst T. R. Expression of human recombinant 72 kDa gelatinase and tissue inhibitor of metalloproteinase-2 (TIMP-2): characterization of complex and free enzyme. Biochem J. 1993 Jan 15;289(Pt 2):411–416. doi: 10.1042/bj2890411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridman R., Fuerst T. R., Bird R. E., Hoyhtya M., Oelkuct M., Kraus S., Komarek D., Liotta L. A., Berman M. L., Stetler-Stevenson W. G. Domain structure of human 72-kDa gelatinase/type IV collagenase. Characterization of proteolytic activity and identification of the tissue inhibitor of metalloproteinase-2 (TIMP-2) binding regions. J Biol Chem. 1992 Aug 5;267(22):15398–15405. [PubMed] [Google Scholar]
- Fridman R., Toth M., Peña D., Mobashery S. Activation of progelatinase B (MMP-9) by gelatinase A (MMP-2). Cancer Res. 1995 Jun 15;55(12):2548–2555. [PubMed] [Google Scholar]
- Gearing A. J., Beckett P., Christodoulou M., Churchill M., Clements J., Davidson A. H., Drummond A. H., Galloway W. A., Gilbert R., Gordon J. L. Processing of tumour necrosis factor-alpha precursor by metalloproteinases. Nature. 1994 Aug 18;370(6490):555–557. doi: 10.1038/370555a0. [DOI] [PubMed] [Google Scholar]
- Givol D., Yayon A. Complexity of FGF receptors: genetic basis for structural diversity and functional specificity. FASEB J. 1992 Dec;6(15):3362–3369. [PubMed] [Google Scholar]
- Goldberg G. I., Marmer B. L., Grant G. A., Eisen A. Z., Wilhelm S., He C. S. Human 72-kilodalton type IV collagenase forms a complex with a tissue inhibitor of metalloproteases designated TIMP-2. Proc Natl Acad Sci U S A. 1989 Nov;86(21):8207–8211. doi: 10.1073/pnas.86.21.8207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross J. L., Moscatelli D., Jaffe E. A., Rifkin D. B. Plasminogen activator and collagenase production by cultured capillary endothelial cells. J Cell Biol. 1982 Dec;95(3):974–981. doi: 10.1083/jcb.95.3.974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanemaaijer R., Koolwijk P., le Clercq L., de Vree W. J., van Hinsbergh V. W. Regulation of matrix metalloproteinase expression in human vein and microvascular endothelial cells. Effects of tumour necrosis factor alpha, interleukin 1 and phorbol ester. Biochem J. 1993 Dec 15;296(Pt 3):803–809. doi: 10.1042/bj2960803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanneken A., Maher P. A., Baird A. High affinity immunoreactive FGF receptors in the extracellular matrix of vascular endothelial cells--implications for the modulation of FGF-2. J Cell Biol. 1995 Mar;128(6):1221–1228. doi: 10.1083/jcb.128.6.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanneken A., Ying W., Ling N., Baird A. Identification of soluble forms of the fibroblast growth factor receptor in blood. Proc Natl Acad Sci U S A. 1994 Sep 13;91(19):9170–9174. doi: 10.1073/pnas.91.19.9170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Höyhtyä M., Fridman R., Komarek D., Porter-Jordan K., Stetler-Stevenson W. G., Liotta L. A., Liang C. M. Immunohistochemical localization of matrix metalloproteinase 2 and its specific inhibitor TIMP-2 in neoplastic tissues with monoclonal antibodies. Int J Cancer. 1994 Feb 15;56(4):500–505. doi: 10.1002/ijc.2910560408. [DOI] [PubMed] [Google Scholar]
- Ishai-Michaeli R., Eldor A., Vlodavsky I. Heparanase activity expressed by platelets, neutrophils, and lymphoma cells releases active fibroblast growth factor from extracellular matrix. Cell Regul. 1990 Oct;1(11):833–842. doi: 10.1091/mbc.1.11.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson D. E., Williams L. T. Structural and functional diversity in the FGF receptor multigene family. Adv Cancer Res. 1993;60:1–41. doi: 10.1016/s0065-230x(08)60821-0. [DOI] [PubMed] [Google Scholar]
- Knudsen B. S., Silverstein R. L., Leung L. L., Harpel P. C., Nachman R. L. Binding of plasminogen to extracellular matrix. J Biol Chem. 1986 Aug 15;261(23):10765–10771. [PubMed] [Google Scholar]
- Kohn E. C., Alessandro R., Spoonster J., Wersto R. P., Liotta L. A. Angiogenesis: role of calcium-mediated signal transduction. Proc Natl Acad Sci U S A. 1995 Feb 28;92(5):1307–1311. doi: 10.1073/pnas.92.5.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liotta L. A., Steeg P. S., Stetler-Stevenson W. G. Cancer metastasis and angiogenesis: an imbalance of positive and negative regulation. Cell. 1991 Jan 25;64(2):327–336. doi: 10.1016/0092-8674(91)90642-c. [DOI] [PubMed] [Google Scholar]
- Matrisian L. M. Metalloproteinases and their inhibitors in matrix remodeling. Trends Genet. 1990 Apr;6(4):121–125. doi: 10.1016/0168-9525(90)90126-q. [DOI] [PubMed] [Google Scholar]
- Menashi S., Vlodavsky I., Ishai-Michaeli R., Legrand Y., Fridman R. The extracellular matrix produced by bovine corneal endothelial cells contains progelatinase A. FEBS Lett. 1995 Mar 13;361(1):61–64. doi: 10.1016/0014-5793(95)00125-s. [DOI] [PubMed] [Google Scholar]
- Miao H. Q., Fritz T. A., Esko J. D., Zimmermann J., Yayon A., Vlodavsky I. Heparan sulfate primed on beta-D-xylosides restores binding of basic fibroblast growth factor. J Cell Biochem. 1995 Feb;57(2):173–184. doi: 10.1002/jcb.240570202. [DOI] [PubMed] [Google Scholar]
- Miyazaki K., Hasegawa M., Funahashi K., Umeda M. A metalloproteinase inhibitor domain in Alzheimer amyloid protein precursor. Nature. 1993 Apr 29;362(6423):839–841. doi: 10.1038/362839a0. [DOI] [PubMed] [Google Scholar]
- Monsky W. L., Kelly T., Lin C. Y., Yeh Y., Stetler-Stevenson W. G., Mueller S. C., Chen W. T. Binding and localization of M(r) 72,000 matrix metalloproteinase at cell surface invadopodia. Cancer Res. 1993 Jul 1;53(13):3159–3164. [PubMed] [Google Scholar]
- Monteagudo C., Merino M. J., San-Juan J., Liotta L. A., Stetler-Stevenson W. G. Immunohistochemical distribution of type IV collagenase in normal, benign, and malignant breast tissue. Am J Pathol. 1990 Mar;136(3):585–592. [PMC free article] [PubMed] [Google Scholar]
- Ochieng J., Fridman R., Nangia-Makker P., Kleiner D. E., Liotta L. A., Stetler-Stevenson W. G., Raz A. Galectin-3 is a novel substrate for human matrix metalloproteinases-2 and -9. Biochemistry. 1994 Nov 29;33(47):14109–14114. doi: 10.1021/bi00251a020. [DOI] [PubMed] [Google Scholar]
- Ornitz D. M., Yayon A., Flanagan J. G., Svahn C. M., Levi E., Leder P. Heparin is required for cell-free binding of basic fibroblast growth factor to a soluble receptor and for mitogenesis in whole cells. Mol Cell Biol. 1992 Jan;12(1):240–247. doi: 10.1128/mcb.12.1.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapraeger A. C., Krufka A., Olwin B. B. Requirement of heparan sulfate for bFGF-mediated fibroblast growth and myoblast differentiation. Science. 1991 Jun 21;252(5013):1705–1708. doi: 10.1126/science.1646484. [DOI] [PubMed] [Google Scholar]
- Roher A. E., Kasunic T. C., Woods A. S., Cotter R. J., Ball M. J., Fridman R. Proteolysis of A beta peptide from Alzheimer disease brain by gelatinase A. Biochem Biophys Res Commun. 1994 Dec 30;205(3):1755–1761. doi: 10.1006/bbrc.1994.2872. [DOI] [PubMed] [Google Scholar]
- Ron D., Reich R., Chedid M., Lengel C., Cohen O. E., Chan A. M., Neufeld G., Miki T., Tronick S. R. Fibroblast growth factor receptor 4 is a high affinity receptor for both acidic and basic fibroblast growth factor but not for keratinocyte growth factor. J Biol Chem. 1993 Mar 15;268(8):5388–5394. [PubMed] [Google Scholar]
- Sato H., Takino T., Okada Y., Cao J., Shinagawa A., Yamamoto E., Seiki M. A matrix metalloproteinase expressed on the surface of invasive tumour cells. Nature. 1994 Jul 7;370(6484):61–65. doi: 10.1038/370061a0. [DOI] [PubMed] [Google Scholar]
- Stetler-Stevenson W. G. Type IV collagenases in tumor invasion and metastasis. Cancer Metastasis Rev. 1990 Dec;9(4):289–303. doi: 10.1007/BF00049520. [DOI] [PubMed] [Google Scholar]
- Takino T., Sato H., Shinagawa A., Seiki M. Identification of the second membrane-type matrix metalloproteinase (MT-MMP-2) gene from a human placenta cDNA library. MT-MMPs form a unique membrane-type subclass in the MMP family. J Biol Chem. 1995 Sep 29;270(39):23013–23020. doi: 10.1074/jbc.270.39.23013. [DOI] [PubMed] [Google Scholar]
- Visscher D. W., Höyhtyä M., Ottosen S. K., Liang C. M., Sarkar F. H., Crissman J. D., Fridman R. Enhanced expression of tissue inhibitor of metalloproteinase-2 (TIMP-2) in the stroma of breast carcinomas correlates with tumor recurrence. Int J Cancer. 1994 Nov 1;59(3):339–344. doi: 10.1002/ijc.2910590308. [DOI] [PubMed] [Google Scholar]
- Vlodavsky I., Bar-Shavit R., Ishai-Michaeli R., Bashkin P., Fuks Z. Extracellular sequestration and release of fibroblast growth factor: a regulatory mechanism? Trends Biochem Sci. 1991 Jul;16(7):268–271. doi: 10.1016/0968-0004(91)90102-2. [DOI] [PubMed] [Google Scholar]
- Woessner J. F., Jr Matrix metalloproteinases and their inhibitors in connective tissue remodeling. FASEB J. 1991 May;5(8):2145–2154. [PubMed] [Google Scholar]
- Yayon A., Klagsbrun M., Esko J. D., Leder P., Ornitz D. M. Cell surface, heparin-like molecules are required for binding of basic fibroblast growth factor to its high affinity receptor. Cell. 1991 Feb 22;64(4):841–848. doi: 10.1016/0092-8674(91)90512-w. [DOI] [PubMed] [Google Scholar]
- Yayon A., Zimmer Y., Shen G. H., Avivi A., Yarden Y., Givol D. A confined variable region confers ligand specificity on fibroblast growth factor receptors: implications for the origin of the immunoglobulin fold. EMBO J. 1992 May;11(5):1885–1890. doi: 10.1002/j.1460-2075.1992.tb05240.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucker S., Conner C., DiMassmo B. I., Ende H., Drews M., Seiki M., Bahou W. F. Thrombin induces the activation of progelatinase A in vascular endothelial cells. Physiologic regulation of angiogenesis. J Biol Chem. 1995 Oct 6;270(40):23730–23738. doi: 10.1074/jbc.270.40.23730. [DOI] [PubMed] [Google Scholar]
- Zucker S., Moll U. M., Lysik R. M., DiMassimo E. I., Schwedes J. W., Liotta L. A. Extraction of type IV collagenase/gelatinase from plasma membranes of human pancreatic cancer cells. Matrix Suppl. 1992;1:411–411. [PubMed] [Google Scholar]