Shared decision making for advanced heart failure has become both more challenging and more crucial as duration of disease and treatment options have increased. High-quality decisions are chosen from medically reasonable options and are aligned with values, goals, and preferences of an informed patient. The top 10 things to know about decision making in advanced heart failure care are listed in Table 1.
Table 1.
Top Ten Things to Know
|
Why Shared Decision Making?
Providers have an ethical and legal mandate to involve patients in medical decisions. Shared decision making recognizes that there are complex trade-offs in the choice of medical care.1 Shared decision making also addresses the ethical need to fully inform patients about the risks and benefits of treatments.2 In the setting of multiple reasonable options for medical care, shared decision making involves clinicians working with patients to ensure that patients’ values, goals, and preferences guide informed decisions that are right for each individual patient.
Grounded in the ethical principle of autonomy,3 judicial decisions (eg, Cruzan v Missouri Department of Health4) and legislative actions (eg, the Patient Self-Determination Act5) have repeatedly affirmed the rights of patients or duly appointed surrogates to choose their medical therapy from among reasonable options.6 The formal process of informed consent before procedural interventions is an embodiment of this concept in that it underscores the clinician’s obligation to ensure that the patient has the opportunity to be informed.3 An informed patient is one who is aware of the diagnosis and prognosis, the nature of the proposed intervention, the risks and benefits of that intervention, and all reasonable alternatives and their associated risks and benefits.7 A major purpose of a high-functioning healthcare system is to provide the resources with which an activated, informed patient can engage in productive discussions with a proactive, prepared healthcare team.8
Shared decision making moves beyond informed consent. It asks that clinicians and patients share information with each other and work toward patient-centered decisions about treatment.9 Shared decision making incorporates the perspective of the patient, who is responsible for articulating goals, values, and preferences as they relate to his or her health care. Shared decision making incorporates the perspective of the clinician, who is responsible for narrowing the diagnostic and treatment options to those that are medically reasonable. Shared decision making is most easily applied to preference-sensitive decisions, in which both clinicians and patients agree that equipoise exists, and decision support helps patients think through, forecast, and deliberate their options. However, in situations in which clinicians hold the view that scientific evidence for benefit strongly outweighs harm, behavioral support (eg, smoking cessation counseling) designed to describe, justify, and recommend specific behavior may also be appropriate and complementary to decision support.10 Finally, certain therapeutic options may be considered unreasonable and therefore independent of patient demands, although situations of medical futility are relatively rare.6 Although not all patients will be able to clearly articulate decisions that are congruent with their stated goals, shared decision making aims to ensure that patients’ values, goals, and preferences are explored and incorporated into the medical decision-making process.
Patient-centered medicine has been suggested as the next phase in health care.11 Shared decision making puts into practice the principle of “patient-centered care,” which the Institute of Medicine has identified as 1 of the 6 pillars of quality,12 with patient-centered care defined as “providing care that is respectful of and responsive to individual patient preferences, needs, and values and ensuring that patient values guide all clinical decisions.”12 The Patient Protection and Affordable Care Act devotes 4 pages to patient-centered care, specifically calling for the development of decision aids, shared decision-making programs, and metrics for the quality of decision making.13
It will be assumed throughout this document that discussions and decision making with patients also include, when appropriate, the family and other individuals involved, such as caregivers and companions. The approach to decision making outlined in this Scientific Statement takes the perspective of the individual patient rather than that of society in general. Although individual medical decisions taken collectively have implications for distributive justice and resource allocation, it is not the responsibility of clinicians, patients, or families to directly factor these global considerations into individual decisions.14 Rather, discussions regarding alternative treatment options, including no treatment, should be focused on meeting a specific individual’s values, goals, and preferences within the context of societal rules and regulations.
Why Advanced Heart Failure?
Heart failure affects 2.4% of the adult population and over 11% of the expanding population >80 years old.15 Estimated total heart failure costs in the United States are projected to reach 44.6 billion by 2015.15 Existing therapies slow, but infrequently reverse, disease progression. As a result, the prevalence of symptomatic heart failure has increased, including a prolongation of the advanced phase of the disease.16 The American Heart Association characterizes the far end of the heart failure continuum as stage D, or “refractory end-stage heart failure,”17 further defined by others,18,19 including the European Society of Cardiology (Table 2).20 These overlapping definitions describe a group of patients for whom symptoms limit daily life despite usual recommended therapies and for whom lasting remission into less symptomatic disease is unlikely. The increasing prevalence, high symptom burden, and possible disease-exchanging therapies (ie, transplantation and mechanical circulatory support) for patients living with advanced heart failure mandate a systematic and thoughtful approach to decision making.
Table 2.
European Society of Cardiology Criteria for Advanced Chronic Heart Failure
|
NYHA indicates New York Heart Association.
Reprinted from Metra et al,20 with permission of the publisher. Copyright © 2007, Oxford University Press.
This Scientific Statement reviews the clinical context for decision making in advanced heart failure and provides guidance on communication techniques to support these decisions. Its goal is primarily to help healthcare providers of all types integrate these concepts into their routine practice to promote the delivery of effective, safe, efficient, timely, equitable, and patient-centered care.12 We recognize that major barriers to the implementation of these concepts are time, training, and resources. We also recognize the limited and inequitable access to experts with formal training in heart failure and palliative care, which leaves many of these responsibilities to be borne by healthcare providers in a general medical setting. If the goals of this document are to be realized, however, the healthcare system will need to make a fundamental commitment to shared decision making, with realignment of incentives to support the tailoring of advanced care to individual patients. Without changes in the structure of the healthcare team and associated reimbursement, these recommendations will remain an unfunded mandate that are unlikely to be fully realized in most practice settings.
Expectations for the Future
Attention to the clinical trajectory is required to calibrate expectations and guide timely decisions.
Predictive models can target high-risk populations but leave wide uncertainties around estimates of survival for an individual.
Difficult discussions now will simplify difficult decisions in the future.
Uncertainty is inevitable and should be included in discussions with patients and family.
Estimating Prognosis in Heart Failure
Assessment of prognosis is the foundation for selection among therapies for life-threatening disease, but this is particularly challenging for heart failure. The clinical course varies dramatically across the spectrum of disease severity and is relatively unpredictable for individual patients (Figure 1).19,21 This contrasts with the more linear decline of patients with advanced cancer, which has traditionally been the model for approaches to end-stage disease. Even late in heart failure, patients often enjoy “good days” and brief interludes of apparent stability, which can lull them and their care providers into postponing vital decisions. Prognosis is further clouded by the unique contrast between unexpected sudden death (ie, lethal arrhythmia) and lingering death with congestive symptoms (ie, progressive pump failure). Frequent reappraisal of the clinical trajectory helps calibrate expectations, guide communication, and inform rational decisions.
Figure 1.
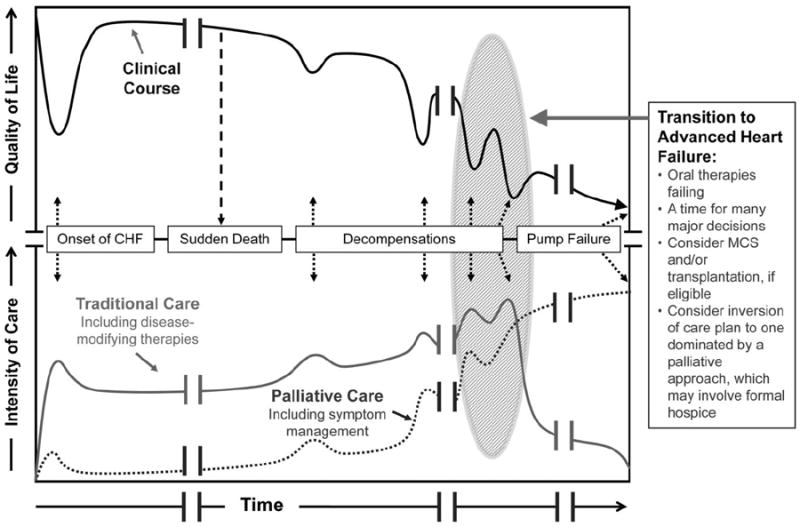
A depiction of the clinical course of heart failure with associated types and intensities of available therapies. Black line: Patients tend to follow a progressive, albeit nonlinear, decline in health-related quality of life as the disease progresses; this course can be interrupted by sudden cardiac death caused by arrhythmia or can end in a more gradual death caused by progressive pump failure. Gray line: At disease onset, multiple oral therapies are prescribed for cardiac dysfunction and/or treatment of comorbidities. As disease severity increases, the intensity of care may increase in parallel, with intensification of diuretics, addition of an implantable cardioverter-defibrillator/cardiac resynchronization therapy for those eligible, and increasing interaction with the medical system through ambulatory visits and hospitalizations, until the time when standard therapies begin to fail (transition to advanced heart failure). Dotted line: Palliative therapies to control symptoms, address quality of life, and enhance communication are relevant throughout the course of heart failure, not just in advanced disease; palliative therapies work hand in hand with traditional therapies designed to prolong survival. The critical transition into advanced heart failure from the medical perspective is often followed by a transition in goals of care from the patient and family perspective, wherein palliative therapies may become the dominant treatment paradigm (for the majority of patients in whom transplantation and mechanical circulatory support are not an option). Clinicians must recognize the transition to advanced heart failure so that therapeutic options can be considered in a timely fashion and patients are able to proactively match medical decisions to clinical realities. CHF indicates chronic heart failure; MCS, mechanical circulatory support. Modified from Lanken et al;21 reprinted with permission of the American Thoracic Society. Copyright © 2012, American Thoracic Society.
More than 100 variables have been associated with mortality and rehospitalization in heart failure.22-27 Examples of prognostic factors include demographics (age, sex, race, insurance status), functional status (New York Heart Association functional class and health-related quality-of-life scores), exercise capacity (peak oxygen consumption, 6-minute walk), cardiac structure and function (cardiac chamber size, ejection fraction), assessments of filling pressures, biomarkers (natriuretic peptides, inflammatory markers), renal and liver dysfunction, comorbidities (diabetes, lung disease), clinical events (defibrillator shocks and recent hospitalizations), psychosocial factors (depression, social isolation), and behavioral factors (eg, adherence to the medical regimen).
A variety of multivariable models have been published in an effort to provide more refined predictions of prognosis in patients with heart failure (Table 3). The most commonly used multivariable instruments for estimating prognosis in symptomatic outpatients are the Heart Failure Survival Score23 and the Seattle Heart Failure Model.22 In patients hospitalized for heart failure, a variety of inpatient models have been developed to predict both in-hospital28 and post-discharge outcomes.26,27,29-31 These inpatient models have highlighted the strength of natriuretic peptides, renal function, and low blood pressure as predictors of survival in patients in this setting.24 Recently, the first model to predict both mortality and quality-of-life outcomes after discharge has been published.32 Although all of these models require complex mathematical formulas to generate risks, the increasing use of health information technology in the delivery of care offers the potential to automatically generate risk profiles from the electronic medical record.
Table 3.
Selected Prognostic Models in Heart Failure
| Key Covariates | Outcome | |
|---|---|---|
| Ambulatory | ||
| Heart Failure Survival Score23 | Peak V̇O2, LVEF, serum sodium, mean BP, HR, ischemic etiology, QRS duration/morphology | All-cause mortality |
| Seattle Heart Failure Model22 (depts.washington.edu/shfm)22a | NYHA function class, ischemic etiology, diuretic dose, LVEF, SBP, sodium, hemoglobin, percent lymphocytes, uric acid, and cholesterol | All-cause mortality, urgent transplantation, or LVAD implantation |
| Hospitalized | ||
| EVEREST Risk Model22 | Age, diabetes, h/o stroke, h/o arrhythmia, β-blocker use, BUN, sodium, BNP, KCCQ scores | The combined end point of mortality or persistently poor quality of life (KCCQ <45) over the 6 mo after discharge |
| EFFECT29 | Age, SBP, respiratory rate, sodium, hemoglobin, BUN, h/o CVA, h/o dementia, h/o COPD, h/o cirrhosis, h/o cancer | 30-d and 1-y mortality |
| ADHERE28 | BUN, SBP, serum creatinine | In-hospital mortality |
| ESCAPE Discharge Score31 | BNP, cardiopulmonary resuscitation or mechanical ventilation during hospitalization, BUN, sodium, age >70 y, daily loop diuretic dose, lack of β-blocker, 6-min walk distance | 6-mo mortality |
V̇O2 indicates oxygen consumption; LVEF, left ventricular ejection fraction; BP, blood pressure; HR, heart rate; NYHA, New York Heart Association; SBP, systolic BP; LVAD, left ventricular assist device; EVEREST, Efficacy of Vasopressin Antagonism in Heart Failure Outcome Study with Tolvaptan; h/o, medical history of; BUN, blood urea nitrogen; BNP, B-type natriuretic peptide; KCCQ, Kansas City Cardiomyopathy Questionnaire; EFFECT, Enhanced Feedback for Effective Cardiac Treatment; CVA, cerebrovascular accident; COPD, chronic obstructive pulmonary disease; ADHERE, Registry for Acute Decompensated Heart Failure Patients; and ESCAPE, Evaluation Study of Congestive Heart Failure and Pulmonary Artery Catheterization Effectiveness.
The application of commonly used ambulatory heart failure models to the advanced heart failure population can result in miscalibrated estimates of life expectancy, with significant underestimation of risk in certain populations.33,34 Therefore, before recommending general use of risk models, adequate discrimination (ie, the ability of a model to accurately distinguish between a patient who will experience the event versus one who will not)35,36 and calibration (ie, the ability of the model to accurately predict the observed probability of an event across levels of risk)37 will need to be validated for broader populations than those from clinical trials.33,34
Prognosis for Both Quantity and Quality of Life
Most prognostic models in heart failure focus on mortality, which is easily determined and highly relevant; however, other clinical outcomes also rank high in importance to individual patients (Figure 2). Multiple studies have documented patients’ willingness to sacrifice survival in exchange for symptom relief, a trade-off that varies between patients and within the same patient over time and is correlated loosely with disease severity39,40 but strongly with do-not-resuscitate status.41 A full discussion of prognosis therefore includes not only the risks of death but also the potential burdens of worsening symptoms, limited functional capacity, loss of independence, reduced social functioning, decreased quality of life, and increased caregiver commitment.42 Unfortunately, much less is known about the risks of these latter outcomes. The only existing model that estimates the risk of unfavorable future quality of life shows important differences from risk models for death, particularly the relative importance of current measures of quality of life.32 More astute anticipation of an unfavorable quality of life until death, in addition to anticipation of death, would better identify patients for whom detailed discussions of prognosis and options are appropriate. In choosing among options, this information gap regarding nonmortality patient-centered outcomes is exacerbated by the lack of rigor in collecting health status information in major trials, although this is improving. Even less is known about the relative impact of the disease and therapies on caregiver burden and quality of life for family members.43-46
Figure 2.
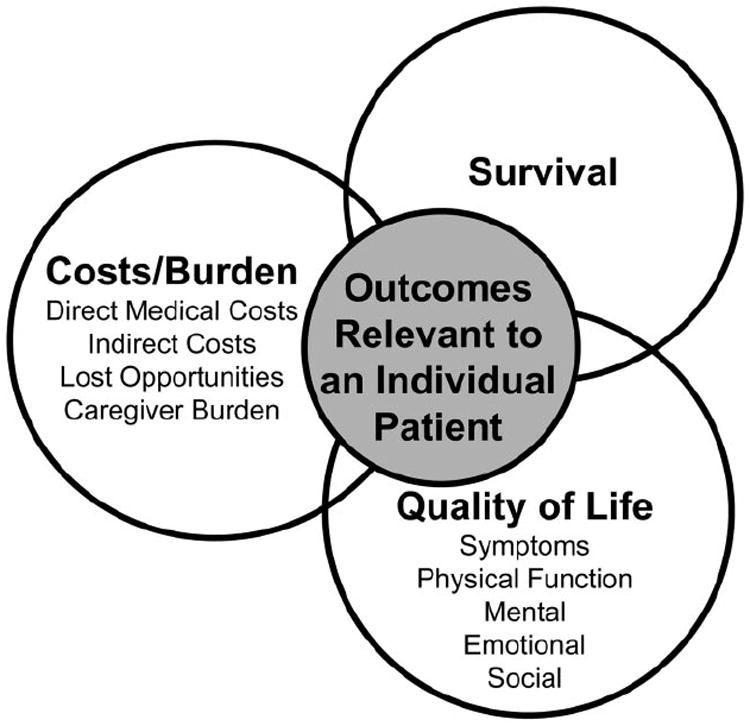
Prognosis is not only about expectations for survival. There are multiple domains that are of varying importance to individual patients. Adapted from Spilker.38
Uncertainty for the Individual
Even under these idealized circumstances, most models designed to predict mortality have only modest accuracy.47 Further complicating practical use, prediction models represent the average survival for a population of patients with characteristics similar to those of the individual patient. A 70% chance of 2-year survival does not directly translate to an individual who will instead be 100% alive or dead at any point in time. For patients with advanced disease, interest often focuses instead on the expected length of time remaining; patients ask the question, “How long do I have?” This point prediction of survival time48 is even more difficult to estimate.49,50 Even if a model fits well for a cohort and the estimated survival curve provides a good fit to the data, it is not clear where along the curve an individual patient will lie. As an example of the difficulty in estimating survival duration, one can consider the median survival estimate (50% survival at time x) as an estimation of the time in which half of the patients will live longer and half will live for a shorter time. Parkes51 defines an “error” in survival as an estimate more than twice as long as the actual survival or less than half the actual survival. That is, if a patient survived for 12 months, a predicted survival of >2 years or <6 months would be considered an error by this definition. This error depends on the variability in survival times for patients, more specifically on the standard deviation of the logarithm of survival time. Using several statistical models of survival, the probability of greater than 2-fold error remains near 50% under realistic assumptions.49
Ultimately, the stochastic nature of heart failure conveys a high level of prognostic uncertainty for most patients. Future events have a certain degree of unpredictability, such that improved understanding of risk tends to add incrementally less prognostic information to existing models. Even a perfect model that includes all possible measurements describes only what has already happened. The trajectory can often be steepened by new conditions or life events, such as myocardial infarction, a serious fall, or the death of a spouse. It is vital to acknowledge uncertainty in discussions about future care.
Need for Accurate Estimates of Risk
Despite limitations of prognostic models, they are generally more accurate than clinical intuition, which is prone to bias. A review of survival predictions among terminally ill cancer patients52 found that physicians consistently overestimated survival, which has been seen in other studies.51,53 For patients discharged from the hospital with advanced-stage heart failure, both physicians’ and nurses’ survival estimates had modest ability to discriminate those who subsequently died from those who lived (with nurses outperforming physicians), but absolute estimates were significantly miscali-brated, again overestimating survival.54 In patients with chronic heart failure, the patient-predicted survival also tended to overestimate survival versus model-based predictions, particularly for younger patients.55 Clinicians need to learn how to leverage objective risk models, while recognizing their limitations and adapting them on the basis of their unique clinical and psychosocial features and serial assessments not generally incorporated into such models.
Anticipation, Timing, and Review
An annual heart failure review with patients should include discussion of current and potential therapies for both anticipated and unanticipated events.
On the day of hospital admission, it is far better to review rather than introduce advanced care decisions, which requires that patient preferences have been discussed previously and documented in the ambulatory setting.
Clinical milestones such as implantable cardioverter-defibrillator (ICD) shocks or recurrent hospitalization should trigger interim review and discussion of treatment options and preferences.
Timing of Discussions
Finding appropriate time to discuss preferences, prognosis, and medical options is a formidable challenge. Such discussions require a major commitment of time, focus, and emotional energy, which is not in synchrony with the frenetic pace and frequent interruptions of clinical practice. Current organizational and reimbursement structures provide strong disincentive to such intense encounters.
As a result, formal discussions about prognosis and decision making are often deferred until more emergent and less favorable occasions, when thoughtful decision making may be impaired. For instance, at the time of presentation for hospital admission with decompensated heart failure, patients are frequently uncomfortable and often require urgent, intensive evaluation and management. Clinicians responsible for delivering care in this setting are typically unfamiliar with the patient and overall disease trajectory. Hasty questions such as, “Do you want us to do everything?” and “Would you want to be kept alive as a vegetable?” can yield inaccurate and conflicting answers. It has been shown that patients deciding resuscitation preferences during an acute hospitalization frequently reverse their decisions over the next few months.56
Therefore, optimal shared decision making requires that patient preferences have been discussed previously and documented in the ambulatory setting. The day of hospital admission is a time to review and possibly update, rather than introduce, advanced care decisions. On the other hand, once the clinical course has become apparent during hospitalization, clinicians can take advantage of the substantial time they have with the patient and family to further address complex medical decisions before discharge. When the expected survival or quality of life is very limited, hospitalization may also afford better access to multidisciplinary teams, palliative care, and other resources than can be marshaled in the outpatient setting. All of these considerations underscore the importance of a proactive, anticipatory, and iterative approach to soliciting patients’ preferences. This should occur both routinely and at the occurrence of milestones that herald a worsening prognosis (Table 4).
Table 4.
Triggers for Formally Assessing Prognosis and Having Conversations About Goals of Care and Voluntary Advance Care Planning
|
ADL indicates activities of daily living; LTC, long-term care; ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin II receptor blocker; ICD, implantable cardioverter-defibrillator; VT, ventricular tachycardia; and VF, ventricular fibrillation.
Annual Heart Failure Review
The “Annual Heart Failure Review” is a concept based on the annual wellness visits that have long been a successful part of primary care. This reflects the principle and practice of “anticipatory guidance,” the psychological preparation of a person to help relieve the fear and anxiety of an event expected to be stressful. When triggered by a scheduled anniversary in the same way as well baby visits or periodic mammography, an automatic annual review can open a broad dialogue with patients and families without the unvoiced concern that it signifies bad news. In heart failure, this may coincide, for example, with an annual influenza vaccination or at 1-year increments roughly originating from the date of diagnosis. It may be convenient to have this review occur in temporal proximity to an annual general medical evaluation, particularly with regard to screening studies, for which the indications might change in the setting of progressive heart disease.
In the annual review visit (Table 5), a variety of tasks could be accomplished. Patients could summarize their recent symptom burden and quality of life. Goals for the coming year and preferences for outcomes including survival, functional capacity, and quality of life could be solicited. A range of prognosis would be estimated and broadly conveyed. Current treatment with drugs and devices could be reviewed relative to indicated treatment based on the patient’s heart failure type, stage, and trajectory. Similarly, evaluation and management of new relevant comorbidities could be reviewed, such as sleep apnea, anemia, and depression. “Voluntary advance care planning,” including formal designation of a healthcare proxy and do-not-resuscitate status, which has been proposed as part of the initial Medicare visit and subsequent “wellness visits,”60(p73406) would be essential and would take place naturally within the context of an annual review.
Table 5.
Selected Components That May Be Included in an Annual Heart Failure Review
|
BB indicates β-blocker; ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin II receptor blocker; AA, aldosterone antagonist; CRT, cardiac resynchronization therapy; ICD, implantable cardioverter-defibrillator; AF, atrial fibrillation; HTN, hypertension; DM, diabetes mellitus; and CKD, chronic kidney disease.
This scheduled review would require considerable face-to-face time between the patient, family, and physician. The results of this discussion should be documented specifically in a designated area of the chart available to all who might be involved in the patient’s current and future care.
Responding to Milestones
Although heart failure is a chronic disease, its clinical course often includes sudden changes. There are several “milestones” in the clinical course of heart failure that can represent an “inflection point” in the overall trajectory (Figure 119,21), such as first ICD shock, rehospitalization, development of cardiorenal syndrome, withdrawal of angiotensin-converting enzyme inhibitors, or intubation. Once the acute condition has been addressed, such events should trigger a focused version of the “Heart Failure Review,” which would include reassessment of prognosis, treatment options, and patient preferences.
Framework of Options
Physicians are responsible for defining the range of options that are medically appropriate.
Presentation of major interventions should always include specific description of alternative approaches, including continuation or withdrawal of ongoing treatments and focus on symptomatic care.
Discussions should include a range of anticipated outcomes, including not only survival but also major adverse events, independence, functional capacity, and quality of life for both patient and caregiver, even if to acknowledge lack of this information for some interventions.
Therapies that may lead to dependence should be weighed carefully before initiation even when anticipated to be temporary (eg, intravenous inotropes, renal replacement therapy, and intubation).
Benefits and risks of noncardiac procedures should be reviewed in the context of competing risks for death and functional limitation attributable to heart failure (eg, hip replacement, repair of asymptomatic aortic aneurysm, or screening tests).
Decisions for major cardiac and noncardiac interventions should include consideration of “what if” situations of unanticipated adversity.
Referral to a palliative care team should be considered for assistance with difficult decision making, symptom management in advanced disease, and caregiver support even as patients continue to receive disease-modifying therapies.
In the face of the increasing complexity of diagnostic and treatment options for heart failure, a framework for classifying various medical decision-making scenarios should help clinicians better anticipate those decisions most likely to occur as the disease progresses to an advanced stage (Table 6). Too frequently, the default assumption is that patients would “want everything done.” Rather, it is the clinicians who are responsible for defining the “everything” set of interventions that are medically reasonable. From these, patients and families can choose those most consistent with their values, preferences, and goals. It is increasingly explicit in quality metrics that those groups or institutions offering specific advanced therapies should include palliative care and access to ongoing care regardless of the therapies chosen.
Table 6.
Framework of Major Medical Decisions in Advanced Heart Failure Faced by Patients and Their Clinicians
| Types of Options | Specific Examples of Interventions | Generally Considered Only for HF With Reduced LVEF | Examples of Uncommon Outcomes That Could Be Anticipated With “What If” Discussions in High-Risk Patients |
|---|---|---|---|
| Major interventions that may improve cardiac function | CABG Valve surgery | Worsened cardiac function/inability to come off bypass or IABP: Place MCS? | |
| Pericardial stripping | Ventilator dependence: Extubate? When? | ||
| Percutaneous valve intervention | Stroke: Feeding tube? Institutional care? | ||
| PCI | Coronary occlusion: Revert to CABG? | ||
| CRT | X | Unable to place coronary sinus lead: Convert to thoracotomy? | |
| Therapies that only reduce the risk of sudden cardiac death | ICD | X | Terminal or permanently disabling disease: Device deactivation? |
| Adjunctive therapies instituted during acute decompensation with potential chronic dependence | Temporary support devices (IABP, percutaneous VAD, ECMO) | X | Unable to wean: Convert to permanent MCS or withdraw? |
| IV inotropes | X | Unable to wean: Transition to home inotropes or discontinue? | |
| Renal replacement therapy (dialysis or ultrafiltration) | Failure of acute injury to resolve: Initiate indefinite hemodialysis or discontinue? | ||
| Advanced surgical therapies to exchange disease | Transplantation | X | Early graft failure or other serious postoperative complications: MCS or withdraw support? |
| Later graft failure: Retransplantation? | |||
| Permanent MCS/LVAD | X | Stroke, infection, or recurrent bleeding: Turn off device? | |
| Noncardiac procedures for comorbidities | Joint replacement Hernia repair | Worsening heart failure causing hemodynamic and/or respiratory collapse: Continue ventilatory support and/or initiate circulatory support? | |
| Resection of pulmonary nodule Asymptomatic aortic aneurysm repair Screening colonoscopy | Not generally to be done, because risks are thought to outweigh potential benefit |
HF indicates heart failure; LVEF, left ventricular ejection fraction; CABG, coronary artery bypass grafting surgery; IABP, intra-aortic balloon pump; MCS, mechanical circulatory support; PCI, percutaneous coronary intervention; CRT, cardiac resynchronization therapy; ICD, implantable cardioverter-defibrillator; VAD, ventricular assist device; ECMO, extracorporeal membranous oxygenation; and IV, intravenous.
Continuation of Stage C Medical Therapies
While anticipating and addressing new options that accompany the transition to advanced stage D heart failure, medical therapy usually includes all stage C therapies.17,61,62 The initial approach to stage D heart failure is optimization of these treatments. The need to decrease or discontinue neurohormonal antagonists is a milestone, as described in Table 4.
Major Interventions That Might Improve Cardiac Function and Clinical Outcomes
High-Risk Cardiac Surgery
Patients may be considered for cardiac surgery for coronary, valvular, and pericardial disease. These surgeries are particularly high risk as a consequence of the patient’s advanced heart failure. These procedures may be pursued either with the hope of improving the heart failure condition or in response to a superimposed diagnosis such as angina or acquired aortic stenosis. It is usually anticipated that there will be substantial residual cardiac dysfunction even if the surgery is successful. Although the intent may be to improve cardiac function, the benefit of cardiac surgery in most patients with advanced chronic heart failure is not established. Even if the cardiac function improves, the surgery and related events may lead to prolonged morbidity and possibly death.63 The potential for protracted postoperative rehabilitation and loss of independence must be considered and included thoughtfully in the shared decision, because surgery inherently increases short-term risk for the prospect of longer-term benefit. Unfortunately, there is limited information about the frequency of these outcomes beyond general estimates of prolonged hospitalization in the Society of Thoracic Surgery risk scores.64
Percutaneous Interventions
Less invasive percutaneous approaches for the treatment of coronary and valvular disease may be appealing in advanced heart failure because of the increased surgical risk among these patients. However, potential benefits depend on a variety of factors and are relatively unknown for the advanced heart failure population; meanwhile, risks of contrast-induced nephropathy65 and 30-day mortality66 are markedly increased in this population. Percutaneous approaches to valvular disease are less well developed than for coronary disease, but the technology is improving rapidly. Catheter approaches to both aortic67 and mitral68 disease have now been shown to be reasonable alternatives to surgery in certain populations. The benefits of valve repair or replacement are less well established in patients with significant heart failure, especially when treating functional (secondary) mitral regurgitation for patients with a dilated left ventricle.69 The acute risk of stroke must be weighed against potential longer-term benefits. Decisions regarding percutaneous interventions should also include consideration of whether emergency “bail out” surgery would be appropriate and feasible.
Pacing Device Therapy
Cardiac resynchronization therapy (CRT) represents a clinical challenge in advanced heart failure. Patients with New York Heart Association functional class IV heart failure have represented a small fraction of patients included in randomized trials of CRT.70 Although there are some reports suggesting that CRT can improve outcomes for patients taking intravenous inotropes, these findings have not been consistent, and the reports have methodological limitations.71,72
Before left ventricular lead implantation or ICD device upgrade, the care team should plan for contingencies. For instance, if the lead cannot be placed transvenously, will there be consideration of an open thoracotomy for placement, with the attendant morbidity of a chest tube and possible recurrent pleural effusion in the setting of chronically elevated right atrial pressures? The risk-benefit ratios of the full complement of possible procedural variations, in the context of patient preferences, should be considered before any procedure so that the patient can provide truly informed consent and a response to potential adverse outcomes is planned a priori. Factors likely to modify the risk-benefit ratio of device implantation, such as noncardiovascular morbidity and acute decompensation, should also be recognized and incorporated into these discussions.73,74
Although CRT pacers and ICDs can be packaged together, their purposes are quite different. CRT, like neurohormonal antagonist therapy, is designed to reverse remodeling and improve cardiac performance. Although CRT has been shown to improve survival, it also can have significant effects on symptom reduction and quality of life in select patients. In contrast, ICDs treat life-threatening arrhythmia without improving symptoms. Patients may not understand the distinction until after the device has discharged or the issue of deactivation is raised. Recommendation for a combined CRT-defibrillator (CRT-D) device should prompt separate discussions on the indications for defibrillator capacity versus cardiac resynchronization, as well as differences in need for monitoring, chances for inappropriate shocks and worsening heart failure, risks for infection and lead malfunction, and options for deactivation.
ICDs to Reduce the Risk of Sudden Cardiac Death
ICDs are fundamentally different than many life-saving therapies for patients with chronic heart failure with reduced ejection fraction. Neurohormonal antagonist medications and CRT improve cardiac function, thereby reducing mortality, reducing hospitalization, and improving quality of life. In contrast, ICDs improve survival by aborting lethal arrhythmias but do not improve cardiac function or heart failure symptoms. Additionally, ICDs can create an additional burden for patients, particularly from inappropriate discharges and prevention of a rapid death. Because ICDs involve this trade-off between reduced risk of sudden cardiac death and an increased risk of hospitalization,75 potential decrease in quality of life,76 and higher likelihood of prolonged death from progressive pump failure, meticulous discussion of absolute risks with and without ICDs are particularly important for informed consent and shared decision making.
Temporary Therapies With Potential Dependence
Some therapies are intended for short-term use to stabilize patients, thereby allowing for recovery from reversible insults or transition to more definitive therapy (ie, cardiac transplantation or permanent mechanical circulatory support). Although initially intended as a temporary intervention, such stabilizing therapies can create indefinite dependence if the patient does not improve as hoped or develops an adverse event (eg, stroke, progressive renal failure) that compromises options for anticipated definitive therapies. Such scenarios are difficult for patients and clinicians and therefore must be anticipated.
Temporary Mechanical Circulatory Support
Short-term circulatory support with intra-aortic balloon pumps or other devices may be initiated when acute or acute-on-chronic hemodynamic instability requires urgent intervention to avoid permanent end-organ dysfunction or death. It may be instituted with the hope of supporting a reversible underlying condition, such as fulminate myocarditis or right-sided heart failure after acute myocardial infarction. It may also be initiated in patients who might be potential candidates for transplantation or permanent circulatory support, in whom (1) there has not been an opportunity to appropriately evaluate their candidacy for more definitive high-dependence therapies, (2) reversibility of end-organ dysfunction is uncertain, or (3) contraindications to more definitive therapies may resolve in the near future. If end-organ dysfunction or contraindications do not resolve, a decision will need to be made about discontinuation. To whatever degree possible, these issues should be addressed before the initiation of short-term support.
Positive Inotropic Agents
Intravenous inotropic agents are commonly initiated in the acute setting for hemodynamic stabilization and to improve end-organ perfusion. Use is most often anticipated to be temporary, with the hope of either clinical improvement or eligibility for more definitive therapies as above. Regardless of intent, initiation of inotropic support for exacerbation of chronic heart failure should be considered a significant clinical milestone (Table 4). When patients fail to wean from intravenous inotropic support, decisions arise concerning its continued chronic use. Therefore, the goals of temporary inotrope use should be established clearly before initiation, and unexpected dependence on this therapy should prompt direct discussions about overall goals of care and the limited range of options available at this juncture.
The decision to arrange for chronic continuous infusions after hospital discharge should be guided by the need for symptom relief and patient preferences. Agreement on the goals of therapy and various “what if” scenarios should be reached before initiation. Nonrandomized data suggest that the number of hospital days may decrease after initiation of chronic inotrope infusion,77 with an increased risk of sudden cardiac death.78,79 However, the majority of patients require hospital readmission after initiation of chronic intravenous inotropic therapy, even if begun with the hope of helping patients to stay at home until death. The use of intermittent infusions to control symptoms is currently not recommended by American Heart Association/American College of Cardiology guidelines (class III recommendation).17
A strategy to continue intravenous inotropic therapy for symptom relief and return home should not trigger implantation of an ICD, unless the patient is awaiting definitive therapy such as transplantation. The majority of patients on home inotropic infusions die by 6 months, and almost all are dead by 1 year, most often of terminal hemodynamic decompensation.
Renal Replacement Therapy
The prevalence of advanced kidney disease increases dramatically with worsening heart failure,80 and measures of renal dysfunction are strong predictors of adverse outcomes in patients with heart failure (Table 3). Conversely, approximately 33% of individuals who commence hemodialysis have a recorded diagnosis of heart failure, and their mortality rates are significantly higher than patients who initiate dialysis without a heart failure diagnosis (adjusted relative risk of 1.22 in the US Renal Data System).81 Dialysis in the setting of advanced heart failure, especially in older patients with other comorbidities or frailty, has been shown to add to patient burden and in high-risk patients may not extend life.82,83 Therefore, the decision to initiate renal replacement therapies (eg, hemodialysis, ultrafiltration) in patients with advanced heart failure should only be made after a clear discussion with the patient about the risks and benefits of dialysis on the patient’s quality of life and prognosis.84
Transplantation and Mechanical Circulatory Support: Exchange of Disease
Cardiac transplantation and mechanical circulatory support offer the potential to fundamentally change the clinical course of heart failure by exchanging it for surgical therapy and the need to adjust to living with a different set of benefits, risks, and burdens. In the case of transplantation, patients must adapt to the risks of organ rejection and immunosuppression and its side effects. For permanently implanted mechanical circulatory support, patients are dependent on a device with major complications of infection and stroke, as well as the potential for continued symptoms and required therapies for right-sided heart dysfunction. Thus, for eligible patients, whether to pursue these therapies represents one of the most difficult decisions that patients and clinicians can make. However, these therapies are limited to a highly selected group of patients. The use of cardiac transplantation is constrained by a limited supply of donor hearts, a situation that will not likely change in the foreseeable future. The use of mechanical circulatory support may increase as the technology improves but is likely to remain inappropriate for the majority of patients with heart failure because of the predominance of heart failure with normal ejection fraction, multiple comorbidities, or very advanced age.15,16 Detailed clinical practice guidelines are available that address the use of these advanced therapies.85-87
Noncardiac Procedures in the Patient With Advanced Heart Failure
The risks and benefits of interventions for noncardiac conditions may be altered significantly in patients with advanced heart failure. When the likelihood of meaningful recovery without the procedure is small, the increase in procedural risk associated with heart failure may be considered acceptable. Examples include both emergent (eg, laparotomy for perforated viscous) and urgent (eg, hip arthroplasty for fracture) surgical procedures. Other procedures, such as knee replacement for degenerative joint disease, must be considered carefully in the context of patient preferences, because complications of the procedure may or may not outweigh the potential benefit. Procedures should be discouraged when they do not offer a tangible improvement in quality of life (eg, repair of asymptomatic abdominal aortic aneurysm). Placement of permanent peritoneal and pleural catheters for the control of volume status is not indicated unless incorporated into a comprehensive palliative plan of care. Similarly, routine preventive care screening tests (eg, mammography, prostate-specific antigen) are typically not appropriate in the context of a significant competing risk of mortality caused by advanced heart failure, yet such tests are frequently ordered at the end of life.88
Anticipating Decisions for Unanticipated Events
The process of clarifying preferences for cardiopulmonary resuscitation, intubation, feeding tubes, implantable defibrillator deactivation, intensive care unit transfer, and other near end-of-life interventions before the occurrence of a near-terminal event or acute-on-chronic decompensation is an important aspect of shared decision making. There is literature on the type and scope of these discussions,89,90 with suggestions to make them an annual event in routine medical care.60(p73406) Such anticipatory discussions should include advance care planning guided by the concept that it is proper to “plan for the worst while hoping for the best.” It is also crucial for the care team to make clear that clinician abandonment will not occur; that is, the clinicians will work with the patient and family in downstream decision making and management.
Palliative Care
Palliative care is interdisciplinary care aimed at improving quality of life for patients by preventing and relieving suffering and supporting families.91 As such, it can be offered simultaneously with all other appropriate medical therapies. Palliative care is not synonymous with end-of-life care or hospice but can encompass them as the disease advances. Palliative care allows for continued disease-modifying therapies while ensuring symptom relief and interventions that address psychosocial, physical, and spiritual needs. This is done in 2 ways: by treating symptoms and by ensuring that patients ’ treatment plans match their values and goals.92-94 The process of shared decision making is a central tenet of palliative care: that the patient and clinician reach an understanding about preferences for life-prolonging therapy, symptom relief, pain control, and end-of-life care. Unlike hospice care (“Use of Hospice Services”), the application of palliative care is based on patient need rather than patient’s prognosis or life expectancy.
Although data on palliative care in patients with heart failure are limited, several guidelines and reviews recommend integration of palliative care for all patients with advanced heart failure.19,89,90 This can and should be done by all clinicians involved in the care of these patients. However, referral to a palliative care team should be considered for assistance with difficult decision making and refractory symptom management in advanced disease, even as patients continue to receive disease-modifying therapies. Palliative care teams can consist of physicians, nurses, social workers, chaplains, and other professionals who work to ensure that patient and caregiver needs are assessed and met. Because of the complex and changing nature of heart failure and the complexity of conversations as they change according to the patient’s underlying heart disease, it is important to integrate palliative care into the care of patients with heart failure before they enter stage D. Even as patients are being considered for transplantation, mechanical circulatory support, or trials of novel therapeutics and pharmacological agents, palliative care can be increasingly integrated to ensure that patients’ symptoms are appropriately controlled and that patients understand the nature of these interventions, as well as the full complement of alternative therapies.95,96 The synergistic relationship between palliative care services and the heart failure team for patients with mechanical circulatory support has been reviewed recently.95
End-of-Life Care Planning
Clinicians should take responsibility for initiating the development of a comprehensive plan for end-of-life care consistent with patient values, preferences, and goals.
Deactivation of an ICD is desirable to avoid unnecessary pain and distress for patients and families at the end of life.
Active discontinuation of mechanical circulatory support is often appropriate and necessary at the end of life.
Planning for Anticipated Death
Although the prognostic uncertainty inherent in heart failure makes it difficult to accurately anticipate the end of life, some patients enter a terminal phase of the disease that may be relatively apparent to the patients and/or their clinicians. In such situations, when the goals of care often transition from a focus on survival to quality of life and ensuring a good death, clinicians should take responsibility for initiating the process of putting into place a comprehensive plan of care consistent with patient values, preferences, and goals.
Passive Withdrawal of Therapies: Deactivation of ICDs
The option and ease of ICD deactivation should be discussed before implantation and again for major changes in clinical status (Table 4) or transitions in goals of care.97 At present, this is done only rarely, thus leaving many patients vulnerable to inappropriate device discharge and unnecessary suffering. A recent survey found that only 1 in 4 next of kin reported that a physician had discussed device deactivation with their deceased family member before death.98 In a nationwide survey of 734 physicians, including 292 cardiologists, 60% had fewer than 3 experiences discussing deactivation of ICDs with patients and/or families.99 Concordant with those findings, a national survey of hospices found that <10% of hospices have a policy regarding deactivation of ICDs, and >50% of hospices had at least 1 patient who had been shocked within the past year.100 For a device near its end-of-battery life, the generator should not be changed without careful review of whether or not active defibrillation is consistent with overall goals of care and anticipated duration of good-quality survival.
Active Withdrawal of Therapies When Patients Are Dependent on Them
Although the legal construct of patient autonomy does not recognize different degrees of dependence on therapies to be withdrawn, clinicians, patients, and families may view scenarios in which withdrawal leads to direct and rapid patient demise as unique and emotionally difficult. Examples include withdrawal of renal replacement therapy, feeding tubes, or pacemaker support for patients dependent on cardiac pacing. An increasingly common scenario is the withdrawal of mechanical circulatory support devices, either temporary or durable, in patients who are not expected to recover to return to a quality of life they consider acceptable.95 The average life span of a patient after implantable left ventricular assist device (LVAD) placement has been increasing over time,101 but morbidity and mortality remain high. Estimated actuarial survival in the HeartMate II destination therapy trial was 58% at 2 years.102 With improvements in medical technology and associated outcomes, patients maintained with mechanical circulatory support may not only be susceptible to death attributable to cardiovascular causes but to other life-limiting disease as well. The discussion about discontinuing device therapy should be part of the “what if” informed consent process before implantation. Subsequently, although there are no specific recommendations to direct when these therapies should be discontinued, it appears that declining quality of life, signs of other organ system failure, or an irreversible catastrophic adverse event such as a major stroke or hemorrhage should trigger serious discussions about device deactivation. In a recent small study of characteristics of patients for whom mechanical circulatory support was electively discontinued, the most common triggers for discontinuation included sepsis, stroke, cancer, renal failure, and impending pump failure.203 Despite the perception that LVADs may impose special ethical dilemmas,91 the Patient Self-Determination Act still broadly applies, giving the patient, or their surrogate decision maker, full autonomy to withdraw support.
Device deactivation can be performed in the hospital or at home, attended by a device-trained individual (ventricular assist device coordinator) and others as requested (hospice nurse, chaplain, etc). Before device deactivation, a discussion with the patient (if able to participate) and family about the patient’s current condition and prognosis, changes in device benefit profile, how the device would be stopped, how symptoms would be managed, readiness to proceed, and anticipated outcome (ie, rapid death) is valuable. Unlike ICDs, which can be deactivated without immediate effect, LVAD discontinuation can result in rapid decompensation and expedite death, particularly with valveless continuous-flow devices. The average time to death after device deactivation is approximately 20 minutes, which indicates that a thoughtful discussion and careful plan should be in place well before the device is discontinued.71 This clinical scenario has been likened to withdrawal of endotracheal intubation and ventilatory support, although patients with LVAD support are more likely to be awake and alert at the time of decision to discontinue support. If patients are on multiple forms of support (eg, mechanical ventilatory support and LVAD support, with an ICD also in place), a coordinated plan to discontinue all of these therapies simultaneously is needed.
Use of Hospice Services
For patients approaching the end of life, hospice may be a viable option to provide symptom care and supportive services for patients and their families, while also ensuring that patients are able to die in their preferred environment. To be enrolled in the Medicare hospice benefit, 2 physicians or a physician and a nurse practitioner (one of whom is often the hospice medical director) must certify that the patient has ≤6 months to live if the disease follows its usual course, and the patient must be willing to forego usual medical services aimed at curing the underlying terminal diagnosis.104 Most private insurers have a hospice benefit similar to that provided under Medicare. Although hospice is provided in a variety of environments,105 it is most commonly provided for patients at home with the goal of keeping them in their home until death. Hospice can offer a number of benefits to enrollees and their families, including interdisciplinary team management, home visits, respite care, and provision of medications and durable medical equipment. Hospice also includes a nurse who can always be contacted to advise on urgent symptom needs and provide reassurance that interventions are appropriate.
Customized care plans may provide comfort and relief for some patients unwilling to accept formal hospice support. In many cases, patients feel they are “not ready for hospice,” and these patients should be referred to palliative care to ensure expert control of their symptoms, as well as support for the family. Likewise, continued education about the benefits of hospice and the fact that families are often more satisfied with hospice care than care provided in the hospital may also help elucidate its benefits.106 One study of Medicare beneficiaries with heart failure who received hospice demonstrated a longer survival (by 81 days) than for those heart failure patients who did not receive hospice.107
Hospice services have been shown to improve patient and family satisfaction with care. Families of those dying with hospice services were more likely to rate their dying experience as “favorable or excellent” than those who died in an institution or at home with only home health services.106 Fewer than half of all patients with heart failure receive hospice. This is, however, a marked increase from <20% of heart failure patients being enrolled in hospice a decade ago.108 Appropriate timing of referral to hospice is important, because the family’s perception of being referred “too late” is associated with greater dissatisfaction and unmet needs.109
Communication and the Decision-Making Process
Trust is the basis for the collaborative shared decision-making process.
Early solicitation of values, goals, and preferences is necessary to guide the range of possible therapy options and decisions.
Shared decision making is an iterative process that evolves over time as a patient’s disease and quality of life change.
Assessment and integration of the emotional readiness of the patient and family are vital to effective communication.
Ask-Tell-Ask provides a useful framework for communicating about prognosis and goals.
Successful conflict resolution involves early recognition of conflict unfolding, with a shift in focus from winning an argument to trying to understand the reasons for conflict.
Decision aids are tools that can enhance shared decision making by presenting numeric data in more understandable ways and assisting patients in clarifying their values.
Most patients and families want accurate and honest conversations with their clinicians.110,111 One study found that 93% of surrogate decision makers felt that avoiding discussions about prognosis was unacceptable.110 Advanced heart failure, with its high degree of prognostic uncertainty and complex trade-offs in the choice of medical care, demands a thoughtful approach to communication and decision making. Ideally, these interactions are not 1-time events but occur as an evolving series of discussions over time, particularly as a patient’s condition changes. Such interactions may be difficult and time consuming, and they often require planning to create a supportive environment for effective communication. These discussions require careful attention to both mental and emotional needs. Clinicians must determine how much quantitative information patients want about prognosis, comparative risks, and benefits for both length and quality of life with available therapies. At the same time, clinicians must attend to the emotional nature of conversations with patients to build trust, clarify core values, and allow for sharper focus on the information. Here, we provide an overview of the tasks and skills, along with sample phrases, that can create high-quality shared decision making (Table 7).
Table 7.
Core Tasks, Skills, and Sample Phrases to Improve Clinician-Patient Communication in Advanced Heart Failure
| Steps in the Roadmap | Elements of the Step | Sample Phrases |
|---|---|---|
| Establish the setting and participants | Determine who should be present and ensure that all appropriate clinicians are present as well | “In preparation for our meeting tomorrow, I’m going to have the cardiothoracic surgeon there to be a part of our conversation. In terms of your family or support network, who is it important that we make sure is there?” |
| Determine what patients know and want to know | ASK what patients/families know | “Tell me about your heart disease; how have you been doing lately?” |
| “What is your understanding of what is occurring now and why we are considering the treatment that we have been discussing?” | ||
| ASK what patients/families want to know | “Sometime patients what to know all the details, whereas other times they just want to know a general outline. What kind of person are you?” | |
| “How much information would you like to know about what is happening with your heart disease?” | ||
| TELL the patient/family the information in a sympathetic and thoughtful manner while also clearing up any misconceptions or unanswered questions | “I think you have a pretty good understanding of what is happening with your heart, but there are a few points I’d like to review and clarify” | |
| ASK the patient or family to repeat back the information that has been delivered | “Now that I’ve clarified a few things about your illness, I want to make sure you understand what I’ve said. Tell me in your own words what we’ve been talking about” | |
| Establish goals and preferences | Use open-ended questions to gain understanding of the patient’s values to determine what is most important to them | “Help me to understand what is important to you. Some patients say they want to live as long as possible, regardless of quality of life. Sometimes patients tell me they are worried that they will be in a great deal of pain or have other uncontrolled symptoms. What is important to you at this point in terms of your health care?” |
| “What are you hoping for?” | ||
| “What is important to you now?” | ||
| “What is your biggest concern right now?” | ||
| “When you think about the future, what are the things you want to avoid?” | ||
| In cases in which the patient is not involved in the conversation, a useful phrase might be, “What would your loved one say right now if he or she were hearing what we are discussing?” | ||
| Work with patient and family to tailor treatments and decisions to goals | Tailor explanation of benefits/burdens of a particular therapy based on goals established | “I think I understand what is important to you now, and it helps me better explain to you the decisions and treatments at hand now. I’d like to take a moment to review the benefits and burdens of each of the treatments based on what you’ve said is important to you at this point” |
| Be willing to make a recommendation based on the patient’s goals | “Would it be helpful if I made a recommendation based on what you’ve said the overall focus of care should be now?” | |
| “Based on what you have told me, if you get sicker and need to go back on a breathing machine again to stay alive, that is very unlikely to provide the kind of life you want to lead. Therefore, I think you should not go back on those machines” | ||
| Acknowledge that there is uncertainty in the course of heart failure | “One of the most difficult things about heart disease is that we can never know for sure exactly what will happen in the next (hours, days, weeks, etc). We must make our best guess and decide what to do based on that information. If things change, we can always readdress this discussion at any time in the future” |
Communication Is Desired, Beneficial, and Dynamic
Open, clear, and accurate communication with patients with heart failure is important for several reasons. First, the majority of patients with serious illness want information about their illness and to be included in the decision making process.116-118 Second, when clinicians have conversations with patients about their prognosis and desires, patients are more likely to receive care that is aligned with their goals and preferences.119-121 These conversations also improve the patient-clinician relationship.122 Finally, when conversations occur, families of deceased patients have better outcomes in terms of the manner in which they cope with loss of their loved one, as well as their own psychological outcome.110,121,123 One randomized trial of an advanced care planning intervention demonstrated that the intervention increased the likelihood that the patient’s preferences were known and followed (86% versus 30%, P<0.001) and decreased family members’ stress, anxiety, and depression.121 Although many of the data about benefits of communication in patients with advanced illness are from the field of oncology, the evidence base demonstrating similar results in cardiology is increasing.124
The biological reality of heart failure makes communication particularly difficult for 2 reasons. First, heart failure is characterized by unpredictable periods of acute illness, followed by improvement in symptoms and function (Figure 1).19,21,125,126 Attending to this uncertainty involves both acknowledging the cognitive aspect of the conversation (eg, explaining to patients and families the unpredictable nature of illness and recognizing the inability of modern medicine to accurately predict life expectancy), while simultaneously addressing the complex emotions associated with the “roller coaster” of heart failure (eg, fear, anxiety, and uncertainty). Second, the chronic nature and unpredictability of heart failure require that communication be viewed as an evolving series of dynamic conversations that take into account the overall goals of the patient and family, the current state of heath, and the shifting balance between benefits and burdens of any treatment or test that is either currently being used or that is being considered. Patients’ preferences may change over time as their illness progresses and their experience with the disease changes, which further underscores the importance of an ongoing dialogue with patients and their families.39,56,127 For example, in 1 study of 936 patients with advanced heart failure, 19% had changed their preferences for resuscitation within 2 months.56
To communicate effectively, clinicians must both determine and then readdress over time patients’ understanding of their heart failure and their goals and treatment preferences (Table 4 for timing) and then determine how to have these conversations within the scope of clinical care. For example, when a patient is being seen for their first office visit after a hospitalization for heart failure, it may be useful to readdress goals of care from a global perspective, asking how the patient’s thoughts about his or her heart disease have changed since the last hospitalization. In the case where emergent decision making is needed, the clinician might acknowledge how the conversation was last addressed and then bring up the specific emergent decision(s) at hand.
A Roadmap to Guide Conversations
Physician training in the conduct of these discussions is limited and needs to be fortified.128,129 Furthermore, the work of shared decision making belongs not only to physicians but to other members of the healthcare team as well, specific to their roles and responsibilities. Although additional training and mentored experience are clearly desirable, this section provides an introductory roadmap of how to effectively communicate with patients with advanced heart failure to facilitate the shared decision-making process. It is meant to represent an idealized version of communication, with the realization that this must be balanced with other competing responsibilities and clinicians’ limited time. The goal is to offer effective strategies to improve conversations and decision making by demonstrating how complex conversations can be broken down into discrete elements, making them easier to accomplish. Not all conversations will include all of these elements every time. The goal is to offer a simple outline (along with some helpful phrases and tools) that may make conversations simpler for the busy clinician. More comprehensive explanations about how to communicate with patients with advanced illness are available.94,112,130
Where Are We on the Road? Ask-Tell-Ask to Determine What Patients Know and Want to Know
Before one can embark on conversations with patients and their families, it is important to establish the right context for the conversation. This includes asking whether the patient wants to have the conversation by themselves or would like other individuals present, remembering that patients often define family in a myriad of ways. Creating the right setting also involves ensuring that the right clinicians are present, or at least have been consulted, before the conversation begins. The individual leading the meeting ideally will have spoken to all the clinicians involved in the care of the patient so all points of view are represented and everyone is “on the same page” in terms of the illness and timely decisions.
Begin by asking the patient and family what they know and want to know. In this system, often called Ask-Tell-Ask,113,131 the clinician begins by asking patients and their families both what they know about their disease or the treatment being considered and how much information they want. Nearly 80% of patients want information about their illness, and this number rises as patients’ disease progresses. A Cochrane review of decision-making trials demonstrated that as patients learn more about the risks and benefits of therapies, the proportion preferring to take an active role in decision making increases to 85%.132 However, the only way to assess patients’ wishes is by asking and providing the patient this locus of control, which generates trust that is essential for collaborative decision making. An explicit way to ask is, “Would you want to know everything about your illness or the treatments we are considering, even if it wasn’t good news?” When patients and families express they do not want certain information, this should be explored further, with the explanation that information may be helpful for improving their understanding and to make sure that the decisions are consistent with the patient’s wishes. Denial and other defense mechanisms should not be ignored but instead carefully addressed and managed (“Emotional Roadblocks” below).
Once basic expectations for information exchange have been established, clinicians convey information to the patient and family in a clear and thoughtful manner, while also clearing up any misconceptions or unanswered questions they might have. This is the “Tell” in Ask-Tell-Ask. It is important to initially focus on the larger picture of the patient’s health, because the ability to cognitively hear information, particularly in stressful situations, is limited.133 Giving all of the medical details may easily overwhelm the patient and may also lead him or her to focus on details that ultimately are not critical. The information should be delivered in simple language with frequent pauses to assess patient and family understanding.
The last “Ask” of the “Ask-Tell-Ask” process involves asking the patient or family to repeat back the information that has been delivered, to assess their understanding. This allows the clinician to determine the level of understanding the patient and/or family have and clarify any elements that may remain unclear in their minds.
The Ask-Tell-Ask technique is meant to be iterative and can be applied to many different levels of the process of communication. For increasingly complicated treatments and situations (eg, destination-therapy mechanical circulatory support), Ask-Tell-Ask is likely to be an extensive recurrent process that will occur over multiple encounters with the patient and family. Particularly complicated decisions may be augmented with decision aids (below).
Where Does the Patient Want the Road to Go? Establishing Values, Goals, and Preferences
One of the core elements of good communication is that it assesses patients’ values, goals, and desired outcomes, thus allowing treatments and care to be as closely tailored to those desires as possible. This is especially true in light of the fact that physicians are often wrong about patients’ desires for care.56 Optimal communication with patients with advanced heart failure does not begin with questions about treatments. Asking a patient during a routine office visit, “Do you want us to try and restart your heart?” is unlikely to be an effective starting point. This task is especially difficult because it involves weighing desired outcomes that may be contradictory (eg, avoiding severe disability while maximizing survival). This step then not only outlines what the patient hopes for but also considers complex trade-offs and situations the patient might consider a “fate worse than death.”
Open-ended questions to gain insight into the patient’s life and values are a useful method for initiating this portion of the conversation. Examples include, “What is important to you now?” or “What are you hoping for?” Another technique involves asking patients to discuss what is important in life outside of the hospital; this helps the clinician to understand what patients are doing in day-to-day life, how much patients value those tasks, and how patients view those tasks in the future. It also may be useful to inquire about a patient’s worries or concerns, using questions such as, “What is your biggest concern right now?” or “When you think about the future, what are the things you want to avoid?” In cases in which the patient is not involved in the conversation, either because of illness severity or because the patient chooses not to take part in the conversation, a useful phrase might be, “What would your loved one say right now if he or she were hearing what we are discussing?”
When advanced heart failure patients discuss their goals, they often discuss quality of life; that is, they typically are not only concerned about how long they will live but also how well they will live. This is especially true given that poor quality of life (limited ability to perform daily activities, significant symptom burden, emotional distress, and social isolation) is often reported by patients with advanced heart failure.42,134,135
After clarifying the patient’s goals, it is often useful to summarize what has been expressed. In addition to ensuring that the clinician has heard and understood these hopes correctly, doing this also demonstrates care for the patient and that the clinician is attending to their needs. This may start with phrases such as, “Let me see if I understand what you are saying.”
Ensuring the Road Is Aligned With the Desired Destination: Tailoring Treatments to Goals
After goals have been clarified, the conversation can then move to discussing the role of specific treatments within the context of the desired outcomes. This involves working with the patient and family to (1) summarize the range of medically reasonable treatments for this particular patient at this particular time and then (2) explain the risks and benefits of each treatment option within the personalized rubric of goals and desires set forth by the patient and the family. Working within this context, the clinician helps the patient understand which treatments are most appropriate or inappropriate, based on their likelihood of getting the patient to the desired outcome. This is, in fact, the core of shared decision making: The clinician does not dictate treatments, nor does the burden of the decision rest solely with the patient and family. Instead, the 2 parties work together to determine which options or treatments make the most sense given the patient/family’s desired outcomes in the context of the current clinical scenario. In some cases, patients or families may be able to come to a decision on their own once the treatments and probable outcomes have been presented. However, even in these cases, families report they want to know what the physician would recommend.136 In other cases, the patient and the family may signal they want more guidance (eg, “What would you do if it were your mother?”) In these cases, it is appropriate to offer a recommendation based on the patient’s stated goals (eg, “Given what you have told me about what is important to her, I think the treatment that makes the most sense to get her to the desired goal is ….”). Uncertainty about outcomes of specific treatments and outcomes should be communicated honestly and openly with patients and their families.
Tips for Navigating Barriers
These complex conversations with patients and families face serious challenges and barriers. This section outlines some of those challenges to inform busy clinicians and increase their awareness of them. Whenever possible, solutions and approaches with which to navigate these potential roadblocks are offered. These elements may be encountered (or solutions utilized) at any point in the decision-making process and thus operate in conjunction with the roadmap described above.
Acknowledging Emotional Roadblocks
Difficult decision making can stimulate complex emotions. Engaging patients in selecting treatments aligned with their informed goals and values requires that clinicians not only present the options clearly but that they also be attentive to patients’ emotional needs. Patients are ill, caregivers are exhausted, and there may be a tremendous amount of fear, anxiety, stress, and perceived loss of control.137 Neuropsy-chological studies have shown that when people are emotionally reactive, cognitive information is not processed accurately.130,133 One small study of family members of patients in the intensive care unit showed a relationship between communication styles and rates of anxiety in caregivers.138 Attending to patients’ emotions may improve their ability to process cognitive data and make better decisions. In addition, responding empathetically has been show to strengthen the patient-clinician relationship, increase patient satisfaction, and make patients more likely to disclose future worries.139
The first element of developing an emotional language for conversations is to recognize that patients are having an emotional reaction to the news that is being delivered and then learning to address the emotional content of the conversation. In one study of communication with family members of patients dying in an intensive care unit, clinicians missed opportunities to respond to the emotional content of the conversation in 29% of the conversations.140 Although at times, patients’ emotional reactions may be clear (especially when they use words such as “scared” or “angry”), at other times the exact emotional content of the discussion may be more veiled, such as when a patient or family says, “I don’t know if I can handle this anymore.” If patients or families keep raising the same issue repeatedly during a meeting, it may indicate that they are having a reaction to an issue that is so strong that it interferes with their ability to process information. For example, when patients ask a question such as, “How did this happen?” it may often be a clue that there is an emotional component to the information and that what is needed is a query about the patient’s emotions and not a cognitive response explaining the cause of a disease or a complication. Addressing the reaction of patients and their families to serious illness is a metric that has been proposed to measure the quality of palliative care programs.93
Once the clinician has recognized that there is an emotional component to the patient’s or family’s reaction, the next essential skill is to respond to it. Although there are data demonstrating that as little as 40 seconds of empathetic comments in conversations can improve patient and family outcomes related to communication, clinicians often need assistance in finding the right words to express their empathy for patients in the course of these complex conversations.141,142 One useful mnemonic device that can help clinicians respond empathetically in conversations is the mnemonic N-U-R-S-E.141 As explained in Table 8, NURSE stands for Naming the emotion expressed in the conversation, demonstrating Understanding of the emotion, Respecting the emotion displayed by the patient or family, Supporting the patient/family, and Exploring the emotion in the context of the discussion. This assists clinicians in demonstrating verbal empathy and ensures that the complex emotional components of the conversation are addressed. More comprehensive reviews regarding the importance of acknowledging patient and family emotions have been published.143,144
Depression and Anxiety
Depression and anxiety are common in patients with heart failure, with prevalence rates ranging from 13% to 77% and from 50% to 70%, respectively.145-147 Furthermore, prevalence rates for depression are almost 4-fold higher in patients with New York Heart Association functional class IV versus class I heart failure and also vary by self-reported symptom severity and health status.148 These indicators of mental health status may affect the decision-making process. Depression is associated with impaired cognition and so can interfere with processing of information, memory, and executive function, which can affect decision making, especially in older adults.149-151 Anxiety, as noted above, can alter processing of information because of emotional reactivity. Thus, in patients with advanced heart failure, these barriers to decision making need to be identified and addressed to enhance discussions about therapeutic options. Screening for depression and anxiety, followed by pharmacological and nonpharmacological interventions (including psychological and/or psychiatric consultation), may be appropriate.
Limitations of Cognition, Literacy, and Numeracy
Current evidence suggests that in general, patients have a poor understanding of their medical interventions and that their preferences are not driving decisions.7 A survey of 3010 adults revealed that people were relatively unaware of the risks of 9 common medical conditions. For example, of patients taking a cholesterol medication, 38% did not know that the treatment was lifelong, and 83% could not correctly identify the most common side effect (muscle pain).152 In the case of ICDs, more than half of patients overrated the benefits of ICD therapy by 500%, thinking that >50 of 100 lives would be saved by the ICD therapy over the next 5 years (the actual estimate is closer to 5–10 per 100).153 In the case of elective percutaneous coronary intervention, patients significantly overestimated the benefits; one study demonstrated that 88% of patients believed that percutaneous coronary intervention would reduce the chance of a recurrent myocardial infarction and 82% believed that it would reduce mortality,154 despite clinical trial data that elective percutaneous coronary intervention has no effect on recurrent myocardial infarction or mortality.155 Another study demonstrated that fewer than half of the patients could recall at least 1 complication of percutaneous coronary intervention despite the fact that most of them expressed a strong interest in participating in decision making. Together, these data suggest a need for improvement in the decision-making process.156
Cognitive impairments compound difficulties with communication, comprehension, and decision making. Mild cognitive decline is seen in 25% to 50% of adults with heart failure.157 A recent comparison of heart failure patients, healthy participants, and medical patients demonstrated that heart failure patients had poorer memory, psychomotor speed, and executive function than the other participant groups.158 Almost one quarter of the heart failure patients had deficits in ≥3 domains of neuropsychological functioning. Those patients most likely to experience cognitive decline were those with the worst heart failure severity.
Limitations in health literacy and numeracy further interfere with understanding and integration of the information discussed as it relates to decision making. Health literacy is the degree to which people have the capacity to obtain, process, and understand the basic health information and services needed to make appropriate health decisions.159 Nearly 10% of the population is functioning at below basic literacy levels.160 Those most likely to be in the below basic level had less than a high school education, spoke no English before starting school, or were Hispanic, black, ≥65 years of age, or had multiple disabilities. Only 12% of the US population had the skills needed to manage their own health care proficiently.161
Not surprisingly, low health literacy is associated with poor self-care162-164 and increased mortality in older adults with chronic illnesses such as heart failure.165-167 To offset these poor outcomes, a recent scientific statement from the Heart Failure Society of America specifies that clinicians must recognize the consequences of low health literacy, screen patients at risk, document literacy levels and learning preferences, and integrate into practice effective strategies to enhance patients’ understanding.168 Furthermore, communicating numeric risk in a graphic form has also been shown to improve comprehension among patients with difficulties in literacy and numeracy132 (“Patient Decision Aids” at http://decisionaid.ohri.ca/169 for examples).
Family Dynamics
Family dynamics can be a barrier to negotiating goals of care. A recent survey of elders (mean age 83 years) and their adult children (mean age 53 years) revealed that although most family units had discussed end-of-life preferences, important barriers to such discussions included fear of death, trust in others to make decisions, family dynamics, and uncertainty about preferences.170 Those factors that facilitated the discussion were acceptance of the reality of death, prior experience with death, religion or spirituality, and a desire to help the family. In addition, there are significant psychological burdens associated with surrogate decision making.170 Interestingly, previous discussion with their loved one about goals appeared to mitigate these burdens, providing an additional reason to raise these issues with both the patient and family early in the course of the illness.
Culture and Religion
Cultural and religious differences in patient preferences are known to exist.171 Awareness of cultural and religious differences can facilitate understanding of patient choices when discussing treatment options, especially when patients decline evidence-based therapies that healthcare professionals perceive as offering more benefit than risk. Although clinicians are not expected to be experts in cultural or religious issues relating to decision making, it is important that they be aware of the influence of these elements on decision making. Conversely, clinicians should also be warned against making assumptions about patient preferences based on perceived cultural or religious expectations. Clinicians should speak candidly with patients using the strategies recommended. Referral to palliative care, chaplaincy, or social work services may help reveal existing religious and cultural differences as they relate to the decision-making process.
Language Differences
According to the US Census Bureau, the population speaking a language other than English at home has increased steadily for the past 3 decades.172 The number of languages and dialects spoken across the country presents major challenges to clinicians seeking to have a meaningful conversation about therapeutic options and end-of-life issues. Even among those patients who have learned English, when English is the second language, the subtleties and nuances of a discussion may be missed. Interpreters are often needed, which can be challenging in terms of finding someone linguistically capable, available, and sufficiently sensitive to communicate both the content and the tone.173 Often, clinicians rely on family members to interpret for them, but family members have their own needs and emotions surrounding these conversations, which makes their use as interpreters potentially problematic.174
Time
One of the largest barriers to these conversations is the time-intensive nature of these conversations, coupled with the time constraints faced by both patients and clinicians in a busy medical setting. Currently, clinicians can bill solely on the basis of time when >50% of the encounter (inpatient or outpatient) is spent on counseling the patient/families or coordinating care. Recent efforts aimed at making it easier for providers to be reimbursed for conversations about goal setting were ultimately abandoned for a variety of reasons.175 Because of the complexity of these conversations, however, realignment of incentives to encourage providers to have these conversations and improve their skills for doing so is necessary and vital.
Resolving Conflict
In some cases, an intervention desired by a patient may appear discordant with the patient’s stated goals and/or medical realities, and clinicians must explain why it is not warranted. This is particularly difficult in our national culture of entitlement and denial of morbidity and mortality. Clinicians must work with patients and their families to explain why a particular treatment is inconsistent with the overall goals of care, using patients’ preferences as a rubric for why the treatment is not appropriate. These discussions can be emotionally charged and adversarial and may require considerable time. Given both the complexities of these conversations and their considerable length, formal involvement of palliative care teams has been shown to improve patient and caregiver satisfaction, effectively control symptoms, and decrease costs for patients with advanced disease.176-179
There are 3 key elements that characterize discussions about not providing a therapy. First, the emphasis of the conversation should be on what treatments will still be provided that will help accomplish the patient’s goals. This ensures the patient does not feel abandoned. Second, one should attend to the emotion behind the request. For example, “I wish I could tell you that doing [specific treatment] will accomplish the goals that you have outlined, but I’m sorry to have to say that it will not.” In general, the “I wish” statement can be beneficial in terms of acknowledging the emotional impact on patients of no longer having options for various therapies (ie, “I wish things were different”). Third, sometimes the patient or family has either misheard or misinterpreted the data that was presented, so it may be helpful to clarify what further information is needed to reconcile any inconsistencies (eg, “Tell me more about how you think CPR would help you”). By asking patients to clarify their reasoning, the clinician can more effectively address misunderstandings and inconsistencies. In this manner, the clinician attends to the emotions to better understand what is behind the request and then uses the NURSE strategy to acknowledge the emotion, rather than creating conflict with patients and their families about the treatment itself.
Decision Support to Assist With Particularly Difficult Conversations
In many cases, the decision at hand may be particularly complex or may require assistive methods to help patients and caregivers understand the potential outcomes and risks. In these cases, a decision support intervention, such as a decision aid or a decision coach, can help enhance conversations between patients and clinicians.
“Decision aids” are tools that help patients and caregivers become involved in decision making by providing information about the options and outcomes and by assisting patients in clarifying their personal values.10,180 Decision aids come in various forms, including booklets, pamphlets, videos, and Web-based systems,169 and are designed to complement, not replace, a clinical encounter. They can be conceptualized broadly as either aids to assist the patient during or independent of the face-to-face encounter.10 A key difference between decision aids and a simple information pamphlet is that decision aids do not simply provide data about the anticipated risks and benefits but also provide guidance to help patients clarify their personal values and make a decision.181 Decision aids can also help patients clarify their values through a simple pros and cons list or an “imagined future” exercise.182 Decision aids attempt to present probabilities of the risks and benefits in ways that patients can understand. In fact, recent innovations have included the calculation and presentation of patient-specific outcomes generated from multivariable models (such as those listed in Table 3, among others), in routine clinical care. In the setting of informed consent for angioplasty, such a tool was demonstrated to improve patients’ understanding of the risks of treatment, decrease anxiety, and improve satisfaction.183 As new models are created that estimate a broader range of outcomes that are important to patients, this concept can be further developed, tested, and applied in advanced heart failure. A Cochrane review of 55 randomized trials of patient decision aids demonstrated that decision aids improved patient knowledge, reduced decisional conflict, increased patients’ participation in decision making, and reduced the number of people remaining undecided with no associated adverse health outcomes.184 However, only 1 trial in this Cochrane review was related to ischemic heart disease, and none were related to heart failure. The fact that substantial evidence suggests that decision aids help patients make better decisions combined with the fact that patients with heart failure face a multitude of complicated decisions indicates that this is an area in need of significant development and research. The work on developing decision aids should begin with the higher-stakes decisions, including use of ICDs, inotropes, LVADs, and transplantation.
An alternative model to decision aids is the “decision coach,” a trained professional, often a nurse, who assists patients in making medical decisions by helping them prepare for a consultation and by empowering them to ask questions of their provider.185 Early research suggests that coaching interventions may have modest effects on knowledge and participation in decision making.186 The Ottawa Decision Support Guide is available for download and can be used for decision coaching (http://decisionaid.ohri.ca/decguide.html).169 Although the use of coaches has not been studied in patients with advanced heart failure, nurses and other providers working with heart failure patients could be trained in decision coaching techniques.
Directions for the Future
Changes in organizational and reimbursement structure will be necessary to reward and integrate decision making into the delivery of patient-centered health care.
Mechanisms for standardizing, integrating, and distributing the work of shared decision making among the healthcare team should be developed and evaluated.
Research to better define comparative functional and quality-of-life outcomes for the major therapies is vital to truly inform and align decision making with patient goals.
Caregiver burdens and expectations should be assessed for all therapies and included as components of decision making.
Content and form of decision aids should reflect contemporary outcomes data and interactive technology.
Skill sets for communication and shared decision making should become part of standard curriculum and training for providers.
Future research is needed in a variety of areas related to shared decision making, including effective communication training, decision support interventions, group visits, health-related quality-of-life measures, and caregiver burden, needs, and outcomes.
Need for Structural and Reimbursement Changes to Emphasize Shared Decision Making
Many busy clinicians may dismiss the above recommendations as impractical given the considerable time needed to complete the detailed communication processes outlined above. The diverse tasks of physicians, nurse practitioners, and physician assistants involved in primary care, general cardiology, and advanced heart failure management limit the capacity to conduct thorough prognostication, communication, and shared decision making for the various patient-centered outcomes, diverse patient and family preferences, and array of treatment options available to these complex patients. Yet, the unique role of clinicians demands that they assume the primary responsibility for advancing shared decision making and promoting patient-centered care.
As such, the routine conduct of these activities must be efficiently integrated into routine care. The more clinicians perform shared decision making, the better they will be at making it a natural part of their routine practice of care. Patients and families could be prepared for important discussions before the clinic visit through a variety of possible mechanisms, facilitating better use of time for the busy clinician. Furthermore, palliative care services are an instrumental resource in helping with the more complex decisions and major milestones that arise in the course of advanced heart failure care. Multidisciplinary team-based care is necessary. Guidelines for the most dramatic therapeutic options, for instance, transplantation and mechanical circulatory support, already recognize the need for coordinated interdisciplinary care.85,87 Only through diverse inclusion of healthcare providers—nurses, nurse practitioners, physician assistants, primary care physicians, medical ethicists, chaplains, social workers, and others—can this shift adequately take place. A cultural change, particularly within cardiology but also more broadly, is necessary.
However, increased efficiency and dedication on the part of healthcare providers can only partially address the need for quality decision making. Ultimately, the US healthcare system primarily reimburses clinicians for doing things, not for deciding which things should and should not be done; both activities are time consuming and involve the expertise of a skilled clinician, yet only one is valued financially. For a healthcare system that has been criticized for overutilization, placing a greater emphasis on the shared decision-making process is likely to serve as a corrective force to achieve greater value from the system.187 A start would be to reimburse clinicians more equitably for conducting a comprehensive annual heart failure review, which would include voluntary advanced care planning.60(p73406) Unfortunately, attempts to specifically reimburse clinicians for these types of activities have met resistance for a variety of reasons.175 Until these policy differences can be reconciled, actions like those by the Institute of Medicine to make shared decision making 1 of the pillars of quality care equate to an unfunded mandate in clinician time and energy.
Training Programs to Improve Communication With Patients With Heart Failure
Skills required to support patients in decision making are not adequately taught in training programs.129 Multiple studies have shown variation and deficiencies in the ability of clinicians to communicate with patients and address end-of-life issues.188-190 Given the important yet difficult task of communication in clinical practice, improving communication should be a core element of the “performance-based” training and certification processes adopted by the Accreditation Council for Graduate Medical Education. The recent establishment of the secondary subspecialty of advanced heart failure and transplant cardiology191 by the American Board of Medical Specialties is an opportunity to formally add communication techniques and shared decision making to the training and certification process for physicians dedicating their career to the care of these patients. Similar training is especially important for general internists and advance practice nurses who will be caring for a large number of patients with advanced heart failure. Requirements for institutional certification, such as The Joint Commission’s Advanced Certification in Heart Failure program,192 offer yet another opportunity to emphasize formal processes that enhance shared decision making.
Several interventions have successfully improved communication skills for clinicians, particularly with regard to end-of-life care. Studies on the Education for Palliative and End-of-Life Care Project, a standardized multicomponent curriculum with an emphasis on improving communication, demonstrated an improved physician knowledge base and confidence in caring for patients at the end of life.193,194 Likewise, work with oncologists found that a program that combined training, role play, and individualized feedback improved participants’ communication patterns, outcomes, and transitions across healthcare settings.195,196 Some nursing-centered interventions have been shown to be successful in changing nursing practice and improving outcomes for patients. The End-of-Life Nursing Education Consortium (ELNEC) program uses a train-the-trainer model, whereby nurses who complete the program are considered trainers who then go back to their home institutions and teach other nurses the core content aimed at improving care for patients with advanced diseases. Across the country, more than 4500 nurses have been enrolled in ELNEC,197 and data from ELNEC have shown that the program improved nursing attitudes and knowledge about end-of-life care.198
The successful programs to improve communication skills in end-of-life care provide a template for comprehensive “communication” training of heart failure clinicians and need to be expanded to the general physician and midlevel provider community. Additionally, mentoring trainees in patient and family meetings can help to ensure that clinicians have obtained the necessary skills to effectively communicate with heart failure patients and their families.
Future Research Directions to Improve Communication With Patients With Heart Failure
Methods for educating clinicians regarding communication and shared decision making remain early in their development. Useful decision aids for commonly encountered medical decisions in heart failure are also largely underdeveloped or unavailable. One company has developed decision aids for patients considering ICD and CRT therapy (http://www.healthwise.org),198a and another has developed a general decision aid for patients with heart failure (http://www.informedmedicaldecisions.org),198b but these have not been studied formally in real-world settings. Several recently funded National Institutes of Health grants are designed to develop and evaluate decision aids among patients with heart failure, with the recognition that the results of this work are several years away.
At a more basic level, our understanding of how patients with advanced heart failure make choices is limited. There is also no consensus in the literature on the best way to measure whether a medical decision was a “good” one.199 Decisional quality, defined as “the extent to which the implemented decision reflects the considered preferences of a well-informed patient,”200 is emerging as another possible measure to assess the quality of decision making, but validated measures to quantify decisional quality are in the early developmental stages.200 An ideal metric to measure decision quality would include domains in knowledge and values and a way to measure value-treatment concordance. Despite lingering questions, decision quality measurement is gaining popularity, and some have proposed that measures of decision quality be included as part of the larger pay-for-performance agenda.187 The Patient Protection and Affordability Care Act calls for “the development of quality measures that allow for assessment of the experience, quality, and use of information provided to and used by patients, caregivers, and authorized representatives.”13
The role of palliative care in patients with advanced heart failure has been far less developed than in cancer,201 and further work to document the synergistic effect of adding palliative care to the clinical care of patients with advanced heart failure is needed. Our understanding of health-related quality of life—“the functional effect of an illness and its consequent therapy on a patient, as perceived by the patient”38—for patients with advanced heart disease is limited as well.202 Although health-related quality-of-life measurements have been developed for patients with symptomatic heart failure,203,204 questions remain about their sensitivity in very advanced stages of disease. There has been some interest in developing self-report instruments to assess quality of life at the end of life, but they have not been thoroughly tested and validated.205 Primarily, there has been relatively slow uptake of health-related quality-of-life instruments in the evaluation of therapies and in the routine care of patients. Our understanding of the burden and quality of life of caregivers of heart failure patients is even more limited, as is knowledge about how best to intervene to maximize caregiver quality of life.
Conclusions
The importance of shared decision making in advanced heart failure cannot be overstated given the complex myriad of treatment options that confront patients, families, and caregivers. We have offered a roadmap for when and how to have conversations with patients to support shared decision making. This process must occur in the context of uncertainties in prognosis, multiple and often competing outcomes, and barriers to communication. Although the promotion of shared decision making may seem daunting to busy practicing clinicians, we have attempted to provide guiding principles and simple tools that can help set future expectations, anticipate major decisions, and promote productive conversations. Our statement is a “call to action,” not only to clinicians within our community directly responsible for facilitating shared decision making but also to those on a national level who would reform and restructure the healthcare medical system to truly support patient-centered care.
Supplementary Material
Table 8.
Using the N-U-R-S-E Mnemonic to Help Express Verbal Empathy When Communicating With Patients With Advanced Heart Disease
| Technique | Sample Language |
|---|---|
| Name the emotion | You seem worried about what will happen if we don’t implant the LVAD. Can you tell me more about that? |
| Understand the emotion | I see why you might be fearful of proceeding with the transplant. Can you help me understand what you’re afraid of? |
| Respect the emotion | You have shown a lot of strength up to this point. Tell me more about what keeps you going |
| Support the patient | Whether or not you choose to have the procedure, I want you to know that I will continue to be your cardiologist and will take care of you no matter what happens |
| Explore the emotion | You mentioned earlier that you’re concerned about what this worsening of your shortness of breath might mean. Can you tell me more about your concerns? |
LVAD indicates left ventricular assist device.
Reprinted from Back et al,113 with permission of the publisher. Copyright © 2005, Wiley & Sons.
Acknowledgments
This statement was approved by the American Heart Association Science Advisory and Coordinating Committee on January 27, 2012.
Appendix
Disclosures
Writing Group Disclosures
| Writing Group Member | Employment | Research Grant | Other Research Support | Speakers’ Bureau/Honoraria | Expert Witness | Ownership Interest | Consultant/Advisory Board | Other |
|---|---|---|---|---|---|---|---|---|
| Larry A. Allen | University of Colorado Denver | AHA†; NHLBI† | None | None | None | None | Amgen*; Robert Wood Johnson Foundation*; Johnson & Johnson* | None |
| Kathleen Grady | Northwestern University | NIH* | None | None | None | None | Heart Failure Consultants* | None |
| Lynne W. Stevenson | Brigham and Women’s Hospital | Medtronic*; NHLBI† | None | Medtronic* | None | None | None | None |
| Robert M. Arnold | University of Pittsburgh | None | None | None | None | None | None | None |
| Nancy R. Cook | Brigham and Women’s Hospital | None | None | None | None | None | None | None |
| G. Michael Felker | Duke University | BG Medicine*; Critical Diagnostics*; NHLBI*; Roche Diagnostics* | None | None | None | None | Amgen*; BG Medicine*; Novartis†; Roche Diagnostics*; Trevena* | None |
| Gary S. Francis | University of Minnesota | None | None | None | None | None | Novartis† | None |
| Nathan E. Goldstein | Mount Sinai School of Medicine | None | None | None | None | None | None | None |
| Paul J. Hauptman | Saint Louis University | None | None | GlaxoSmithKline*; Otsuka Pharmaceuticals* | None | None | BioControl Medical†; BTG*; Cardiokine*; EvaHeart*; Otsuka Pharmaceuticals* | None |
| Edward P. Havranek | Denver Health and Hospital Authority/University of Colorado School of Medicine | NHLBI† | None | None | None | None | None | None |
| Harlan M. Krumholz | Yale University | Medtronic, Inc.† | None | None | None | None | UnitedHealth† | None |
| Donna Mancini | Columbia University | None | None | None | None | None | None | None |
| Daniel D. Matlock | University of Colorado Denver | Foundation for Informed Medical Decision Making† | None | None | None | None | None | None |
| Barbara Riegel | University of Pennsylvania | NIH† | Kynett Foundation* | None | None | None | None | None |
| John A. Spertus | University of Missouri, Kansas City | ACCF†; AHA†; Amgen†; Bristol-Myers Squibb/Sanofi†; EvaHeart†; Eli Lilly†; NHLBI† | None | None | None | Health Outcomes Sciences† | Amgen*; St Jude Medical*; United HealthCare* | KCCQ Copyright† |
This table represents the relationships of writing group members that may be perceived as actual or reasonably perceived conflicts of interest as reported on the Disclosure Questionnaire, which all members of the writing group are required to complete and submit. A relationship is considered to be “significant” if (1) the person receives $10 000 or more during any 12-month period, or 5% or more of the person’s gross income; or (2) the person owns 5% or more of the voting stock or share of the entity, or owns $10 000 or more of the fair market value of the entity. A relationship is considered to be “modest” if it is less than “significant” under the preceding definition.
Modest.
Significant.
Reviewer Disclosures
| Reviewer | Employment | Research Grant | Other Research Support | Speakers’ Bureau/Honoraria | Expert Witness | Ownership Interest | Consultant/Advisory Board | Other |
|---|---|---|---|---|---|---|---|---|
| Rebecca Boxer | Case Western Reserve University | None | None | None | None | None | None | None |
| Harleah Buck | Pennsylvania State University | None | None | None | None | None | None | None |
| Gregg Fonarow | University of California Los Angeles | None | None | None | None | None | None | None |
| Jessie Gruman | Center for Advancing Health | None | None | None | None | None | None | None |
| Michael Stanley Kiernan | Tufts Medical Center | None | None | None | None | None | None | None |
| Susan Pressler | University of Michigan | None | None | None | None | None | None | None |
| Winifred Teuteberg | University of Pittsburgh | None | None | None | None | None | None | None |
| Angelo Volandes | Harvard Medical School | None | None | None | None | None | None | Nous Foundation, Inc.* |
This table represents the relationships of reviewers that may be perceived as actual or reasonably perceived conflicts of interest as reported on the Disclosure Questionnaire, which all reviewers are required to complete and submit. A relationship is considered to be “significant” if (1) the person receives $10 000 or more during any 12-month period, or 5% or more of the person’s gross income; or (2) the person owns 5% or more of the voting stock or share of the entity, or owns $10 000 or more of the fair market value of the entity. A relationship is considered to be “modest” if it is less than “significant” under the preceding definition.
Footnotes
Expert peer review of AHA Scientific Statements is conducted by the AHA Office of Science Operations. For more on AHA statements and guidelines development, visit http://my.americanheart.org/statements and select the “Policies and Development” link.
The American Heart Association makes every effort to avoid any actual or potential conflicts of interest that may arise as a result of an outside relationship or a personal, professional, or business interest of a member of the writing panel. Specifically, all members of the writing group are required to complete and submit a Disclosure Questionnaire showing all such relationships that might be perceived as real or potential conflicts of interest.
The online-only Data Supplement is available with this article at http://circ.ahajournals.org/lookup/suppl/doi:10.1161/CIR.0b013e31824f2173/-/DC1
References
- 1.Elwyn G, Edwards A, Kinnersley P, Grol R. Shared decision making and the concept of equipoise: the competences of involving patients in healthcare choices. Br J Gen Pract. 2000;50:892–899. [PMC free article] [PubMed] [Google Scholar]
- 2.Frosch DL, Kaplan RM. Shared decision making in clinical medicine: past research and future directions. Am J Prev Med. 1999;17:285–294. doi: 10.1016/s0749-3797(99)00097-5. [DOI] [PubMed] [Google Scholar]
- 3.Beauchamp TL, Childress JF. Principles of Biomedical Ethics. 6. New York, NY: Oxford University Press; 2008. [Google Scholar]
- 4.Cruzan v Director, Missouri Department of Health. 1990 497 US 261. [Google Scholar]
- 5.The Patient Self-Determination Act of 1990. 1990 42 USC 1395 cc (a) [Google Scholar]
- 6.Schneiderman LJ. Defining medical futility and improving medical care. J Bioeth Inq. 2011;8:123–131. doi: 10.1007/s11673-011-9293-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Krumholz HM. Informed consent to promote patient-centered care. JAMA. 2010;303:1190–1191. doi: 10.1001/jama.2010.309. [DOI] [PubMed] [Google Scholar]
- 8.Wagner EH. Chronic disease management: what will it take to improve care for chronic illness? Eff Clin Pract. 1998;1:2–4. [PubMed] [Google Scholar]
- 9.Charles C, Gafni A, Whelan T. Shared decision-making in the medical encounter: what does it mean? (or it takes at least two to tango) Soc Sci Med. 1997;44:681–692. doi: 10.1016/s0277-9536(96)00221-3. [DOI] [PubMed] [Google Scholar]
- 10.Elwyn G, Frosch D, Volandes AE, Edwards A, Montori VM. Investing in deliberation: a definition and classification of decision support interventions for people facing difficult health decisions. Med Decis Making. 2010;30:701–711. doi: 10.1177/0272989X10386231. [DOI] [PubMed] [Google Scholar]
- 11.Krumholz HM. Patient-centered medicine: the next phase in health care. Circ Cardiovasc Qual Outcomes. 2011;4:374–375. doi: 10.1161/CIRCOUTCOMES.111.962217. [DOI] [PubMed] [Google Scholar]
- 12.The Institute of Medicine. Crossing the Quality Chasm: A New Health System for the 21st Century. Washington, DC: National Academy Press; 2001. [PubMed] [Google Scholar]
- 13.The Patient Protection and Affordable Care Act. 2010 Pub L No. 111-148, 124 Stat 119. [Google Scholar]
- 14.Jonsen AR, Siegler M, Winslade WJ. Clinical Ethics: A Practical Approach to Ethical Decisions in Clinical Medicine. New York, NY: Macmillan; 1982. [Google Scholar]
- 15.Roger VL, Go AS, Lloyd-Jones DM, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Makuc DM, Marcus GM, Marelli A, Matchar DB, Moy CS, Mozaffarian D, Mussolino ME, Nichol G, Paynter NP, Soliman EZ, Sorlie PD, Sotoodehnia N, Turan TN, Virani SS, Wong ND, Woo D, Turner MB on behalf of the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2012 update: a report from the American Heart Association. Circulation. 2012;125:e2–e220. doi: 10.1161/CIR.0b013e31823ac046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cubbon RM, Gale CP, Kearney LC, Schechter CB, Brooksby WP, Nolan J, Fox KA, Rajwani A, Baig W, Groves D, Barlow P, Fisher AC, Batin PD, Kahn MB, Zaman AG, Shah AM, Byrne JA, Lindsay SJ, Sapsford RJ, Wheatcroft SB, Witte KK, Kearney MT. Changing characteristics and mode of death associated with chronic heart failure caused by left ventricular systolic dysfunction: a study across therapeutic eras. Circ Heart Fail. 2011;4:396–403. doi: 10.1161/CIRCHEARTFAILURE.110.959882. [DOI] [PubMed] [Google Scholar]
- 17.Hunt SA, Abraham WT, Chin MH, Feldman AM, Francis GS, Ganiats TG, Jessup M, Konstam MA, Mancini DM, Michl K, Oates JA, Rahko PS, Silver MA, Stevenson LW, Yancy CW. 2009 Focused update incorporated into the ACC/AHA 2005 guidelines for the diagnosis and management of heart failure in adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines: developed in collaboration with the International Society for Heart and Lung Transplantation. Circulation. 2009;119:e391–e479. doi: 10.1161/CIRCULATIONAHA.109.192065. published correction appears in Circulation. 2010;121:e258. [DOI] [PubMed] [Google Scholar]
- 18.Adams KF, Jr, Zannad F. Clinical definition and epidemiology of advanced heart failure. Am Heart J. 1998;135(pt 2):S204–S215. doi: 10.1016/s0002-8703(98)70251-0. [DOI] [PubMed] [Google Scholar]
- 19.Goodlin SJ, Hauptman PJ, Arnold R, Grady K, Hershberger RE, Kutner J, Masoudi F, Spertus J, Dracup K, Cleary JF, Medak R, Crispell K, Piña I, Stuart B, Whitney C, Rector T, Teno J, Renlund DG. Consensus statement: palliative and supportive care in advanced heart failure. J Card Fail. 2004;10:200–209. doi: 10.1016/j.cardfail.2003.09.006. [DOI] [PubMed] [Google Scholar]
- 20.Metra M, Ponikowski P, Dickstein K, McMurray JJ, Gavazzi A, Bergh CH, Fraser AG, Jaarsma T, Pitsis A, Mohacsi P, Böhm M, Anker S, Dargie H, Brutsaert D, Komajda M Heart Failure Association of the European Society of Cardiology. Advanced chronic heart failure: a position statement from the Study Group on Advanced Heart Failure of the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail. 2007;9:684–694. doi: 10.1016/j.ejheart.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 21.Lanken PN, Terry PB, Delisser HM, Fahy BF, Hansen-Flaschen J, Heffner JE, Levy M, Mularski RA, Osborne ML, Prendergast TJ, Rocker G, Sibbald WJ, Wilfond B, Yankaskas JR ATS End-of-Life Care Task Force. An official American Thoracic Society clinical policy statement: palliative care for patients with respiratory diseases and critical illnesses. Am J Respir Crit Care Med. 2008;177:912–927. doi: 10.1164/rccm.200605-587ST. [DOI] [PubMed] [Google Scholar]
- 22.Levy WC, Mozaffarian D, Linker DT, Sutradhar SC, Anker SD, Cropp AB, Anand I, Maggioni A, Burton P, Sullivan MD, Pitt B, Poole-Wilson PA, Mann DL, Packer M. The Seattle Heart Failure Model: prediction of survival in heart failure. Circulation. 2006;113:1424–1433. doi: 10.1161/CIRCULATIONAHA.105.584102. [DOI] [PubMed] [Google Scholar]
- 22a.Seattle Heart Failure Model. University of Washington; [February 23, 2012]. http://depts.washington.edu/shfm/ [Google Scholar]
- 23.Aaronson KD, Schwartz JS, Chen TM, Wong KL, Goin JE, Mancini DM. Development and prospective validation of a clinical index to predict survival in ambulatory patients referred for cardiac transplant evaluation. Circulation. 1997;95:2660–2667. doi: 10.1161/01.cir.95.12.2660. [DOI] [PubMed] [Google Scholar]
- 24.Fonarow GC. Epidemiology and risk stratification in acute heart failure. Am Heart J. 2008;155:200–207. doi: 10.1016/j.ahj.2006.10.043. [DOI] [PubMed] [Google Scholar]
- 25.Ross JS, Mulvey GK, Stauffer B, Patlolla V, Bernheim SM, Keenan PS, Krumholz HM. Statistical models and patient predictors of readmission for heart failure: a systematic review. Arch Intern Med. 2008;168:1371–1386. doi: 10.1001/archinte.168.13.1371. [DOI] [PubMed] [Google Scholar]
- 26.Krumholz HM, Wang Y, Mattera JA, Han LF, Ingber MJ, Roman S, Normand SL. An administrative claims model suitable for profiling hospital performance based on 30-day mortality rates among patients with heart failure. Circulation. 2006;113:1693–1701. doi: 10.1161/CIRCULATIONAHA.105.611194. [DOI] [PubMed] [Google Scholar]
- 27.Keenan PS, Normand SL, Lin Z, Drye EE, Bhat KR, Ross JS, Schuur JD, Stauffer BD, Bernheim SM, Epstein AJ, Wang Y, Herrin J, Chen J, Federer JJ, Mattera JA, Krumholz HM. An administrative claims measure suitable for profiling hospital performance on the basis of 30-day all-cause readmission rates among patients with heart failure. Circ Cardiovasc Qual Outcomes. 2008;1:29–37. doi: 10.1161/CIRCOUTCOMES.108.802686. [DOI] [PubMed] [Google Scholar]
- 28.Fonarow GC, Adams KF, Jr, Abraham WT, Yancy CW, Boscardin WJ ADHERE Scientific Advisory Committee, Study Group, and Investigators. Risk stratification for in-hospital mortality in acutely decompensated heart failure: classification and regression tree analysis. JAMA. 2005;293:572–580. doi: 10.1001/jama.293.5.572. [DOI] [PubMed] [Google Scholar]
- 29.Lee DS, Austin PC, Rouleau JL, Liu PP, Naimark D, Tu JV. Predicting mortality among patients hospitalized for heart failure: derivation and validation of a clinical model. JAMA. 2003;290:2581–2587. doi: 10.1001/jama.290.19.2581. [DOI] [PubMed] [Google Scholar]
- 30.Felker GM, Leimberger JD, Califf RM, Cuffe MS, Massie BM, Adams KF, Jr, Gheorghiade M, O’Connor CM. Risk stratification after hospitalization for decompensated heart failure. J Card Fail. 2004;10:460–466. doi: 10.1016/j.cardfail.2004.02.011. [DOI] [PubMed] [Google Scholar]
- 31.O’Connor CM, Hasselblad V, Mehta RH, Tasissa G, Califf RM, Fiuzat M, Rogers JG, Leier CV, Stevenson LW. Triage after hospitalization with advanced heart failure: the ESCAPE (Evaluation Study of Congestive Heart Failure and Pulmonary Artery Catheterization Effectiveness) risk model and discharge score. J Am Coll Cardiol. 2010;55:872–878. doi: 10.1016/j.jacc.2009.08.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Allen LA, Gheorghiade M, Reid KJ, Dunlay SM, Chan PS, Hauptman PJ, Zannad F, Konstam MA, Spertus JA. Identifying patients hospitalized with heart failure at risk for unfavorable future quality of life. Circ Cardiovasc Qual Outcomes. 2011;4:389–398. doi: 10.1161/CIRCOUTCOMES.110.958009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gorodeski EZ, Chu EC, Chow CH, Levy WC, Hsich E, Starling RC. Application of the Seattle Heart Failure Model in ambulatory patients presented to an advanced heart failure therapeutics committee. Circ Heart Fail. 2010;3:706–714. doi: 10.1161/CIRCHEARTFAILURE.110.944280. [DOI] [PubMed] [Google Scholar]
- 34.Kalogeropoulos AP, Georgiopoulou VV, Giamouzis G, Smith AL, Agha SA, Waheed S, Laskar S, Puskas J, Dunbar S, Vega D, Levy WC, Butler J. Utility of the Seattle Heart Failure Model in patients with advanced heart failure. J Am Coll Cardiol. 2009;53:334–342. doi: 10.1016/j.jacc.2008.10.023. [DOI] [PubMed] [Google Scholar]
- 35.Harrell FE. Regression Modeling Strategies: With Application to Linear Models, Logistic Regression, and Survival Analysis. New York, NY: Springer-Verlag; 2001. [Google Scholar]
- 36.Pencina MJ, D’Agostino RB, Sr, D’Agostino RB, Jr, Vasan RS. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med. 2008;27:157–172. doi: 10.1002/sim.2929. [DOI] [PubMed] [Google Scholar]
- 37.Cook NR, Ridker PM. Advances in measuring the effect of individual predictors of cardiovascular risk: the role of reclassification measures. Ann Intern Med. 2009;150:795–802. doi: 10.7326/0003-4819-150-11-200906020-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Spilker B, editor. Quality of Life and Pharmacoeconomics in Clinical Trials. 2. Philadelphia, PA: Lippincott Williams & Wilkins; 1996. [Google Scholar]
- 39.Stevenson LW, Hellkamp AS, Leier CV, Sopko G, Koelling T, Warnica JW, Abraham WT, Kasper EK, Rogers JG, Califf RM, Schramm EE, O’Connor CM. Changing preferences for survival after hospitalization with advanced heart failure. J Am Coll Cardiol. 2008;52:1702–1708. doi: 10.1016/j.jacc.2008.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lewis EF, Johnson PA, Johnson W, Collins C, Griffin L, Stevenson LW. Preferences for quality of life or survival expressed by patients with heart failure. J Heart Lung Transplant. 2001;20:1016–1024. doi: 10.1016/s1053-2498(01)00298-4. [DOI] [PubMed] [Google Scholar]
- 41.Dev S, Clare R, Felker GM, Fiuzat M, Stevenson LW, O’Connor CM. Link between decisions regarding resuscitation and preferences for quality over length of life with heart failure. Eur Heart J. 2012;14:45–53. doi: 10.1093/eurjhf/hfr142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Grady KL, Jalowiec A, White-Williams C, Pifarre R, Kirklin JK, Bourge RC, Costanzo MR. Predictors of quality of life in patients with advanced heart failure awaiting transplantation. J Heart Lung Transplant. 1995;14(pt 1):2–10. [PubMed] [Google Scholar]
- 43.Pressler SJ, Gradus-Pizlo I, Chubinski SD, Smith G, Wheeler S, Wu J, Sloan R. Family caregiver outcomes in heart failure. Am J Crit Care. 2009;18:149–159. doi: 10.4037/ajcc2009300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Scott LD. Caregiving and care receiving among a technologically dependent heart failure population. ANS Adv Nurs Sci. 2000;23:82–97. doi: 10.1097/00012272-200012000-00008. [DOI] [PubMed] [Google Scholar]
- 45.Hooley PJ, Butler G, Howlett JG. The relationship of quality of life, depression, and caregiver burden in outpatients with congestive heart failure. Congest Heart Fail. 2005;11:303–310. doi: 10.1111/j.1527-5299.2005.03620.x. [DOI] [PubMed] [Google Scholar]
- 46.Luttik ML, Jaarsma T, Veeger N, Tijssen J, Sanderman R, van Veldhuisen DJ. Caregiver burden in partners of heart failure patients: limited influence of disease severity. Eur J Heart Fail. 2007;9:695–701. doi: 10.1016/j.ejheart.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 47.Siontis GC, Tzoulaki I, Ioannidis JP. Predicting death: an empirical evaluation of predictive tools for mortality. Arch Intern Med. 2011;171:1721–1726. doi: 10.1001/archinternmed.2011.334. [DOI] [PubMed] [Google Scholar]
- 48.Henderson R, Keiding N. Individual survival time prediction using statistical models. J Med Ethics. 2005;31:703–706. doi: 10.1136/jme.2005.012427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Henderson R, Jones M, Stare J. Accuracy of point predictions in survival analysis. Stat Med. 2001;20:3083–3096. doi: 10.1002/sim.913. [DOI] [PubMed] [Google Scholar]
- 50.Glare P, Sinclair C, Downing M, Stone P, Maltoni M, Vigano A. Predicting survival in patients with advanced disease. Eur J Cancer. 2008;44:1146–1156. doi: 10.1016/j.ejca.2008.02.030. [DOI] [PubMed] [Google Scholar]
- 51.Parkes CM. Accuracy of predictions of survival in later stages of cancer. BMJ. 1972;2:29–31. doi: 10.1136/bmj.2.5804.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Glare P, Virik K, Jones M, Hudson M, Eychmuller S, Simes J, Christakis N. A systematic review of physicians’ survival predictions in terminally ill cancer patients. BMJ. 2003;327:195–198. doi: 10.1136/bmj.327.7408.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Christakis NA, Lamont EB. Extent and determinants of error in doctors’ prognoses in terminally ill patients: prospective cohort study. BMJ. 2000;320:469–472. doi: 10.1136/bmj.320.7233.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yamokoski LM, Hasselblad V, Moser DK, Binanay C, Conway GA, Glotzer JM, Hartman KA, Stevenson LW, Leier CV. Prediction of rehospitalization and death in severe heart failure by physicians and nurses of the ESCAPE trial. J Card Fail. 2007;13:8–13. doi: 10.1016/j.cardfail.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 55.Allen LA, Yager JE, Funk MJ, Levy WC, Tulsky JA, Bowers MT, Dodson GC, O’Connor CM, Felker GM. Discordance between patient-predicted and model-predicted life expectancy among ambulatory patients with heart failure. JAMA. 2008;299:2533–2542. doi: 10.1001/jama.299.21.2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Krumholz HM, Phillips RS, Hamel MB, Teno JM, Bellamy P, Broste SK, Califf RM, Vidaillet H, Davis RB, Muhlbaier LH, Connors AF, Jr, Lynn J, Goldman L. Resuscitation preferences among patients with severe congestive heart failure: results from the SUPPORT project: Study to Understand Prognoses and Preferences for Outcomes and Risks of Treatments. Circulation. 1998;98:648–655. doi: 10.1161/01.cir.98.7.648. [DOI] [PubMed] [Google Scholar]
- 57.Solomon SD, Dobson J, Pocock S, Skali H, McMurray JJ, Granger CB, Yusuf S, Swedberg K, Young JB, Michelson EL, Pfeffer MA Candesartan in Heart failure: Assessment of Reduction in Mortality and morbidity (CHARM) Investigators. Influence of nonfatal hospitalization for heart failure on subsequent mortality in patients with chronic heart failure. Circulation. 2007;116:1482–1487. doi: 10.1161/CIRCULATIONAHA.107.696906. [DOI] [PubMed] [Google Scholar]
- 58.Kittleson M, Hurwitz S, Shah MR, Nohria A, Lewis E, Givertz M, Fang J, Jarcho J, Mudge G, Stevenson LW. Development of circulatory-renal limitations to angiotensin-converting enzyme inhibitors identifies patients with severe heart failure and early mortality. J Am Coll Cardiol. 2003;41:2029–2035. doi: 10.1016/s0735-1097(03)00417-0. [DOI] [PubMed] [Google Scholar]
- 59.Mishkin JD, Saxonhouse SJ, Woo GW, Burkart TA, Miles WM, Conti JB, Schofield RS, Sears SF, Aranda JM., Jr Appropriate evaluation and treatment of heart failure patients after implantable cardioverter-defibrillator discharge: time to go beyond the initial shock. J Am Coll Cardiol. 2009;54:1993–2000. doi: 10.1016/j.jacc.2009.07.039. [DOI] [PubMed] [Google Scholar]
- 60.Centers for Medicare and Medicaid Services (CMS), Department of Health and Human Services. Medicare program: payment policies under the physician fee. Fed Regist. 2010;75:73369–73418. [Google Scholar]
- 61.Lindenfeld J, Albert NM, Boehmer JP, Collins SP, Ezekowitz JA, Givertz MM, Katz SD, Klapholz M, Moser DK, Rogers JG, Starling RC, Stevenson WG, Tang WH, Teerlink JR, Walsh MN Heart Failure Society of America. HFSA 2010 comprehensive heart failure practice guideline. J Card Fail. 2010;16:e1–e194. doi: 10.1016/j.cardfail.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 62.Dickstein K, Cohen-Solal A, Filippatos G, McMurray JJ, Ponikowski P, Poole-Wilson PA, Strömberg A, van Veldhuisen DJ, Atar D, Hoes AW, Keren A, Mebazaa A, Nieminen M, Priori SG, Swedberg K. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2008: the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2008 of the European Society of Cardiology: developed in collaboration with the Heart Failure Association of the ESC (HFA) and endorsed by the European Society of Intensive Care Medicine (ESICM) Eur Heart J. 2008;29:2388–2442. doi: 10.1093/eurheartj/ehn309. published corrections appear in Eur Heart J. 2010;12:416 and Eur Heart J. 2010;31:624. [DOI] [PubMed] [Google Scholar]
- 63.Velazquez EJ, Lee KL, Deja MA, Jain A, Sopko G, Marchenko A, Ali IS, Pohost G, Gradinac S, Abraham WT, Yii M, Prabhakaran D, Szwed H, Ferrazzi P, Petrie MC, O’Connor CM, Panchavinnin P, She L, Bonow RO, Rankin GR, Jones RH, Rouleau JL STICH Investigators. Coronary-artery bypass surgery in patients with left ventricular dysfunction. N Engl J Med. 2011;364:1607–1616. doi: 10.1056/NEJMoa1100356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. [February 23, 2012];The Society of Thoracic Surgeons National Database. [database online]. http://www.sts.org/national-database.
- 65.Mehran R, Aymong ED, Nikolsky E, Lasic Z, Iakovou I, Fahy M, Mintz GS, Lansky AJ, Moses JW, Stone GW, Leon MB, Dangas G. A simple risk score for prediction of contrast-induced nephropathy after percutaneous coronary intervention: development and initial validation. J Am Coll Cardiol. 2004;44:1393–1399. doi: 10.1016/j.jacc.2004.06.068. [DOI] [PubMed] [Google Scholar]
- 66.Chowdhary S, Ivanov J, Mackie K, Seidelin PH, Dzavik V. The Toronto score for in-hospital mortality after percutaneous coronary interventions. Am Heart J. 2009;157:156–163. doi: 10.1016/j.ahj.2008.08.026. [DOI] [PubMed] [Google Scholar]
- 67.Smith C. Transcatheter valve implantation compared to surgical valve replacement for severe aortic stenosis: the PARTNER Cohort A Study. Paper presented at: American College of Cardiology Scientific Sessions/I2 Summit; April 3, 2011; New Orleans, LA. [Google Scholar]
- 68.Feldman T, Foster E, Glower DG, Kar S, Rinaldi MJ, Fail PS, Smalling RW, Siegel R, Rose GA, Engeron E, Loghin C, Trento A, Skipper ER, Fudge T, Letsou GV, Massaro JM, Mauri L EVEREST II Investigators. Percutaneous repair or surgery for mitral regurgitation. N Engl J Med. 2011;364:1395–1406. doi: 10.1056/NEJMoa1009355. published correction appears in N Engl J Med. 2011;365:189. [DOI] [PubMed] [Google Scholar]
- 69.Franzen O, van der Heyden J, Baldus S, Schluter M, Schillinger W, Butter C, Hoffmann R, Corti R, Pedrazzini G, Swaans MJ, Neuss M, Rudolph V, Surder D, Grünenfelder J, Eulenburg C, Reichenspurner H, Meinertz T, Auricchio A. MitraClip therapy in patients with end-stage systolic heart failure. Eur J Heart Fail. 2011;13:569–576. doi: 10.1093/eurjhf/hfr029. [DOI] [PubMed] [Google Scholar]
- 70.Lindenfeld J, Feldman AM, Saxon L, Boehmer J, Carson P, Ghali JK, Anand I, Singh S, Steinberg JS, Jaski B, DeMarco T, Mann D, Yong P, Galle E, Ecklund F, Bristow M. Effects of cardiac resynchronization therapy with or without a defibrillator on survival and hospitalizations in patients with New York Heart Association class IV heart failure. Circulation. 2007;115:204–212. doi: 10.1161/CIRCULATIONAHA.106.629261. [DOI] [PubMed] [Google Scholar]
- 71.Bhattacharya S, Abebe K, Simon M, Saba S, Adelstein E. Role of cardiac resynchronization in end-stage heart failure patients requiring inotrope therapy. J Card Fail. 2010;16:931–937. doi: 10.1016/j.cardfail.2010.07.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Begg GA, Witte KK. Closing the door after the horse has bolted: device therapy in patients with end-stage heart failure. J Card Fail. 2010;16:938–939. doi: 10.1016/j.cardfail.2010.08.013. [DOI] [PubMed] [Google Scholar]
- 73.Swindle JP, Rich MW, McCann P, Burroughs TE, Hauptman PJ. Implantable cardiac device procedures in older patients: use and in-hospital outcomes. Arch Intern Med. 2010;170:631–637. doi: 10.1001/archinternmed.2010.30. [DOI] [PubMed] [Google Scholar]
- 74.Swindle J, Burroughs TE, Schnitzler MA, Hauptman PJ. Short-term mortality and cost associated with cardiac device implantation in patients hospitalized with heart failure. Am Heart J. 2008;156:322–328. doi: 10.1016/j.ahj.2008.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Nazarian S, Maisel WH, Miles JS, Tsang S, Stevenson LW, Stevenson WG. Impact of implantable cardioverter defibrillators on survival and recurrent hospitalization in advanced heart failure. Am Heart J. 2005;150:955–960. doi: 10.1016/j.ahj.2005.01.012. [DOI] [PubMed] [Google Scholar]
- 76.Passman R, Subacius H, Ruo B, Schaechter A, Howard A, Sears SF, Kadish A. Implantable cardioverter defibrillators and quality of life: results from the Defibrillators in Nonischemic Cardiomyopathy Treatment Evaluation Study. Arch Intern Med. 2007;167:2226–2232. doi: 10.1001/archinte.167.20.2226. [DOI] [PubMed] [Google Scholar]
- 77.Hauptman PJ, Mikolajczak P, George A, Mohr CJ, Hoover R, Swindle J, Schnitzler MA. Chronic inotropic therapy in end-stage heart failure. Am Heart J. 2006;152:1096.e1091–e1098. doi: 10.1016/j.ahj.2006.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Thackray S, Easthaugh J, Freemantle N, Cleland JG. The effectiveness and relative effectiveness of intravenous inotropic drugs acting through the adrenergic pathway in patients with heart failure: a meta-regression analysis. Eur J Heart Fail. 2002;4:515–529. doi: 10.1016/s1388-9842(02)00041-7. [DOI] [PubMed] [Google Scholar]
- 79.Oliva F, Latini R, Politi A, Staszewsky L, Maggioni AP, Nicolis E, Mauri F. Intermittent 6-month low-dose dobutamine infusion in severe heart failure: DICE multicenter trial. Am Heart J. 1999;138(pt 1):247–253. doi: 10.1016/s0002-8703(99)70108-0. [DOI] [PubMed] [Google Scholar]
- 80.Ronco C, Haapio M, House AA, Anavekar N, Bellomo R. Cardiorenal syndrome. J Am Coll Cardiol. 2008;52:1527–1539. doi: 10.1016/j.jacc.2008.07.051. [DOI] [PubMed] [Google Scholar]
- 81.Stack AG, Mohammed A, Hanley A, Mutwali A, Nguyen H. Survival trends of US dialysis patients with heart failure: 1995 to 2005. Clin J Am Soc Nephrol. 2011;6:1982–1989. doi: 10.2215/CJN.01130211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Smith C, Da Silva-Gane M, Chandna S, Warwicker P, Greenwood R, Farrington K. Choosing not to dialyse: evaluation of planned non-dialytic management in a cohort of patients with end-stage renal failure. Nephron Clin Pract. 2003;95:c40–c46. doi: 10.1159/000073708. [DOI] [PubMed] [Google Scholar]
- 83.Murtagh FE, Marsh JE, Donohoe P, Ekbal NJ, Sheerin NS, Harris FE. Dialysis or not? A comparative survival study of patients over 75 years with chronic kidney disease stage 5. Nephrol Dial Transplant. 2007;22:1955–1962. doi: 10.1093/ndt/gfm153. [DOI] [PubMed] [Google Scholar]
- 84.Renal Physicians Association. Shared Decision-Making in the Appropriate Initiation of and Withdrawal From Dialysis: Clinical Practice Guideline. 2. Rockville, MD: Agency for Healthcare Research and Quality; Oct, 2010. [August 9, 2011]. http://www.guidelines.gov/content.aspx?id=24176. [Google Scholar]
- 85.Costanzo MR, Dipchand A, Starling R, Anderson A, Chan M, Desai S, Fedson S, Fisher P, Gonzales-Stawinski G, Martinelli L, McGiffin D, Smith J, Taylor D, Meiser B, Webber S, Baran D, Carboni M, Dengler T, Feldman D, Frigerio M, Kfoury A, Kim D, Kobashigawa J, Shullo M, Stehlik J, Teuteberg J, Uber P, Zuckermann A, Hunt S, Burch M, Bhat G, Canter C, Chinnock R, Crespo-Leiro M, Delgado R, Dobbels F, Grady K, Kao W, Lamour J, Parry G, Patel J, Pini D, Towbin J, Wolfel G, Delgado D, Eisen H, Goldberg L, Hosenpud J, Johnson M, Keogh A, Lewis C, O’Connell J, Rogers J, Ross H, Russell S, Vanhaecke J. The International Society of Heart and Lung Transplantation Guidelines for the care of heart transplant recipients. J Heart Lung Transplant. 2010;29:914–956. doi: 10.1016/j.healun.2010.05.034. [DOI] [PubMed] [Google Scholar]
- 86.Mehra MR, Kobashigawa J, Starling R, Russell S, Uber PA, Parameshwar J, Mohacsi P, Augustine S, Aaronson K, Barr M. Listing criteria for heart transplantation: International Society for Heart and Lung Transplantation guidelines for the care of cardiac transplant candidates–2006. J Heart Lung Transplant. 2006;25:1024–1042. doi: 10.1016/j.healun.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 87.Slaughter MS, Pagani FD, Rogers JG, Miller LW, Sun B, Russell SD, Starling RC, Chen L, Boyle AJ, Chillcott S, Adamson RM, Blood MS, Camacho MT, Idrissi KA, Petty M, Sobieski M, Wright S, Myers TJ, Farrar DJ HeartMate II Clinical Investigators. Clinical management of continuous-flow left ventricular assist devices in advanced heart failure. J Heart Lung Transplant. 2010;29(suppl):S1–S39. doi: 10.1016/j.healun.2010.01.011. [DOI] [PubMed] [Google Scholar]
- 88.Sima CS, Panageas KS, Schrag D. Cancer screening among patients with advanced cancer. JAMA. 2010;304:1584–1591. doi: 10.1001/jama.2010.1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Goodlin SJ. Palliative care in congestive heart failure. J Am Coll Cardiol. 2009;54:386–396. doi: 10.1016/j.jacc.2009.02.078. [DOI] [PubMed] [Google Scholar]
- 90.Adler ED, Goldfinger JZ, Kalman J, Park ME, Meier DE. Palliative care in the treatment of advanced heart failure. Circulation. 2009;120:2597–2606. doi: 10.1161/CIRCULATIONAHA.109.869123. [DOI] [PubMed] [Google Scholar]
- 91.Rizzieri AG, Verheijde JL, Rady MY, McGregor JL. Ethical challenges with the left ventricular assist device as a destination therapy. Philos Ethics Humanit Med. 2008;3:20. doi: 10.1186/1747-5341-3-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.National Consensus Project for Quality Palliative Care. [February 23, 2012];Clinical practice guidelines for quality palliative care. (2). 2009 http://www.nationalconsensusproject.org.
- 93.National Quality Forum. [February 23, 2012];A national framework and preferred practices for palliative and hospice care quality. 2006 Dec; http://www.qualityforum.org/Publications/2006/12/A_National_Framework_and_Preferred_Practices_for_Palliative_and_Hospice_Care_Quality.aspx.
- 94.Morrison RS, Meier DE. Clinical practice: palliative care. N Engl J Med. 2004;350:2582–2590. doi: 10.1056/NEJMcp035232. [DOI] [PubMed] [Google Scholar]
- 95.Goldstein NE, May CW, Meier DE. Comprehensive care for mechanical circulatory support: a new frontier for synergy with palliative care. Circ Heart Fail. 2011;4:519–527. doi: 10.1161/CIRCHEARTFAILURE.110.957241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Swetz KM, Freeman MR, AbouEzzeddine OF, Carter KA, Boilson BA, Ottenberg AL, Park SJ, Mueller PS. Palliative medicine consultation for preparedness planning in patients receiving left ventricular assist devices as destination therapy. Mayo Clin Proc. 2011;86:493–500. doi: 10.4065/mcp.2010.0747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Padeletti L, Arnar DO, Boncinelli L, Brachman J, Camm JA, Daubert JC, Kassam S, Deliens L, Glikson M, Hayes D, Israel C, Lampert R, Lobban T, Raatikainen P, Siegal G, Vardas P, Kirchhof P, Becker R, Cosio F, Loh P, Cobbe S, Grace A, Morgan J. EHRA expert consensus statement on the management of cardiovascular implantable electronic devices in patients nearing end of life or requesting withdrawal of therapy. Europace. 2010;12:1480–1489. doi: 10.1093/europace/euq275. published correction appears in Europace. 2011;13:599. [DOI] [PubMed] [Google Scholar]
- 98.Kelley AS, Reid MC, Miller DH, Fins JJ, Lachs MS. Implantable cardioverter-defibrillator deactivation at the end of life: a physician survey. Am Heart J. 2009;157:702–708.e1. doi: 10.1016/j.ahj.2008.12.011. [DOI] [PubMed] [Google Scholar]
- 99.Hauptman PJ, Swindle J, Hussain Z, Biener L, Burroughs TE. Physician attitudes toward end-stage heart failure: a national survey. Am J Med. 2008;121:127–135. doi: 10.1016/j.amjmed.2007.08.035. [DOI] [PubMed] [Google Scholar]
- 100.Goldstein N, Carlson M, Livote E, Kutner JS. Brief communication: management of implantable cardioverter-defibrillators in hospice: a nationwide survey. Ann Intern Med. 2010;152:296–299. doi: 10.1059/0003-4819-152-5-201003020-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Long JW, Healy AH, Rasmusson BY, Cowley CG, Nelson KE, Kfoury AG, Clayson SE, Reid BB, Moore SA, Blank DU, Renlund DG. Improving outcomes with long-term “destination” therapy using left ventricular assist devices. J Thorac Cardiovasc Surg. 2008;135:1353–1360. doi: 10.1016/j.jtcvs.2006.09.124. [DOI] [PubMed] [Google Scholar]
- 102.Slaughter MS, Rogers JG, Milano CA, Russell SD, Conte JV, Feldman D, Sun B, Tatooles AJ, Delgado RM, 3rd, Long JW, Wozniak TC, Ghumman W, Farrar DJ, Frazier OH HeartMate II Investigators. Advanced heart failure treated with continuous-flow left ventricular assist device. N Engl J Med. 2009;361:2241–2251. doi: 10.1056/NEJMoa0909938. [DOI] [PubMed] [Google Scholar]
- 103.Brush S, Budge D, Alharethi R, McCormick AJ, MacPherson JE, Reid BB, Ledford ID, Smith HK, Stoker S, Clayson SE, Doty JR, Caine WT, Drakos S, Kfoury AG. End-of-life decision making and implementation in recipients of a destination left ventricular assist device. J Heart Lung Transplant. 2010;29:1337–1341. doi: 10.1016/j.healun.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 104.Department of Health and Human Services, Centers for Medicare & Medicaid Services. Medicare and Medicaid Programs: Hospice Conditions of Participation. [February 23, 2012];Fed Regist. 2008 73:32088–32220. To be codified at 42 CFR Part 418 §408(a). http://edocket.access.gpo.gov/2008/pdf/08-1305.pdf. [Google Scholar]
- 105.Bain KT, Maxwell TL, Strassels SA, Whellan DJ. Hospice use among patients with heart failure. Am Heart J. 2009;158:118–125. doi: 10.1016/j.ahj.2009.05.013. [DOI] [PubMed] [Google Scholar]
- 106.Teno JM, Clarridge BR, Casey V, Welch LC, Wetle T, Shield R, Mor V. Family perspectives on end-of-life care at the last place of care. JAMA. 2004;291:88–93. doi: 10.1001/jama.291.1.88. [DOI] [PubMed] [Google Scholar]
- 107.Connor SR, Pyenson B, Fitch K, Spence C, Iwasaki K. Comparing hospice and nonhospice patient survival among patients who die within a three-year window. J Pain Symptom Manage. 2007;33:238–246. doi: 10.1016/j.jpainsymman.2006.10.010. [DOI] [PubMed] [Google Scholar]
- 108.Unroe KT, Greiner MA, Hernandez AF, Whellan DJ, Kaul P, Schulman KA, Peterson ED, Curtis LH. Resource use in the last 6 months of life among Medicare beneficiaries with heart failure, 2000–2007. Arch Intern Med. 2011;171:196–203. doi: 10.1001/archinternmed.2010.371. [DOI] [PubMed] [Google Scholar]
- 109.Teno JM, Shu JE, Casarett D, Spence C, Rhodes R, Connor S. Timing of referral to hospice and quality of care: length of stay and bereaved family members’ perceptions of the timing of hospice referral. J Pain Symptom Manage. 2007;34:120–125. doi: 10.1016/j.jpainsymman.2007.04.014. [DOI] [PubMed] [Google Scholar]
- 110.Apatira L, Boyd EA, Malvar G, Evans LR, Luce JM, Lo B, White DB. Hope, truth, and preparing for death: perspectives of surrogate decision makers. Ann Intern Med. 2008;149:861–868. doi: 10.7326/0003-4819-149-12-200812160-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Golin CE, Wenger NS, Liu H, Dawson NV, Teno JM, Desbiens NA, Lynn J, Oye RK, Phillips RS. A prospective study of patient-physician communication about resuscitation. J Am Geriatr Soc. 2000;48(suppl):S52–S60. doi: 10.1111/j.1532-5415.2000.tb03141.x. [DOI] [PubMed] [Google Scholar]
- 112.Emanuel LL, von Gunten CF, Ferris FD, editors. The Education in Palliative and End-of-Life (EPEC) curriculum. Chicago, IL: EPEC Project; 2003. [Google Scholar]
- 113.Back AL, Arnold RM, Baile WF, Tulsky JA, Fryer-Edwards K. Approaching difficult communication tasks in oncology. CA Cancer J Clin. 2005;55:164–177. doi: 10.3322/canjclin.55.3.164. [DOI] [PubMed] [Google Scholar]
- 114.Wiegand DL, Kalowes PG. Withdrawal of cardiac medications and devices. AACN Adv Crit Care. 2007;18:415–425. doi: 10.1097/01.AACN.0000298634.45653.81. [DOI] [PubMed] [Google Scholar]
- 115.Goldstein NE, Back AL, Morrison RS. Titrating guidance: a model to guide physicians in assisting patients and family members who are facing complex decisions. Arch Intern Med. 2008;168:1733–1739. doi: 10.1001/archinte.168.16.1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Singer PA, Martin DK, Kelner M. Quality end-of-life care: patients’ perspectives. JAMA. 1999;281:163–168. doi: 10.1001/jama.281.2.163. [DOI] [PubMed] [Google Scholar]
- 117.Nicolasora N, Pannala R, Mountantonakis S, Shanmugam B, DeGirolamo A, Amoateng-Adjepong Y, Manthous CA. If asked, hospitalized patients will choose whether to receive life-sustaining therapies. J Hosp Med. 2006;1:161–167. doi: 10.1002/jhm.78. [DOI] [PubMed] [Google Scholar]
- 118.Fried TR, O’Leary JR. Using the experiences of bereaved caregivers to inform patient- and caregiver-centered advance care planning. J Gen Intern Med. 2008;23:1602–1607. doi: 10.1007/s11606-008-0748-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kirchhoff KT, Hammes BJ, Kehl KA, Briggs LA, Brown RL. Effect of a disease-specific planning intervention on surrogate understanding of patient goals for future medical treatment. J Am Geriatr Soc. 2010;58:1233–1240. doi: 10.1111/j.1532-5415.2010.02760.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Silveira MJ, Kim SY, Langa KM. Advance directives and outcomes of surrogate decision making before death. N Engl J Med. 2010;362:1211–1218. doi: 10.1056/NEJMsa0907901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Detering KM, Hancock AD, Reade MC, Silvester W. The impact of advance care planning on end of life care in elderly patients: randomised controlled trial. BMJ. 2010;340:c1345. doi: 10.1136/bmj.c1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Mack JW, Block SD, Nilsson M, Wright A, Trice E, Friedlander R, Paulk E, Prigerson HG. Measuring therapeutic alliance between oncologists and patients with advanced cancer: the Human Connection Scale. Cancer. 2009;115:3302–3311. doi: 10.1002/cncr.24360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Wright AA, Zhang B, Ray A, Mack JW, Trice E, Balboni T, Mitchell SL, Jackson VA, Block SD, Maciejewski PK, Prigerson HG. Associations between end-of-life discussions, patient mental health, medical care near death, and caregiver bereavement adjustment. JAMA. 2008;300:1665–1673. doi: 10.1001/jama.300.14.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Caldwell PH, Arthur HM, Demers C. Preferences of patients with heart failure for prognosis communication. Can J Cardiol. 2007;23:791–796. doi: 10.1016/s0828-282x(07)70829-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Lunney JR, Lynn J, Foley DJ, Lipson S, Guralnik JM. Patterns of functional decline at the end of life. JAMA. 2003;289:2387–2392. doi: 10.1001/jama.289.18.2387. [DOI] [PubMed] [Google Scholar]
- 126.Lynn J, Goldstein NE. Advance care planning for fatal chronic illness: avoiding commonplace errors and unwarranted suffering. Ann Intern Med. 2003;138:812–818. doi: 10.7326/0003-4819-138-10-200305200-00009. [DOI] [PubMed] [Google Scholar]
- 127.Fried TR, Bradley EH, Towle VR, Allore H. Understanding the treatment preferences of seriously ill patients. N Engl J Med. 2002;346:1061–1066. doi: 10.1056/NEJMsa012528. [DOI] [PubMed] [Google Scholar]
- 128.Accreditation Council for Graduate Medical Education. [February 23, 2012]; http://www.acgme.org/acWebsite/navPages/nav_IRC.asp.
- 129.Elwyn G, Edwards A, Gwyn R, Grol R. Towards a feasible model for shared decision making: focus group study with general practice registrars. BMJ. 1999;319:753–756. doi: 10.1136/bmj.319.7212.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Back AL, Arnold R, Tulsky JA. Mastering Communication with Seriously Ill Patients: Balancing Honesty With Empathy and Hope. New York, NY: Cambridge University Press; 2009. [Google Scholar]
- 131.Goodlin SJ, Quill TE, Arnold RM. Communication and decision-making about prognosis in heart failure care. J Card Fail. 2008;14:106–113. doi: 10.1016/j.cardfail.2007.10.022. [DOI] [PubMed] [Google Scholar]
- 132.O’Connor AM, Stacey D, Rovner D, Holmes-Rovner M, Tetroe J, Llewellyn-Thomas H, Entwistle V, Rostom A, Fiset V, Barry M, Jones J. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev. 2001;(3) doi: 10.1002/14651858.CD001431. CD001431. [DOI] [PubMed] [Google Scholar]
- 133.Knight SJ, Emanuel L. Processes of adjustment to end-of-life losses: a reintegration model. J Palliat Med. 2007;10:1190–1198. doi: 10.1089/jpm.2006.0068. [DOI] [PubMed] [Google Scholar]
- 134.Dracup K, Walden JA, Stevenson LW, Brecht ML. Quality of life in patients with advanced heart failure. J Heart Lung Transplant. 1992;11(pt 1):273–279. [PubMed] [Google Scholar]
- 135.Hallas CN, Wray J, Andreou P, Banner NR. Depression and perceptions about heart failure predict quality of life in patients with advanced heart failure. Heart Lung. 2010;40:111–121. doi: 10.1016/j.hrtlng.2009.12.008. [DOI] [PubMed] [Google Scholar]
- 136.Johnson SK, Bautista CA, Hong SY, Weissfeld L, White DB. An empirical study of surrogates’ preferred level of control over value-laden life support decisions in intensive care units. Am J Respir Crit Care Med. 2011;183:915–921. doi: 10.1164/rccm.201008-1214OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Nelson JE, Puntillo KA, Pronovost PJ, Walker AS, McAdam JL, Ilaoa D, Penrod J. In their own words: patients and families define high-quality palliative care in the intensive care unit. Crit Care Med. 2010;38:808–818. doi: 10.1097/ccm.0b013e3181c5887c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Anderson WG, Arnold RM, Angus DC, Bryce CL. Passive decision-making preference is associated with anxiety and depression in relatives of patients in the intensive care unit. J Crit Care. 2009;24:249–254. doi: 10.1016/j.jcrc.2007.12.010. [DOI] [PubMed] [Google Scholar]
- 139.Street RL, Jr, Makoul G, Arora NK, Epstein RM. How does communication heal? Pathways linking clinician-patient communication to health outcomes. Patient Educ Couns. 2009;74:295–301. doi: 10.1016/j.pec.2008.11.015. [DOI] [PubMed] [Google Scholar]
- 140.Curtis JR, Engelberg RA, Wenrich MD, Shannon SE, Treece PD, Rubenfeld GD. Missed opportunities during family conferences about end-of-life care in the intensive care unit. Am J Respir Crit Care Med. 2005;171:844–849. doi: 10.1164/rccm.200409-1267OC. [DOI] [PubMed] [Google Scholar]
- 141.Back AL, Anderson WG, Bunch L, Marr LA, Wallace JA, Yang HB, Arnold RM. Communication about cancer near the end of life. Cancer. 2008;113(suppl):1897–1910. doi: 10.1002/cncr.23653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Fogarty LA, Curbow BA, Wingard JR, McDonnell K, Somerfield MR. Can 40 seconds of compassion reduce patient anxiety? J Clin Oncol. 1999;17:371–379. doi: 10.1200/JCO.1999.17.1.371. [DOI] [PubMed] [Google Scholar]
- 143.Tulsky JA. Beyond advance directives: importance of communication skills at the end of life. JAMA. 2005;294:359–365. doi: 10.1001/jama.294.3.359. [DOI] [PubMed] [Google Scholar]
- 144.Schaefer KG, Block SD. Physician communication with families in the ICU: evidence-based strategies for improvement. Curr Opin Crit Care. 2009;15:569–577. doi: 10.1097/MCC.0b013e328332f524. [DOI] [PubMed] [Google Scholar]
- 145.Thomas SA, Friedmann E, Khatta M, Cook LK, Lann AL. Depression in patients with heart failure: physiologic effects, incidence, and relation to mortality. AACN Clin Issues. 2003;14:3–12. doi: 10.1097/00044067-200302000-00002. [DOI] [PubMed] [Google Scholar]
- 146.Vaccarino V, Kasl SV, Abramson J, Krumholz HM. Depressive symptoms and risk of functional decline and death in patients with heart failure. J Am Coll Cardiol. 2001;38:199–205. doi: 10.1016/s0735-1097(01)01334-1. [DOI] [PubMed] [Google Scholar]
- 147.Moser DK, Doering LV, Chung ML. Vulnerabilities of patients recovering from an exacerbation of chronic heart failure. Am Heart J. 2005;150:984. doi: 10.1016/j.ahj.2005.07.028. [DOI] [PubMed] [Google Scholar]
- 148.Rutledge T, Reis VA, Linke SE, Greenberg BH, Mills PJ. Depression in heart failure: a meta-analytic review of prevalence, intervention effects, and associations with clinical outcomes. J Am Coll Cardiol. 2006;48:1527–1537. doi: 10.1016/j.jacc.2006.06.055. [DOI] [PubMed] [Google Scholar]
- 149.Thomas AJ, O’Brien JT. Depression and cognition in older adults. Curr Opin Psychiatry. 2008;21:8–13. doi: 10.1097/YCO.0b013e3282f2139b. [DOI] [PubMed] [Google Scholar]
- 150.Butters MA, Whyte EM, Nebes RD, Begley AE, Dew MA, Mulsant BH, Zmuda MD, Bhalla R, Meltzer CC, Pollock BG, Reynolds CF, 3rd, Becker JT. The nature and determinants of neuropsychological functioning in late-life depression. Arch Gen Psychiatry. 2004;61:587–595. doi: 10.1001/archpsyc.61.6.587. [DOI] [PubMed] [Google Scholar]
- 151.Bhalla RK, Butters MA, Mulsant BH, Begley AE, Zmuda MD, Schoderbek B, Pollock BG, Reynolds CF, 3rd, Becker JT. Persistence of neuropsychologic deficits in the remitted state of late-life depression. Am J Geriatr Psychiatry. 2006;14:419–427. doi: 10.1097/01.JGP.0000203130.45421.69. [DOI] [PubMed] [Google Scholar]
- 152.Fagerlin A, Sepucha KR, Couper MP, Levin CA, Singer E, Zikmund-Fisher BJ. Patients’ knowledge about 9 common health conditions: the DECISIONS survey. Med Decis Making. 2010;30(suppl):35S–52S. doi: 10.1177/0272989X10378700. [DOI] [PubMed] [Google Scholar]
- 153.Stewart GC, Weintraub JR, Pratibhu PP, Semigran MJ, Camuso JM, Brooks K, Tsang SW, Anello MS, Nguyen VT, Lewis EF, Nohria A, Desai AS, Givertz MM, Stevenson LW. Patient expectations from implantable defibrillators to prevent death in heart failure. J Card Fail. 2010;16:106–113. doi: 10.1016/j.cardfail.2009.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Rothberg MB, Sivalingam SK, Ashraf J, Visintainer P, Joelson J, Kleppel R, Vallurupalli N, Schweiger MJ. Patients’ and cardiologists’ perceptions of the benefits of percutaneous coronary intervention for stable coronary disease. Ann Intern Med. 2010;153:307–313. doi: 10.7326/0003-4819-153-5-201009070-00005. [DOI] [PubMed] [Google Scholar]
- 155.Boden WE, O’Rourke RA, Teo KK, Hartigan PM, Maron DJ, Kostuk WJ, Knudtson M, Dada M, Casperson P, Harris CL, Chaitman BR, Shaw L, Gosselin G, Nawaz S, Title LM, Gau G, Blaustein AS, Booth DC, Bates ER, Spertus JA, Berman DS, Mancini GB, Weintraub WS COURAGE Trial Research Group. Optimal medical therapy with or without PCI for stable coronary disease. N Engl J Med. 2007;356:1503–1516. doi: 10.1056/NEJMoa070829. [DOI] [PubMed] [Google Scholar]
- 156.Holmboe ES, Fiellin DA, Cusanelli E, Remetz M, Krumholz HM. Perceptions of benefit and risk of patients undergoing first-time elective percutaneous coronary revascularization. J Gen Intern Med. 2000;15:632–637. doi: 10.1046/j.1525-1497.2000.90823.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Pressler SJ. Cognitive functioning and chronic heart failure: a review of the literature (2002-July 2007) J Cardiovasc Nurs. 2008;23:239–249. doi: 10.1097/01.JCN.0000305096.09710.ec. [DOI] [PubMed] [Google Scholar]
- 158.Pressler SJ, Subramanian U, Kareken D, Perkins SM, Gradus-Pizlo I, Sauvé MJ, Ding Y, Kim J, Sloan R, Jaynes H, Shaw RM. Cognitive deficits in chronic heart failure. Nurs Res. 2010;59:127–139. doi: 10.1097/NNR.0b013e3181d1a747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Nielsen-Bohlman L, Panzer AM, Kindig DA, editors. Institute of Medicine, Committee on Health Literacy. Health Literacy: A Prescription to End Confusion. Washington, DC: National Academies Press; 2004. [PubMed] [Google Scholar]
- 160.Kutner M, Greenberg E, Yin Y, Boyle B, Hsu Y-c, Dunleavy E. Literacy in Everyday Life: Results From the 2003 National Assessment of Adult Literacy. Washington, DC: US Dept of Education, National Center for Education Statistics; 2007. Publication No. NCES 2007-480. [Google Scholar]
- 161.America’s Health Literacy: Why We Need Accessible Health Information: An Issue Brief From the US Department of Health and Human Services. Rockville, MD: Agency for Healthcare Research and Quality; 2008. [Google Scholar]
- 162.Morrow DG, Weiner M, Steinley D, Young J, Murray MD. Patients’ health literacy and experience with instructions: influence preferences for heart failure medication instructions. J Aging Health. 2007;19:575–593. doi: 10.1177/0898264307304448. [DOI] [PubMed] [Google Scholar]
- 163.Morrow D, Clark D, Tu W, Wu J, Weiner M, Steinley D, Murray MD. Correlates of health literacy in patients with chronic heart failure. Gerontologist. 2006;46:669–676. doi: 10.1093/geront/46.5.669. [DOI] [PubMed] [Google Scholar]
- 164.Murray MD, Tu W, Wu J, Morrow D, Smith F, Brater DC. Factors associated with exacerbation of heart failure include treatment adherence and health literacy skills. Clin Pharmacol Ther. 2009;85:651–658. doi: 10.1038/clpt.2009.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Baker DW, Wolf MS, Feinglass J, Thompson JA. Health literacy, cognitive abilities, and mortality among elderly persons. J Gen Intern Med. 2008;23:723–726. doi: 10.1007/s11606-008-0566-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Sudore RL, Yaffe K, Satterfield S, Harris TB, Mehta KM, Simonsick EM, Newman AB, Rosano C, Rooks R, Rubin SM, Ayonayon HN, Schillinger D. Limited literacy and mortality in the elderly: the Health, Aging, and Body Composition Study. J Gen Intern Med. 2006;21:806–812. doi: 10.1111/j.1525-1497.2006.00539.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Peterson PN, Shetterly SM, Clarke CL, Bekelman DB, Chan PS, Allen LA, Matlock DD, Magid DJ, Masoudi FA. Health literacy and outcomes among patients with heart failure. JAMA. 2011;305:1695–1701. doi: 10.1001/jama.2011.512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Evangelista LS, Rasmusson KD, Laramee AS, Barr J, Ammon SE, Dunbar S, Ziesche S, Patterson JH, Yancy CW. Health literacy and the patient with heart failure: implications for patient care and research: a consensus statement of the Heart Failure Society of America. J Card Fail. 2010;16:9–16. doi: 10.1016/j.cardfail.2009.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Ottawa Hospital Research Institute. [February 23, 2012];Patient decision aids. http://decisionaid.ohri.ca/
- 170.Glass AP, Nahapetyan L. Discussions by elders and adult children about end-of-life preparation and preferences. Prev Chronic Dis. 2008;5:A08. [PMC free article] [PubMed] [Google Scholar]
- 171.Blackhall LJ, Frank G, Murphy ST, Michel V, Palmer JM, Azen SP. Ethnicity and attitudes towards life sustaining technology. Soc Sci Med. 1999;48:1779–1789. doi: 10.1016/s0277-9536(99)00077-5. [DOI] [PubMed] [Google Scholar]
- 172.US Census Bureau. [February 23, 2012]; http://www.census.gov/hhes/socdemo/language/
- 173.Baker DW, Parker RM, Williams MV, Coates WC, Pitkin K. Use and effectiveness of interpreters in an emergency department. JAMA. 1996;275:783–788. [PubMed] [Google Scholar]
- 174.Leanza Y, Boivin I, Rosenberg E. Interruptions and resistance: a comparison of medical consultations with family and trained interpreters. Soc Sci Med. 2010;70:1888–1895. doi: 10.1016/j.socscimed.2010.02.036. [DOI] [PubMed] [Google Scholar]
- 175.Pear R. U.S. Alters Rule on Paying for End-of-Life Planning. [February 23, 2012];New York Times. 2011 Jan 4; http://www.nytimes.com/2011/01/05/health/policy/05health.html?_r=1.
- 176.Temel JS, Greer JA, Muzikansky A, Gallagher ER, Admane S, Jackson VA, Dahlin CM, Blinderman CD, Jacobsen J, Pirl WF, Billings JA, Lynch TJ. Early palliative care for patients with metastatic non-small-cell lung cancer. N Engl J Med. 2010;363:733–742. doi: 10.1056/NEJMoa1000678. [DOI] [PubMed] [Google Scholar]
- 177.Morrison RS, Penrod JD, Cassel JB, Caust-Ellenbogen M, Litke A, Spragens L, Meier DE. Cost savings associated with US hospital palliative care consultation programs. Arch Intern Med. 2008;168:1783–1790. doi: 10.1001/archinte.168.16.1783. [DOI] [PubMed] [Google Scholar]
- 178.Bakitas M, Lyons KD, Hegel MT, Balan S, Brokaw FC, Seville J, Hull JG, Li Z, Tosteson TD, Byock IR, Ahles TA. Effects of a palliative care intervention on clinical outcomes in patients with advanced cancer: the Project ENABLE II randomized controlled trial. JAMA. 2009;302:741–749. doi: 10.1001/jama.2009.1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Morrison RS, Dietrich J, Ladwig S, Quill T, Sacco J, Tangeman J, Meier DE. Palliative care consultation teams cut hospital costs for Medicaid beneficiaries. Health Aff (Millwood) 2011;30:454–463. doi: 10.1377/hlthaff.2010.0929. [DOI] [PubMed] [Google Scholar]
- 180.O’Connor AM, Bennett C, Stacey D, Barry MJ, Col NF, Eden KB, Entwistle V, Fiset V, Holmes-Rovner M, Khangura S, Llewellyn-Thomas H, Rovner DR. Do patient decision aids meet effectiveness criteria of the International Patient Decision Aid Standards Collaboration? A systematic review and meta-analysis. Med Decis Making. 2007;27:554–574. doi: 10.1177/0272989X07307319. [DOI] [PubMed] [Google Scholar]
- 181.O’Connor AM, Wennberg JE, Legare F, Llewellyn-Thomas HA, Moulton BW, Sepucha KR, Sodano AG, King JS. Toward the “tipping point”: decision aids and informed patient choice. Health Aff (Millwood) 2007;26:716–725. doi: 10.1377/hlthaff.26.3.716. [DOI] [PubMed] [Google Scholar]
- 182.Payne J, Bettman J, Schadke D. Measuring constructed preferences: towards a building code. J Risk Uncertain. 1999;19:243–270. [Google Scholar]
- 183.Arnold SV, Decker C, Ahmad H, Olabiyi O, Mundluru S, Reid KJ, Soto GE, Gansert S, Spertus JA. Converting the informed consent from a perfunctory process to an evidence-based foundation for patient decision making. Circ Cardiovasc Qual Outcomes. 2008;1:21–28. doi: 10.1161/CIRCOUTCOMES.108.791863. [DOI] [PubMed] [Google Scholar]
- 184.O’Connor AM, Bennett CL, Stacey D, Barry M, Col NF, Eden KB, Entwistle VA, Fiset V, Holmes-Rovner M, Khangura S, Llewellyn-Thomas H, Rovner D. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev. 2009;(3) doi: 10.1002/14651858.CD001431.pub2. CD001431. [DOI] [PubMed] [Google Scholar]
- 185.Stacey D, Murray MA, Légaré F, Sandy D, Menard P, O’Connor A. Decision coaching to support shared decision making: a framework, evidence, and implications for nursing practice, education, and policy. Worldviews Evid Based Nurs. 2008;5:25–35. doi: 10.1111/j.1741-6787.2007.00108.x. [DOI] [PubMed] [Google Scholar]
- 186.O’Connor AM, Stacey D, Légaré F. Coaching to support patients in making decisions. BMJ. 2008;336:228–229. doi: 10.1136/bmj.39435.643275.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Wennberg JE, O’Connor AM, Collins ED, Weinstein JN. Extending the P4P agenda, part 1: how Medicare can improve patient decision making and reduce unnecessary care. Health Aff (Millwood) 2007;26:1564–1574. doi: 10.1377/hlthaff.26.6.1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Larochelle MR, Rodriguez KL, Arnold RM, Barnato AE. Hospital staff attributions of the causes of physician variation in end-of-life treatment intensity. Palliat Med. 2009;23:460–470. doi: 10.1177/0269216309103664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Sprung CL, Woodcock T, Sjokvist P, Ricou B, Bulow HH, Lippert A, Maia P, Cohen S, Baras M, Hovilehto S, Ledoux D, Phelan D, Wennberg E, Schobersberger W. Reasons, considerations, difficulties and documentation of end-of-life decisions in European intensive care units: the ETHICUS Study. Intensive Care Med. 2008;34:271–277. doi: 10.1007/s00134-007-0927-1. [DOI] [PubMed] [Google Scholar]
- 190.Calam B, Far S, Andrew R. Discussions of “code status” on a family practice teaching ward: what barriers do family physicians face? CMAJ. 2000;163:1255–1259. [PMC free article] [PubMed] [Google Scholar]
- 191.Konstam MA, Jessup M, Francis GS, Mann DL, Greenberg B. Advanced heart failure and transplant cardiology: a subspecialty is born. J Card Fail. 2009;15:98–100. doi: 10.1016/j.cardfail.2008.12.012. [DOI] [PubMed] [Google Scholar]
- 192.The Joint Commission. [July 20, 2011];Advanced Certification in Heart Failure. www.jointcommission.org/assets/1/18/Advanced%20Heart%20Failure%20Requirements1.PDF.
- 193.Robinson K, Sutton S, von Gunten CF, Ferris FD, Molodyko N, Martinez J, Emanuel LL. Assessment of the Education for Physicians on End-of-Life Care (EPEC) Project. J Palliat Med. 2004;7:637–645. doi: 10.1089/jpm.2004.7.637. [DOI] [PubMed] [Google Scholar]
- 194.VanGeest JB. Process evaluation of an educational intervention to improve end-of-life care: the Education for Physicians on End-of-Life Care (EPEC) program. Am J Hosp Palliat Care. 2001;18:233–238. doi: 10.1177/104990910101800407. [DOI] [PubMed] [Google Scholar]
- 195.Back AL, Arnold RM, Baile WF, Fryer-Edwards KA, Alexander SC, Barley GE, Gooley TA, Tulsky JA. Efficacy of communication skills training for giving bad news and discussing transitions to palliative care. Arch Intern Med. 2007;167:453–460. doi: 10.1001/archinte.167.5.453. [DOI] [PubMed] [Google Scholar]
- 196.Back AL, Arnold RM, Tulsky JA, Baile WF, Fryer-Edwards KA. Teaching communication skills to medical oncology fellows. J Clin Oncol. 2003;21:2433–2436. doi: 10.1200/JCO.2003.09.073. [DOI] [PubMed] [Google Scholar]
- 197.Advance Planning and Compassionate Care Act of 2002, S 2857, 107th Congress, 2nd Sess. 2002 [Google Scholar]
- 198.Kelly K, Ersek M, Virani R, Malloy P, Ferrell B End-of-Life Nursing Education Consortium. Geriatric Training Program: improving palliative care in community geriatric care settings. J Gerontol Nurs. 2008;34:28–35. doi: 10.3928/00989134-20080501-06. [DOI] [PubMed] [Google Scholar]
- 198a. [February 23, 2012];Healthwise. http://www.healthwise.org/
- 198b.The Foundation for Informed Medical Decision Making. [February 23, 2012]; http://www.informedmedicaldecisions.org/
- 199.Kennedy AD. On what basis should the effectiveness of decision aids be judged? Health Expect. 2003;6:255–268. doi: 10.1046/j.1369-6513.2003.00240.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Sepucha KR, Fowler FJ, Jr, Mulley AG., Jr Policy support for patient-centered care: the need for measurable improvements in decision quality. Health Aff (Millwood) 2004;(Suppl Variation):VAR54–VAR62. doi: 10.1377/hlthaff.var.54. [DOI] [PubMed] [Google Scholar]
- 201.Bekelman DB, Havranek EP. Palliative care for patients with acute decompensated heart failure: an underused service? Nat Clin Pract Cardiovasc Med. 2008;5:250–251. doi: 10.1038/ncpcardio1154. [DOI] [PubMed] [Google Scholar]
- 202.Stewart AL, Teno J, Patrick DL, Lynn J. The concept of quality of life of dying persons in the context of health care. J Pain Symptom Manage. 1999;17:93–108. doi: 10.1016/s0885-3924(98)00131-6. [DOI] [PubMed] [Google Scholar]
- 203.Green CP, Porter CB, Bresnahan DR, Spertus JA. Development and evaluation of the Kansas City Cardiomyopathy Questionnaire: a new health status measure for heart failure. J Am Coll Cardiol. 2000;35:1245–1255. doi: 10.1016/s0735-1097(00)00531-3. [DOI] [PubMed] [Google Scholar]
- 204.Rector TS, Cohn JN Pimobendan Multicenter Research Group. Assessment of patient outcome with the Minnesota Living with Heart Failure questionnaire: reliability and validity during a randomized, double-blind, placebo-controlled trial of pimobendan. Am Heart J. 1992;124:1017–1025. doi: 10.1016/0002-8703(92)90986-6. [DOI] [PubMed] [Google Scholar]
- 205.Steinhauser KE, Bosworth HB, Clipp EC, McNeilly M, Christakis NA, Parker J, Tulsky JA. Initial assessment of a new instrument to measure quality of life at the end of life. J Palliat Med. 2002;5:829–841. doi: 10.1089/10966210260499014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


