Abstract
Background: Circadian rhythm has been shown to be related to glucose metabolism and risk of diabetes, probably through effects on energy balance. Recent genome-wide association studies identified variants in circadian rhythm–related genes (CRY2 and MTNR1B) associated with glucose homeostasis.
Objective: We tested whether CRY2 and MTNR1B genotypes affected changes in measures of energy expenditure in response to a weight-loss diet intervention in a 2-y randomized clinical trial, the POUNDS (Preventing Overweight Using Novel Dietary Strategies) LOST Trial.
Design: The variants CRY2 rs11605924 (n = 721) and MTNR1B rs10830963 (n = 722) were genotyped in overweight or obese adults who were randomly assigned to 1 of 4 weight-loss diets that differed in their proportions of macronutrients. Respiratory quotient (RQ) and resting metabolic rate (RMR) were measured.
Results: By 2 y of diet intervention, the A allele of CRY2 rs11605924 was significantly associated with a greater reduction in RQ (P = 0.03) and a greater increase in RMR and RMR/kg (both P = 0.04). The G allele of MTNR1B rs10830963 was significantly associated with a greater increase in RQ (P = 0.01) but was not related to changes in RMR and RMR/kg. In addition, we found significant gene-diet fat interactions for both CRY2 (P-interaction = 0.02) and MTNR1B (P-interaction < 0.001) in relation to 2-y changes in RQ.
Conclusions: Our data indicate that variants in the circadian-related genes CRY2 and MTNR1B may affect long-term changes in energy expenditure, and dietary fat intake may modify the genetic effects. This trial was registered at www.clinicaltrials.gov as NCT00072995.
INTRODUCTION
Compelling evidence has related disturbed circadian rhythms to obesity and diabetes—a disorder of abnormal glucose metabolism (1, 2). Recent genome-wide association studies identified a group of genetic variants determining fasting glucose concentrations. Interestingly, 2 of the glucose-associated genes—cryptochrome 2 (CRY2) (3) and melatonin receptor 1B (MTNR1B)—(4) are involved in the regulation of circadian rhythms.
One of the principal pathways that link diurnal rhythm and glucose metabolism is through alteration in balance between energy expenditure and intake. Recent evidence has shown that glucose homeostasis and energy metabolism are controlled basically along with each other through a central pacemaker in the circadian system (5). Energy expenditure, which is under tight regulation of the circadian rhythm (6–9), plays a key role in glucose metabolism (10).
In this study, we examined the associations of the genetic variants at CRY2 and MTNR1B loci, which are related to both glucose metabolism and circadian rhythms, with 2-y change in measures of energy expenditure in response to a weight-loss diet intervention in a randomized clinical trial. In addition, we assessed the potential gene-diet interactions.
SUBJECTS AND METHODS
Study population
The POUNDS (Preventing Overweight Using Novel Dietary Strategies)4 LOST trial was conducted from October 2004 through December 2007 at 2 sites: Harvard School of Public Health and Brigham and Women's Hospital in Boston, MA, and the Pennington Biomedical Research Center of Louisiana State University System, Baton Rouge, LA. The study design and sample collection have been described in detail previously (11). The study population included 811 overweight or obese [BMI (in kg/m2): 25–40] participants aged 30–70 y. Major criteria for exclusion were the presence of diabetes or unstable cardiovascular disease, the use of medications that affect body weight, and insufficient motivation as assessed by interview and questionnaire (12). Individuals with type 2 diabetes controlled with diet, or with hypertension or hyperlipidemia treated with diet or drugs, were eligible to participate. Participants were randomly assigned to 1 of 4 diets; the target percentages of energy derived from fat, protein, and carbohydrate, respectively, in the 4 diets were 20%, 15%, and 65%; 20%, 25%, and 55%; 40%, 15%, and 45%; and 40%, 25% and 35%. By constituting a 2-by-2 factorial design, 2 diets were low-fat (20%), 2 were high-fat (40%), 2 were average-protein (15%), and 2 were high-protein (25%). After 2 y, 80% of the participants (n = 645) completed the trial.
Food provision or compliance of diets was tracked through a computer tracking system. A computer tracking system was used to self-monitor behaviors and provide feedback. Dietitians and other study staff entered the data into the computer tracking system via a World Wide Web–based application each time contact was made with a participant (individual or group sessions or via telephone, e-mail, or mail). The counselor entered objective data consisting of body weight (measured at an intervention session), attendance, intervention make-up sessions, and number of days of food diaries and physical activity records completed. We added the mean values of calorie intakes and biomarkers of adherence at 6 mo and at 2 y and at baseline for comparison.
In the current study, data were analyzed among the 721 and 722 participants with CRY2 rs11605924 and MTNR1B rs10830963 genotypes, respectively. Of the study participants, 61% were women, 80% were white, 15% were African American, 3.5% were Hispanic, and 1.5% were Asian or other ethnic groups by self-report. The study was approved by the human subjects committee at each institution and by a data and safety monitoring board appointed by the National Heart, Lung, and Blood Institute. All participants provided written informed consent.
Measurements of adiposity and other variables
Body weight and waist circumference were measured in the morning before breakfast on 2 d at baseline, 6 mo, and 2 y. BMI was calculated as weight (kg)/height (m)2. Dietary intake was assessed in a random sample of 50% of the participants, by a review of the 5-d diet record at baseline, and by 24-h recall during a telephone interview on 3 nonconsecutive days at 6 mo and at 2 y. Fasting blood samples were obtained on one day. Analyses of serum lipids and glucose were performed at the Clinical Laboratory at Pennington. Triglycerides, total cholesterol, LDL cholesterol, and HDL cholesterol were measured on the Synchron CX7 (Beckman Coulter). LDL cholesterol was calculated for each participant according to the following formula: LDL cholesterol = total cholesterol − HDL cholesterol − triglycerides/5 (13), except when triglyceride concentration exceeded 400 mg/dL, in which case LDL cholesterol was measured directly in all samples of the participants. Glucose and insulin were measured by using an immunoassay with chemiluminescent detection on the Immulite (Diagnostic Products Corporation). Glycated hemoglobin was measured on a Synchron CX5 (Beckman Coulter). Blood pressure was measured on 2 d at baseline and at 6, 12, and 24 mo with an automated device (IntelliSense Professional Digital Blood Pressure Monitor; HEM907XL Omron HealthCare) by using methods established in other large NIH trials (14). The calibration was evaluated at regular intervals by using a mercury manometer.
Measurements of energy expenditure
Respiratory quotient (RQ) and resting metabolic rate (RMR) were obtained by DeltaTrac II metabolic cart. RMR was used to estimate energy requirements. For the measurement of energy expenditure and substrate oxidation under resting conditions, an open-circuit ventilated-hood setup was used for indirect calorimetry. This procedure has advantages over direct calorimetry, which measures the amount of heat released by an individual. The advantage of indirect calorimetry over direct calorimetry is that the method is simpler and cheaper, the response time for changes in energy expenditure is faster, and, when carbon dioxide production and urinary nitrogen excretion are simultaneously measured, it is possible to calculate substrate oxidation rates as well. The Deltatrac II Metabolic Monitor, which has a 25-L transparent plastic canopy through which a constant ambient airflow of ∼40 L/min is pulled, was placed over the volunteer's head while he or she was resting comfortably. By burning 5 mL ethanol and determining the amount of carbon dioxide recovered, the exact airflow was calculated for each system individually. Oxygen and carbon dioxide concentrations in constant airflow were continuously measured while the concentrations of the ambient air were measured every 4 min for carbon dioxide and for oxygen by using a differential oxygen sensor. Before each measurement, the analyzers were calibrated by using standard gases containing ∼95% O2 and ∼5% CO2. The accuracy of the Deltratrac is 96%.
For measurement of RMR, the participant arrived in the morning after fasting overnight for 12 h and having performed a minimal amount of physical activity. The participants were also instructed not to consume any caffeine or alcohol during the evening and morning preceding each test and to not perform any strenuous activities. On arrival, the volunteer rested for 30 min before the measurement. Oxygen consumption and carbon dioxide production were then measured for 30 min, and energy expenditure was calculated. RQ, a ratio of metabolic gas exchange, was computed as the quantity of carbon dioxide produced divided by the amount of oxygen consumed per unit time. Of the 810 (baseline), 641 (6 mo), and 497 (24 mo) participants with RMR and RQ measurements, 720, 582, and 456 were genotyped in CRY2 rs11605924, and 721, 582, and 455 were genotyped in MTNR1B rs10830963.
Genotyping
DNA was extracted from the buffy-coat fraction of centrifuged blood by using the QIAmp Blood Kit (Qiagen). The single nucleotide polymorphisms (SNPs) CRY2 rs11605924 and MTNR1B rs10830963 were successfully genotyped in 721 and 722 participants, respectively, by using the OpenArray SNP Genotyping System (BioTrove). Replicated quality-control samples (10%) were included in every genotyping plate with >99% concordance (15). The genotype distribution was in Hardy-Weinberg equilibrium in all participants or in whites (P > 0.05).
Statistical analysis
The Hardy-Weinberg equilibrium and comparison of categorical variables were assessed by using a chi-square test. Differences in continuous variables at baseline by genotype were tested by using a general linear models. Appropriate adjustments, such as age, sex, ethnicity, baseline value for the respective outcome, calorie intake, and weight change, were used in the analysis. Linear mixed models, with time used as a repeated measurement factor, were applied to test genetic associations with the trajectory of changes in RMR, RQ, and weight in the participants who provided measurements at baseline, 6 mo, and 2 y. Time was considered as a categorical variable in the mixed models. For gene-diet interactions, data were pooled for 2 factorial comparisons: low fat compared with high fat or low protein compared with high protein (11). An interaction term of genotype-by-diet was included in the general linear model. All reported P values were 2-sided, and a P value of 0.05 was considered statistically significant. All data were analyzed with PASW Statistics 18 (SPSS).
RESULTS
Characteristics of the study population
The baseline characteristics of the participants, according to CRY2 rs11605924 and MTNR1B rs10830963 genotypes, are shown in Table 1. The distributions of the 2 variants were in Hardy-Weinberg equilibrium in the study sample (P > 0.05), and the minor allele frequencies were 0.47 and 0.26, respectively. The distribution of CRY2 rs11605924 was different by sex (P = 0.004), ethnicity, and age (P < 0.0001). Baseline concentrations of glucose, insulin, RMR, and RQ were not correlated with the CRY2 genotype. The distribution of MTNR1B rs10830963 was different by ethnicity (P < 0.0001). The MTNR1B genotype was marginally correlated with baseline fasting glucose (P = 0.07) and diastolic blood pressure (P = 0.05), but not with insulin concentration and measures of energy expenditure.
TABLE 1.
Baseline characteristics of the study participants according to CRY2 and MTNR1B genotypes1
|
CRY2 rs11605924 |
MTNR1B rs10830963 |
|||||||||
| Characteristic | AA (n = 211) | AC (n = 342) | CC (n = 168) | Total (n = 721) | P2 | CC (n = 393) | CG (n = 273) | GG (n = 56) | Total (n = 722) | P2 |
| Age (y) | 49.02 ± 9.323 | 51.41 ± 9.00 | 52.76 ± 8.99 | 51.03 ± 9.19 | <0.0001 | 50.77 ± 9.18 | 51.04 ± 9.05 | 53.00 ± 10.28 | 51.04 ± 9.23 | 0.23 |
| Sex [n (%)] | 0.004 | 0.72 | ||||||||
| Men | 62 (29.4) | 146 (42.7) | 72 (42.9) | 280 (38.8) | 149 (37.9) | 112 (41.0) | 22 (39.3) | 283 (39.2) | ||
| Women | 14 (70.6) | 196 (57.3) | 96 (57.1) | 441 (61.2) | 244 (62.1) | 161 (59.0) | 34 (60.7) | 439 (60.8) | ||
| Ethnicity [n (%)] | <0.0001 | 0.0001 | ||||||||
| White | 115 (54.5) | 298 (87.1) | 163 (97.0) | 576 (79.9) | 279 (71.0) | 247 (90.5) | 49 (87.5) | 575 (79.6) | ||
| Black | 78 (37.0) | 30 (8.8) | 1 (0.6) | 109 (15.1) | 96 (24.4) | 12 (4.4) | 3 (5.4) | 111 (15.4) | ||
| Hispanic | 10 (4.7) | 12 (3.5) | 3 (1.8) | 25 (3.5) | 13 (3.3) | 10 (3.7) | 2 (3.6) | 25 (3.5) | ||
| Asian or other | 8 (3.8) | 2 (0.6) | 1 (0.6) | 11 (1.5) | 5 (1.3) | 4 (1.5) | 2 (3.6) | 11 (1.5) | ||
| Weight (kg) | 93.24 ± 14.61 | 93.22 ± 16.05 | 93.21 ± 15.77 | 93.22 ± 15.55 | NS | 93.67 ± 14.96 | 92.84 ± 15.98 | 92.00 ± 17.49 | 93.23 ± 15.54 | 0.65 |
| WC (cm) | 102.78 ± 12.05 | 103.93 ± 13.58 | 103.94 ± 13.32 | 103.59 ± 13.08 | 0.5 | 103.87 ± 12.99 | 103.17 ± 13.18 | 103.92 ± 13.37 | 103.61 ± 13.08 | 0.78 |
| BMI (kg/m2) | 33.04 ± 3.79 | 32.47 ± 3.94 | 32.54 ± 3.78 | 32.65 ± 3.86 | 0.2 | 32.92 ± 3.83 | 32.28 ± 3.91 | 32.54 ± 3.73 | 32.65 ± 3.86 | 0.10 |
| Glucose (mg/dL) | 91.13 ± 12.54 | 92.55 ± 11.51 | 91.67 ± 11.49 | 91.93 ± 11.82 | 0.37 | 91.56 ± 12.19 | 91.34 ± 10.42 | 96.96 ± 14.33 | 91.90 ± 11.82 | 0. 07 |
| TC (mg/dL) | 199.22 ± 35.86 | 202.68 ± 36.64 | 204.67 ± 39.14 | 202.13 ± 37.02 | 0.33 | 199.97 ± 37.27 | 204.36 ± 37.24 | 206.16 ± 33.90 | 202.11 ± 37.04 | 0.22 |
| LDL-C (mg/dL) | 125.25 ± 32.07 | 124.46 ± 31.22 | 127.38 ± 33.86 | 125.37 ± 32.08 | 0.62 | 125.01 ± 31.97 | 125.86 ± 32.67 | 126.38 ± 31.25 | 125.44 ± 32.14 | 0.92 |
| HDL-C (mg/dL) | 49.94 ± 13.31 | 48.02 ± 13.98 | 48.87 ± 15.42 | 48.78 ± 14.14 | 0.29 | 48.19 ± 13.27 | 48.94 ± 13.81 | 51.20 ± 20.29 | 48.71 ± 14.13 | 0.31 |
| TG (mg/dL) | 124.06 ± 84.98 | 154.40 ± 85.54 | 147.24 ± 84.99 | 143.85 ± 86.13 | 0.68 | 137.36 ± 86.80 | 151.43 ± 85.40 | 151.55 ± 84.46 | 143.78 ± 86.26 | 0.09 |
| Insulin (IU/mL) | 11.96 ± 8.59 | 12.19 ± 7.26 | 12.37 ± 7.67 | 12.16 ± 7.76 | 0.87 | 12.41 ± 7.28 | 11.34 ± 7.99 | 13.93 ± 9.15 | 12.12 ± 7.73 | 0.87 |
| HOMA | 2.75 ± 2.12 | 2.86 ± 1.85 | 2.87 ± 1.96 | 2.83 ± 1.96 | 0.77 | 2.87 ± 1.82 | 2.62 ± 2.03 | 3.43 ± 2.35 | 2.82 ± 1.96 | 0.56 |
| SBP (mm Hg) | 118.94 ± 13.37 | 120.14 ± 13.19 | 119.44 ± 13.76 | 119.62 ± 13.37 | 0.58 | 119.89 ± 13.30 | 119.86 ± 13.82 | 116.91 ± 10.78 | 119.65 ± 13.33 | 0.27 |
| DBP (mm Hg) | 75.34 ± 9.51 | 75.57 ± 9.54 | 75.83 ± 8.71 | 75.56 ± 9.33 | 0.88 | 75.73 ± 9.09 | 75.91 ± 9.83 | 72.69 ± 7.67 | 75.56 ± 9.30 | 0.054 |
| RMR (kcal/d) | 1524.9 ± 283.6 | 1575.9 ± 308.0 | 1566.1 ± 313.8 | 1558.7 ± 302.8 | 0.35 | 1553.7 ± 298.2 | 1567.1 ± 310.8 | 1554.3 ± 316.6 | 1558.8 ± 304.1 | 0.85 |
| RQ | 0.840 ± 0.049 | 0.840 ± 0.040 | 0.845 ± 0.039 | 0.841 ± 0.043 | 0.14 | 0.841 ± 0.042 | 0.843 ± 0.044 | 0.831 ± 0.042 | 0.841 ± 0.043 | 0.18 |
DBP, diastolic blood pressure; HDL-C, HDL cholesterol; LDL-C, LDL cholesterol; RMR, resting metabolic rate; RQ, respiratory quotient; SBP, systolic blood pressure; TC, total cholesterol; TG, triglyceride; WC, waist circumference.
P values were calculated by chi-square test for categorical variables and ANOVA for continuous variables. All significant values were adjusted for age, sex, and ethnicity in the regression model.
Mean ± SD (all such values).
Genotype effects on 2-y change in energy expenditure measures
We examined the genotype effects on 2-y changes in measures of energy expenditure in the whole study population. After adjustment for age, sex, ethnicity, diet, weight loss, and baseline values for respective outcomes, we found that carrying the A allele of CRY2 rs11605924 was significantly associated with more reduction in RQ (P = 0.03), but greater increase in RMR and RMR/kg at 2 y (both P = 0.04). The G allele of MTNR1B rs10830963 was significantly associated with a greater increase in RQ (P = 0.01), but was not related to changes in RMR and RMR/kg (Table 2).
TABLE 2.
Genotype effect on 2-y change in energy expenditure measures after the intervention
|
CRY2 rs11605924 (A/C) |
MTNR1B rs10830963 (C/G) |
|||||||||
| Month 24 − baseline | Genotype | N | Mean ± SE | β | P1 | Genotype | N | Mean ± SE | β | P1 |
| Respiratory quotient | AA | 115 | −0.017 ± 0.005 | 0.047 | 0.03 | CC | 243 | −0.015 ± 0.00352 | 0.117 | 0.01 |
| AC | 239 | −0.012 ± 0.003 | CG | 168 | −0.013 ± 0.00422 | |||||
| CC | 102 | −0.006 ± 0.005 | GG | 44 | 0.015 ± 0.00752 | |||||
| Total | 456 | −0.012 ± 0.002 | Total | 454 | −0.011 ± 0.002 | |||||
| Change in resting metabolic rate (%) | AA | 115 | 2.15 ± 1.333 | −0.094 | 0.04 | CC | 243 | −0.58 ± 0.89 | 0.019 | 0.65 |
| AC | 239 | −0.87 ± 0.88 | CG | 168 | 0.88 ± 1.17 | |||||
| CC | 102 | −2.8 ± 1.513 | GG | 44 | −3.27 ± 2.29 | |||||
| Total | 456 | −0.53 ± 0.66 | Total | 454 | −0.30 ± 0.68 | |||||
| Resting metabolic rate (kcal/kg) | AA | 115 | 1.11 ± 0.224 | −0.094 | 0.04 | CC | 243 | 0.72 ± 0.15 | 0.029 | 0.51 |
| AC | 239 | 0.89 ± 0.15 | CG | 168 | 1.17 ± 0.20 | |||||
| CC | 102 | 0.42 ± 0.264 | GG | 44 | 0.67 ± 0.36 | |||||
| Total | 456 | 0.84 ± 0.11 | Total | 455 | 0.88 ± 0.11 | |||||
An overall P value compares all 3 genotypes after adjustment for age, sex, ethnicity, diet, weight loss, and baseline value of each variable in the linear regression model.
A post hoc analysis using Tukey's procedure for pairwise comparison showed significant differences in respiratory quotient changes between the CC and GG genotypes (P = 0.002) and the CG and GG genotypes (P = 0.005).
A post hoc analysis using Tukey's procedure for pairwise comparison showed significant differences in percentage change in resting metabolic rate between the AA and CC genotypes (P = 0.02).
A post hoc analysis using Tukey's procedure for pairwise comparison showed significant differences in resting metabolic rate/weight changes between the AA and CC genotypes (P = 0.03).
No association was found between these 2 SNPs and weight loss and changes in blood pressure, glucose, insulin, glycated hemoglobin, lipids (see Supplementary Table 1 under “Supplemental data” in the online issue), and body compositions (see Supplementary Table 2 under “Supplemental data” in the online issue). We found no significant correlation between these SNPs and changes in calorie intake and biomarkers of adherence (P > 0.05) (Table 3).
TABLE 3.
Repeated measurements of energy intake per day and biomarkers of diet adherence in the study participants according to CRY2 and MTNR1B genotypes
|
CRY2 rs11605924 |
MTNR1B rs10830963 |
|||||||||
| AA | AC | CC | Total | P1 | CC | CG | GG | Total | P1 | |
| Baseline | ||||||||||
| Dietary intake per day | ||||||||||
| n | 102 | 186 | 83 | 371 | 208 | 133 | 29 | 370 | ||
| Energy (kcal) | 1900.03 ± 509.212 | 2006.47 ± 586.30 | 1947.79 ± 548.50 | 1964.08 ± 558.05 | 0.28 | 1945.33 ± 555.35 | 2016.31 ± 572.33 | 1883.62 ± 447.69 | 1966.01 ± 554.15 | 0.36 |
| Biomarkers of adherence | ||||||||||
| n | 211 | 342 | 168 | 721 | 393 | 273 | 56 | 722 | ||
| Urinary nitrogen (g) | 11.80 ± 3.90 | 12.43 ± 4.61 | 12.36 ± 4.66 | 12.23 ± 4.43 | 0.24 | 11.97 ± 4.58 | 12.41 ± 4.15 | 12.76 ± 4.67 | 12.20 ± 4.43 | 0.28 |
| 6 mo | ||||||||||
| Dietary intake per day | ||||||||||
| n | 83 | 163 | 79 | 325 | 172 | 128 | 25 | 325 | ||
| Energy (kcal) | 1511.78 ± 493.68 | 1690.96 ± 560.23 | 1582.16 ± 451.07 | 1618.75 ± 522.95 | 0.712 | 1614.93 ± 545.94 | 1642.45 ± 519.52 | 1524.75 ± 279.73 | 1618.83 ± 519.43 | 0.58 |
| Biomarkers of adherence | ||||||||||
| n | 133 | 269 | 125 | 527 | 277 | 202 | 47 | 526 | ||
| Urinary nitrogen (g) | 11.48 ± 4.39 | 11.78 ± 4.69 | 11.29 ± 4.46 | 11.59 ± 4.56 | 0.58 | 11.75 ± 4.85 | 11.45 ± 4.24 | 11.14 ± 4.19 | 11.58 ± 4.56 | 0.60 |
| 2 y | ||||||||||
| Dietary intake per day | ||||||||||
| n | 47 | 79 | 42 | 168 | 88 | 65 | 16 | 169 | ||
| Energy (kcal) | 1402.07 ± 390.45 | 1556.53 ± 443.40 | 1600.74 ± 621.68 | 1524.37 ± 484.63 | 0.11 | 1545.77 ± 518.38 | 1494.30 ± 462.77 | 1504.37 ± 381.87 | 1522.05 ± 484.13 | 0.8 |
| Biomarkers of adherence | ||||||||||
| n | 89 | 194 | 83 | 366 | 194 | 136 | 36 | 366 | ||
| Urinary nitrogen (g) | 12.39 ± 4.10 | 11.90 ± 4.47 | 11.82 ± 5.17 | 12.00 ± 4.54 | 0.64 | 11.80 ± 4.38 | 12.12 ± 4.75 | 12.34 ± 4.52 | 11.97 ± 4.52 | 0.71 |
P values were calculated by ANOVA. All P values were from models adjusted for age, sex, and ethnicity.
Mean ± SD (all such values).
Gene-by-diet interactions on energy expenditure measures
We next tested whether the genotype effects of CRY2 rs11605924 and MTNR1B rs10830963 on energy expenditure differed by diet interventions. Both genotypes showed significant interactions with dietary fat intake in relation to the 2-y change in RQ. We found that the A allele of CRY2 rs11605924 was significantly associated with a greater reduction in RQ in the high-fat diet group as compared with the low-fat diet group (P-interaction = 0.02). The G allele of MTNR1B rs10830963 was significantly associated with a greater increase in RQ in the low-fat diet as compared with the high-fat diet (P-interaction < 0.001) (Figure 1).
FIGURE 1.
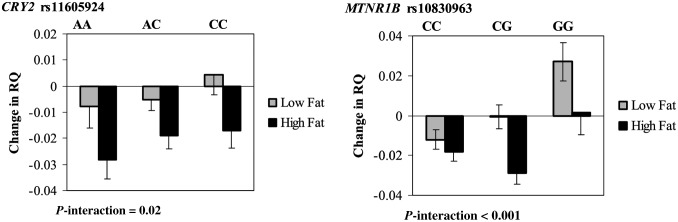
Interaction between the CRY2 or MTNR1B genotype and dietary fat intervention on change in RQ at 2 y. P-interactions between single nucleotide polymorphism and the low-fat or high-fat diet after adjustment for age, sex, ethnicity, and baseline weight are presented. Data included 57 and 58 (AA), 122 and 117 (AC), and 53 and 49 (CC) participants in the low-fat group and the high-fat group, respectively, at 2 y (total n = 456) in the CRY2 gene and 123 and 120 (CC), 86 and 82 (CG), and 24 and 20 (GG) participants in the low-fat group and the high-fat group, respectively, at 2 y (total n = 455) in the MTNR1B gene. A post hoc analysis that used Tukey's procedure showed significant differences in RQ change between the CC and GG genotypes in the low-fat diet group (P = 0.004). RQ, respiratory quotient.
The tests for the genotype-by-dietary fat interactions were not significant in relation to changes in other energy expenditure measures (RMR and RMR/kg) for both SNPs. In addition, the CRY2 and MTNR1B genotypes did not interact with dietary protein intake on changes in energy expenditure measures.
The trajectory of changes in response to diet
In secondary analyses, we also assessed the trajectory of change patterns in energy expenditure by using repeated measures at 6 mo and 2 y. The genotype-by-dietary fat interactions on changes in RQ remained significant for both CRY2 rs11605924 (P-interaction = 0.03; Figure 2) and MTNR1B rs10830963 (P-interaction < 0.0001; Figure 3). Participants with the CRY2 rs11605924 AA genotype had a consistently greater reduction in RQ in the high-fat diet group than in the low-fat diet group across the 2-y intervention (Figure 2). Participants with the MTNR1B rs10830963 GG genotype had a consistently greater increase in RQ in the low-fat diet group than in the high-fat diet group across the 2-y intervention (Figure 3). The gene-by-dietary fat interactions appeared more evident at the end of the 2-y intervention than at 6 mo, especially for MTNR1B rs10830963.
FIGURE 2.
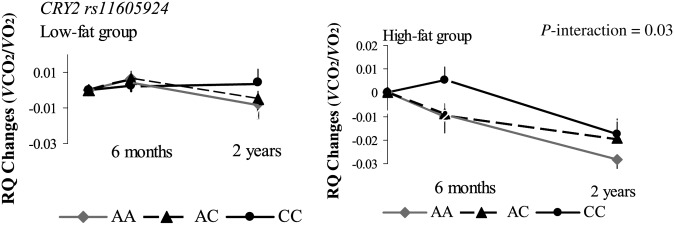
Interaction between the CRY2 rs11605924 genotype and the dietary fat intervention on RQ changes during a 2-y intervention. P-interactions between single nucleotide polymorphism and the low-fat or high-fat diet after adjustment for age, sex, ethnicity, and baseline weight are presented. Data included 110 and 101 (AA), 170 and 171 (AC), and 83 and 85 (CC) participants in the low-fat group and the high-fat group, respectively, at baseline (total n = 720); 80 and 72 (AA), 145 and 147 (AC), and 70 and 68 (CC) participants in the low-fat group and the high-fat group, respectively, at 6 mo (total n = 582); and 57 and 58 (AA), 122 and 117 (AC), and 53 and 49 (CC) participants in the low-fat group and the high-fat group, respectively, at 2 y (total n = 456). A post hoc analysis showed no significant difference between pairwise analyses of the genotypes. RQ, respiratory quotient; VCO2, carbon dioxide consumption; VO2, oxygen consumption.
FIGURE 3.
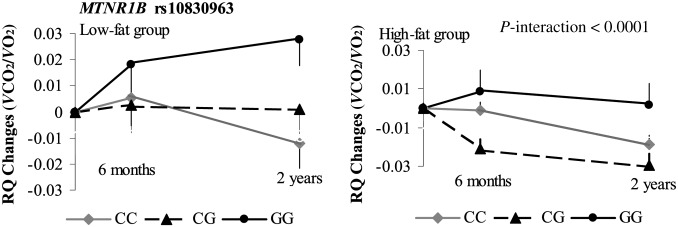
Interaction between MTNR1B rs10830963 genotype and the dietary fat intervention on RQ changes during the 2-y intervention. P-interactions between single nucleotide polymorphism and the low-fat or high-fat diet after adjustment for age, sex, ethnicity and baseline weight are presented. Data included 198 and 194 (CC), 134 and 139 (CG), and 31 and 25 (GG) participants in the low-fat group and the high-fat group, respectively, at baseline (total n = 721); 156 and 152 (CC), 113 and 111 (CG), and 27 and 23 (GG) participants in the low-fat group and the high-fat group, respectively, at 6 mo (total n = 582); and 123 and 120 (CC), 86 and 82 (CG), and 24 and 20 (GG) participants in the low-fat group and the high-fat group, respectively, at 2 y (total n = 455). A post hoc analysis that used Tukey's procedure showed significant differences in the 2-y RQ change between CC and GG genotypes in the low-fat diet group (P = 0.004) and in the 6-mo RQ change between the CC and CG (P = 0.008) and CG and GG (P = 0.03) genotypes in the high-fat diet group. RQ, respiratory quotient; VCO2, carbon dioxide consumption; VO2, oxygen consumption.
DISCUSSION
In the 2-y randomized weight-loss diet intervention trial, we found significant associations of CRY2 rs11605924 with changes in measures of energy expenditure, including RQ, RMR, and RMR/kg, and of the MTNR1B rs10830963 with changes in RQ. In addition, we observed significant interactions of dietary fat intake with CRY2 and MTNR1B genotypes on changes in RQ during the 2-y intervention.
It has been estimated that ∼10% of genes in a given tissue type exhibit a diurnal pattern (16). Recent genome-wide association studies identified a group of genomic loci determining blood concentrations of fasting glucose. Interestingly, 2 glucose-related loci, CRY2 and MTNR1B, both located on chromosome 11, are involved in circadian pathways (17–21). CRY2 regulates the expression of multiple clock-controlled genes (17–20) and plays a key role in feedback loops in circadian pathway (22–24). CRY2 knockout mice showed a lengthened circadian period (25), and it was found that the gene played an important role in the direction of diurnal cycle in the suprachiasmatic nucleus (26). MTNR1B is also involved in the circadian pathway through cross-talk with melatonin as a circulating hormone primarily released from the pineal gland (21, 27).
Our findings provide novel and important evidence linking the circadian-related genetic variants and energy expenditure; however, the results from this study suggest that these genetic variants might not have a functional effect on cardiometabolic parameters. Disturbance of energy homeostasis has been related to various health problems, including cardiovascular disease and type 2 diabetes (28). Our findings may open avenues for further investigations of the genetic variants related to circadian rhythms with these diseases, in long-term prospective studies and experimental analyses, to illustrate whether the observed genetic effects on energy expenditure and metabolic disorders are functional.
Recently, compelling evidence has shown that diurnal rhythm is closely related to glucose metabolism. Of the potential mechanisms, a prevailing explanation is that glucose metabolism is regulated (10) through circadian control of energy expenditure–related genes (6–9). It has been documented that a balance of energy expenditure and intake plays a pivotal role in the regulation of glucose homeostasis (29).
Interestingly, we found that dietary fat intake significantly modified the effect of CRY2 and MTNR1B variants, especially on changes in RQ—a parameter that measures fuel utilization. RQ exhibits circadian rhythm in human and rodents (30–32). There is evidence that a high-fat diet might affect the circadian expression of metabolic factors and obesity (33). The effects of high-fat feeding on the rhythmic mRNA expression of clock genes (34) or circadian rhythmicity balance (35) have also been observed in animals. However, little is known about the potential mechanisms underlying the modulation of dietary fat on the CRY2 and MTNR1B variants, and further experimental studies are warranted to clarify the precise mechanisms.
To our knowledge, this is the first investigation of the effects of the glucose- and circadian rhythm–related genetic variants on the response of energy expenditure to weight-loss diet interventions. However, many caveats need to be considered when interpreting our findings. Briefly, the relatively small sample size might limit the power to detect very moderate genetic effects or interactions. In addition, confounding might exist and influence our analyses. However, we did carefully adjust for the important factors that may affect energy expenditure measures, such as age, weight, dietary intake, sex, and ethnicity. We acknowledge that the measurement of clock parameters would strengthen the current study. We used genetic markers related to circadian rhythm. According to the Mendelian randomization theory, a genetic variant could be a better marker than biomarkers in causal inference, because it is less likely to be affected by confounding and reverse causation (36). We examined the exact measures of RMR and RQ only in participants who arrived in the morning after a 12-h overnight fast, had performed a minimal amount of physical activity, and did not consume any caffeine or alcohol during the evening and morning preceding each test. We acknowledge that replication in diverse populations is needed to verify our findings.
In summary, we found that genotypes of glucose- and circadian rhythm–related loci (CRY2 and MTNR1B) affected long-term changes in energy expenditure in response to dietary intervention, and dietary fat intake might modify the genetic effects.
Supplementary Material
Acknowledgments
We are particularly grateful to all the participants in the trial for their dedication and contribution to the research.
The authors’ responsibilities were as follows—KM and LQ: designed the research; KM, MX, QQ, and LQ: conducted the research; KM and LQ: analyzed the data; KM, MX, and LQ: wrote the manuscript; GAB, FS, LdJ, and GAB: provided the materials; and LQ: had primary responsibility for the final content of the manuscript. All authors read and approved the final manuscript. No potential conflicts of interest relevant to this article were reported.
Footnotes
Abbreviations used: POUNDS, Preventing Overweight Using Novel Dietary Strategies; RMR, resting metabolic rate; RQ, respiratory quotient; SNP, single nucleotide polymorphism.
REFERENCES
- 1.Shi SQ, Ansari TS, McGuinness OP, Wasserman DH, Johnson CH. Circadian disruption leads to insulin resistance and obesity. Curr Biol 2013;23:372–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bass J, Takahashi JS. Circadian integration of metabolism and energetics. Science 2010;330:1349–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dupuis J, Langenberg C, Prokopenko I, Saxena R, Soranzo N, Jackson AU, Wheeler E, Glazer NL, Bouatia-Naji N, Gloyn AL, et al. New genetic loci implicated in fasting glucose homeostasis and their impact on type 2 diabetes risk. Nat Genet 2010;42:105–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Prokopenko I, Langenberg C, Florez JC, Saxena R, Soranzo N, Thorleifsson G, Loos RJ, Manning AK, Jackson AU, Aulchenko Y, et al. Variants in MTNR1B influence fasting glucose levels. Nat Genet 2009;41:77–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coomans CP, van den Berg SA, Lucassen EA, Houben T, Pronk AC, van der Spek RD, Kalsbeek A, Biermasz NR, Willems van Dijk K, Romijn JA, et al. The suprachiasmatic nucleus controls circadian energy metabolism and hepatic insulin sensitivity. Diabetes 2013;62:1102–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oishi K, Shirai H, Ishida N. CLOCK is involved in the circadian transactivation of peroxisome-proliferator-activated receptor alpha (PPARalpha) in mice. Biochem J 2005;386:575–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu C, Li S, Liu T, Borjigin J, Lin JD. Transcriptional coactivator PGC-1alpha integrates the mammalian clock and energy metabolism. Nature 2007;447:477–81. [DOI] [PubMed] [Google Scholar]
- 8.Grimaldi B, Sassone-Corsi P. Circadian rhythms: metabolic clockwork. Nature 2007;447(7143):386ndash7. [DOI] [PubMed]
- 9.Lamia KA, Sachdeva UM, DiTacchio L, Williams EC, Alvarez JG, Egan DF, Vasquez DS, Juguilon H, Panda S, Shaw RJ, et al. AMPK regulates the circadian clock by cryptochrome phosphorylation and degradation. Science 2009;326:437–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kersten S, Desvergne B, Wahli W. Roles of PPARs in health and disease. Nature 2000;405:421–4. [DOI] [PubMed] [Google Scholar]
- 11.Sacks FM, Bray GA, Carey VJ, Smith SR, Ryan DH, Anton SD, McManus K, Champagne CM, Bishop LM, Laranjo N, et al. Comparison of weight-loss diets with different compositions of fat, protein, and carbohydrates. N Engl J Med 2009;360:859–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang X, Qi Q, Zhang C, Smith SR, Hu FB, Sacks FM, Bray GA, Qi L. FTO genotype and 2-year change in body composition and fat distribution in response to weight-loss diets: the POUNDS LOST Trial. Diabetes 2012;61:3005–11. Diabetes 2013;62:662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 1972;18:499–502. [PubMed] [Google Scholar]
- 14.Appel LJ, Sacks FM, Carey VJ, Obarzanek E, Swain JF, Miller ER, 3rd, Conlin PR, Erlinger TP, Rosner BA, Laranjo NM, et al. Effects of protein, monounsaturated fat, and carbohydrate intake on blood pressure and serum lipids: results of the OmniHeart randomized trial. JAMA 2005;294:2455–64. [DOI] [PubMed] [Google Scholar]
- 15.Qi Q, Bray GA, Smith SR, Hu FB, Sacks FM, Qi L. Insulin receptor substrate 1 gene variation modifies insulin resistance response to weight-loss diets in a 2-year randomized trial: the preventing overweight using novel dietary strategies (POUNDS LOST) trial. Circulation 2011;124:563–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mirsky HP, Liu A, Welsh D, Kay S, Doyle FJ., III A model of the cell-autonomous mammalian circadian clock. Proc Natl Acad Sci USA 2009;106:11107–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Darlington TK, Wager-Smith K, Ceriani M, Staknis D, Gekakis N, Steeves T, Weitz C, Takahashi J, Kay S. Closing the circadian loop: CLOCK-induced transcription of its own inhibitors per and tim. Science 1998;280:1599–603. [DOI] [PubMed] [Google Scholar]
- 18.Gekakis N, Staknis D, Nguyen H, Davis F, Wilsbacher L, King D, Takahashi J, Weitz C. Role of the CLOCK protein in the mammalian circadian mechanism. Science 1998;280:1564–9. [DOI] [PubMed] [Google Scholar]
- 19.Duffield GE, Best J, Meurers B, Bittner A, Loros J, Dunlap J. Circadian programs of transcriptional activation, signaling, and protein turnover revealed by microarray analysis of mammalian cells. Curr Biol 2002;12:551–7. [DOI] [PubMed] [Google Scholar]
- 20.Narasimamurthy R, Hatori M, Nayak S, Liu F, Panda S, Verma I. Circadian clock protein cryptochrome regulates the expression of proinflammatory cytokines. Proc Natl Acad Sci USA 2012;109:12662–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nagorny C, Lyssenko V. Tired of diabetes genetics? circadian rhythms and diabetes: the MTNR1B Story? Curr Diab Rep 2012;12:667–72. [DOI] [PubMed] [Google Scholar]
- 22.Dardente H, Fortier EE, Martineau V, Cermakian N. Cryptochromes impair phosphorylation of transcriptional activators in the clock: a general mechanism for circadian repression. Biochem J 2007;402:525–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hirota T, Lee JW, St John PC, Sawa M, Iwaisako K, Noguchi T, Pongsawakul PY, Sonntag T, Welsh DK, Brenner DA, et al. Identification of small molecule activators of cryptochrome. Science 2012;337:1094–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Padmanabhan K, Robles MS, Westerling T, Weitz CJ. Feedback regulation of transcriptional termination by the mammalian circadian clock PERIOD complex. Science 2012;337:599–602. [DOI] [PubMed] [Google Scholar]
- 25.van der Horst GT, Muijtjens M, Kobayashi K, Takano R, Kanno S, Takao M, de Wit J, Verkerk A, Eker AP, van Leenen D, et al. Mammalian Cry1 and Cry2 are essential for maintenance of circadian rhythms. Nature 1999;398:627–30. [DOI] [PubMed] [Google Scholar]
- 26.Anand SN, Maywood ES, Chesham JE, Joynson G, Banks GT, Hastings MH, Nolan PM. Distinct and separable roles for endogenous CRY1 and CRY2 within the circadian molecular clockwork of the suprachiasmatic nucleus, as revealed by the Fbxl3(Afh) mutation. J Neurosci 2013;33:7145–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mulder H, Nagorny CL, Lyssenko V, Groop L. Melatonin receptors in pancreatic islets: good morning to a novel type 2 diabetes gene. Diabetologia 2009;52:1240–9. [DOI] [PubMed] [Google Scholar]
- 28.Korner J, Woods SC, Woodworth KA. Regulation of energy homeostasis and health consequences in obesity. Am J Med 2009;122:S12–8. [DOI] [PubMed] [Google Scholar]
- 29.Kitazawa M. Circadian rhythms, metabolism, and insulin sensitivity: transcriptional networks in animal models. Curr Diab Rep 2013;13:223–8. [DOI] [PubMed] [Google Scholar]
- 30.Ravussin E, Burnand B, Schutz Y, Jequier E. Twenty-four-hour energy expenditure and resting metabolic rate in obese, moderately obese, and control subjects. Am J Clin Nutr 1982;35:566–73. [DOI] [PubMed] [Google Scholar]
- 31.Rubal A, Choshniak I, Haim A. Daily rhythms of metabolic rate and body temperature of two murids from extremely different habitats. Chronobiol Int 1992;9:341–9. [DOI] [PubMed] [Google Scholar]
- 32.Satoh Y, Kawai H, Kudo N, Kawashima Y, Mitsumoto A. Time-restricted feeding entrains daily rhythms of energy metabolism in mice. Am J Physiol Regul Integr Comp Physiol 2006;290:R1276–83. [DOI] [PubMed] [Google Scholar]
- 33.Sherman H, Genzer Y, Cohen R, Chapnik N, Madar Z, Froy O. Timed high-fat diet resets circadian metabolism and prevents obesity. FASEB J 2012;26:3493–502. [DOI] [PubMed] [Google Scholar]
- 34.Yanagihara H, Ando H, Hayashi Y, Obi Y, Fujimura A. High-fat feeding exerts minimal effects on rhythmic mRNA expression of clock genes in mouse peripheral tissues. Chronobiol Int 2006;23:905–14. [DOI] [PubMed] [Google Scholar]
- 35.Cano P, Jimenez-Ortega V, Larrad A, Reyes Toso CF, Cardinali DP, Esquifino AI. Effect of a high-fat diet on 24-h pattern of circulating levels of prolactin, luteinizing hormone, testosterone, corticosterone, thyroid-stimulating hormone and glucose, and pineal melatonin content, in rats. Endocrine 2008;33:118–25. [DOI] [PubMed] [Google Scholar]
- 36.Qi L. Mendelian randomization in nutritional epidemiology. Nutr Rev 2009;67:439–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


