Abstract
Antisera specific for each of the 21 homogenous ribosomal proteins from 30S subunits of Escherichia coli were used to investigate, by immunodiffusion and immunoprecipitation, whether there are any extensive sequence homologies among these proteins.
No immunological crossreactions were detected between any of the proteins. Therefore, we conclude that no significant common sequences exist among any of the 21 30S ribosomal proteins of E. coli.
Keywords: double-diffusion, Heidelberger curves, immunoprecipitation, rabbit, IgG
Full text
PDF

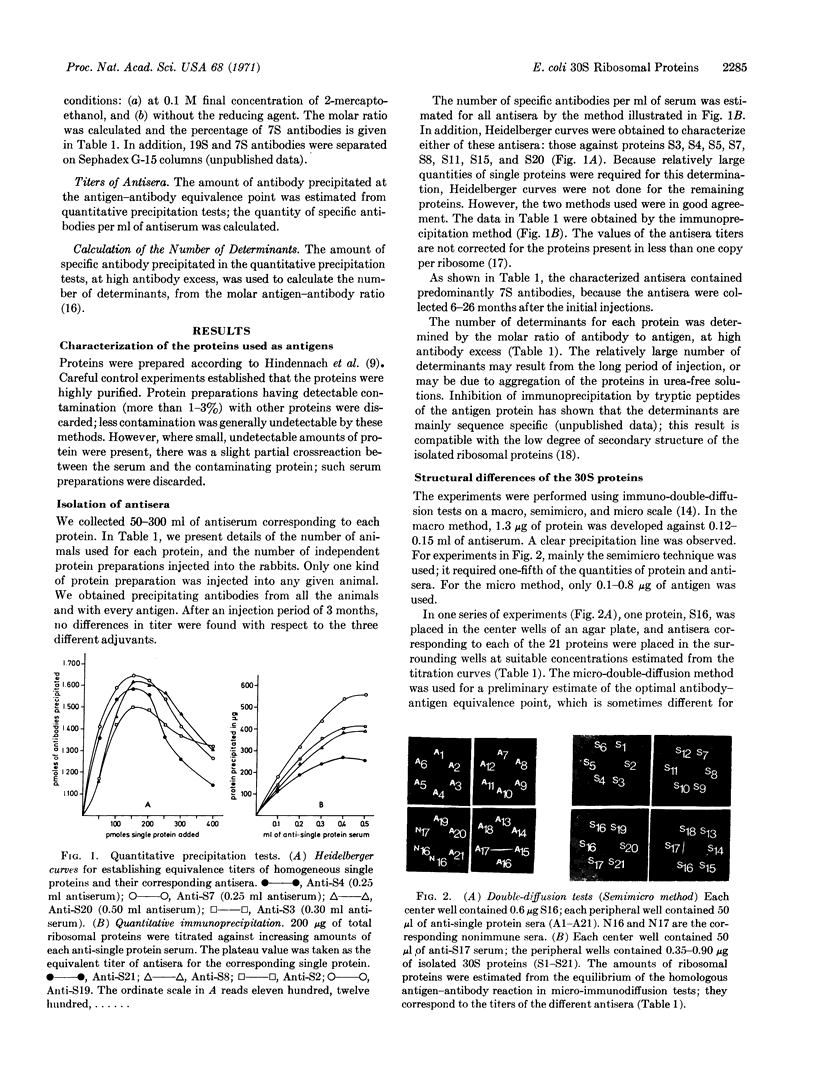


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atassi M. Z., Saplin B. J. Immunochemistry of sperm whale myoglobin. I. The specific interaction of some tryptic peptides and of peptides containing all the reactive regions of the antigen. Biochemistry. 1968 Feb;7(2):688–698. doi: 10.1021/bi00842a026. [DOI] [PubMed] [Google Scholar]
- BENJAMINI E., YOUNG J. D., SHIMIZU M., LEUNG C. Y. IMMUNOCHEMICAL STUDIES ON THE TOBACCO MOSAIC VIRUS PROTEIN. I. THE IMMUNOLOGICAL RELATIONSHIP OF THE TRYPTIC PEPTIDES OF TOBACCO MOSAIC VIRUS PROTEIN TO THE WHOLE PROTEIN. Biochemistry. 1964 Aug;3:1115–1120. doi: 10.1021/bi00896a018. [DOI] [PubMed] [Google Scholar]
- Birge E. A., Kurland C. G. Reversion of a streptomycin-dependent strain of Escherichia coli. Mol Gen Genet. 1970;109(4):356–369. doi: 10.1007/BF00267704. [DOI] [PubMed] [Google Scholar]
- Craven G. R., Voynow P., Hardy S. J., Kurland C. G. The ribosomal proteins of Escherichia coli. II. Chemical and physical characterization of the 30S ribosomal proteins. Biochemistry. 1969 Jul;8(7):2906–2915. doi: 10.1021/bi00835a032. [DOI] [PubMed] [Google Scholar]
- Deusser E., Stöffler G., Wittmann H. G. Ribosomal proteins. XVI. Altered S4 proteins in Escherichia coli revertants from streptomycin dependence to independence. Mol Gen Genet. 1970;109(4):298–302. doi: 10.1007/BF00267699. [DOI] [PubMed] [Google Scholar]
- Dzionara M., Kaltschmidt E., Wittmann H. G. Ribosomal proteins. 8. Molecular weights of isolated ribosomal proteins of Escherichia coli. Proc Natl Acad Sci U S A. 1970 Dec;67(4):1909–1913. doi: 10.1073/pnas.67.4.1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dzionara M. Ribosomal proteins. Secondary structure of individual ribosomal proteins of E. coli studied by circular dichroism. FEBS Lett. 1970 Jun 8;8(4):197–200. doi: 10.1016/0014-5793(70)80262-9. [DOI] [PubMed] [Google Scholar]
- Fogel S., Sypherd P. S. Chemical basis for heterogeneity of ribosomal proteins. Proc Natl Acad Sci U S A. 1968 Apr;59(4):1329–1336. doi: 10.1073/pnas.59.4.1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gesteland R. F. Unfolding of Escherichia coli ribosomes by removal of magnesium. J Mol Biol. 1966 Jul;18(2):356–371. doi: 10.1016/s0022-2836(66)80253-x. [DOI] [PubMed] [Google Scholar]
- Hardy S. J., Kurland C. G., Voynow P., Mora G. The ribosomal proteins of Escherichia coli. I. Purification of the 30S ribosomal proteins. Biochemistry. 1969 Jul;8(7):2897–2905. doi: 10.1021/bi00835a031. [DOI] [PubMed] [Google Scholar]
- Kaltschmidt E., Dzionara M., Donner D., Wittmann H. G. Ribosomal proteins. I. Isolation, amino acid composition, molecular weights and peptide mapping of proteins from E. coli ribosomes. Mol Gen Genet. 1967;100(4):364–373. doi: 10.1007/BF00334063. [DOI] [PubMed] [Google Scholar]
- Kaltschmidt E., Dzionara M., Wittmann H. G. Ribosomal proteins. XV. Amino acid compositions of isolated ribosomal proteins from 30S and 50S subunits of Escherichia coli. Mol Gen Genet. 1970;109(4):292–297. doi: 10.1007/BF00267698. [DOI] [PubMed] [Google Scholar]
- Kaltschmidt E., Stöffler G., Dzionara M., Wittmann H. G. Ribosomal proteins. XVII. Comparative studies on ribosomal proteins of four strains of Escherichia coli. Mol Gen Genet. 1970;109(4):303–308. doi: 10.1007/BF00267700. [DOI] [PubMed] [Google Scholar]
- Kaltschmidt E., Wittmann H. G. Ribosomal proteins. VII. Two-dimensional polyacrylamide gel electrophoresis for fingerprinting of ribosomal proteins. Anal Biochem. 1970 Aug;36(2):401–412. doi: 10.1016/0003-2697(70)90376-3. [DOI] [PubMed] [Google Scholar]
- Kaltschmidt E., Wittmann H. G. Ribosomal proteins. XII. Number of proteins in small and large ribosomal subunits of Escherichia coli as determined by two-dimensional gel electrophoresis. Proc Natl Acad Sci U S A. 1970 Nov;67(3):1276–1282. doi: 10.1073/pnas.67.3.1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurland C. G., Voynow P., Hardy S. J., Randall L., Lutter L. Physical and functional heterogeneity of E. coli ribosomes. Cold Spring Harb Symp Quant Biol. 1969;34:17–24. doi: 10.1101/sqb.1969.034.01.006. [DOI] [PubMed] [Google Scholar]
- LEBOY P. S., COX E. C., FLAKS J. G. THE CHROMOSOMAL SITE SPECIFYING A RIBOSOMAL PROTEIN IN ESCHERICHIA COLI. Proc Natl Acad Sci U S A. 1964 Dec;52:1367–1374. doi: 10.1073/pnas.52.6.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mizushima S., Nomura M. Assembly mapping of 30S ribosomal proteins from E. coli. Nature. 1970 Jun 27;226(5252):1214–1214. doi: 10.1038/2261214a0. [DOI] [PubMed] [Google Scholar]
- Moore P. B., Traut R. R., Noller H., Pearson P., Delius H. Ribosomal proteins of Escherichia coli. II. Proteins from the 30 s subunit. J Mol Biol. 1968 Feb 14;31(3):441–461. doi: 10.1016/0022-2836(68)90420-8. [DOI] [PubMed] [Google Scholar]
- OUCHTERLONY O. Diffusion-in-gel methods for immunological analysis. Prog Allergy. 1958;5:1–78. [PubMed] [Google Scholar]
- Salas M., Hille M. B., Last J. A., Wahba A. J., Ochoa S. Translation of the genetic message, ii. Effect of initiation factors on the binding of formyl-methionyl-trna to ribosomes. Proc Natl Acad Sci U S A. 1967 Feb;57(2):387–394. doi: 10.1073/pnas.57.2.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaup H. W., Green M., Kurland C. G. Molecular interactions of ribosomal components. I. Identification of RNA binding sites for individual 30S ribosomal proteins. Mol Gen Genet. 1970;109(3):193–205. doi: 10.1007/BF00267007. [DOI] [PubMed] [Google Scholar]
- Sela M. Immunological studies with synthetic polypeptides. Adv Immunol. 1966;5:29–129. doi: 10.1016/s0065-2776(08)60272-2. [DOI] [PubMed] [Google Scholar]
- Stöffler G., Deusser E., Wittmann H. G., Apirion D. Ribosomal proteins. XIX. Altered S5 ribosomal protein in an Escherichia coli revertant from strptomycin dependence to independence. Mol Gen Genet. 1971;111(4):334–341. doi: 10.1007/BF00569785. [DOI] [PubMed] [Google Scholar]
- Stöffler G. Immunologische Untersuchungen mit Antiseren gegen isolierte ribosomale Proteine aus 30S and 50S-Untereinheiten aus E. coli. Hoppe Seylers Z Physiol Chem. 1969 Oct;350(10):1166–1166. [PubMed] [Google Scholar]
- Stöffler G. Ribosomal proteins. II. A rapid micromethod for electrophoretic separation of Escherichia coli ribosomal protein on gelatinized cellulose acetate. Mol Gen Genet. 1967;100(4):374–377. doi: 10.1007/BF00334064. [DOI] [PubMed] [Google Scholar]
- Traut R. R., Delius H., Ahmad-Zadeh C., Bickle T. A., Pearson P., Tissières A. Ribosomal proteins of E. Coli: stoichiometry and implications for ribosome structure. Cold Spring Harb Symp Quant Biol. 1969;34:25–38. doi: 10.1101/sqb.1969.034.01.007. [DOI] [PubMed] [Google Scholar]
- Wittmann H. G., Stöfflet G., Hindennach I., Kurland C. G., Birge E. A., Randall-Hazelbauer L., Nomura M., Kaltschmidt E., Mizushima S., Traut R. R. Correlation of 30S ribosomal proteins of Escherichia coli isolated in different laboratories. Mol Gen Genet. 1971;111(4):327–333. doi: 10.1007/BF00569784. [DOI] [PubMed] [Google Scholar]




