Abstract
A model for protein synthesis is proposed in which the donor for the peptide elongation reaction is peptidyl-5S RNA. Space-filling models show that peptide bond formation between peptidyl-5S RNA and aminoacyl-tRNA is eminently feasible from a stereochemical point of view. The peptide is transferred to 5S RNA, while at the same time the deacylated tRNA is exchanged by a new aminoacyl-tRNA acceptor. Two peptidyl transferases are required by the model, both of which have sites for binding the termini of both aminoacyl-tRNA and peptidyl-5S RNA. The model makes detailed predictions about the properties of the transferases.
Keywords: peptidyl transferases
Full text
PDF
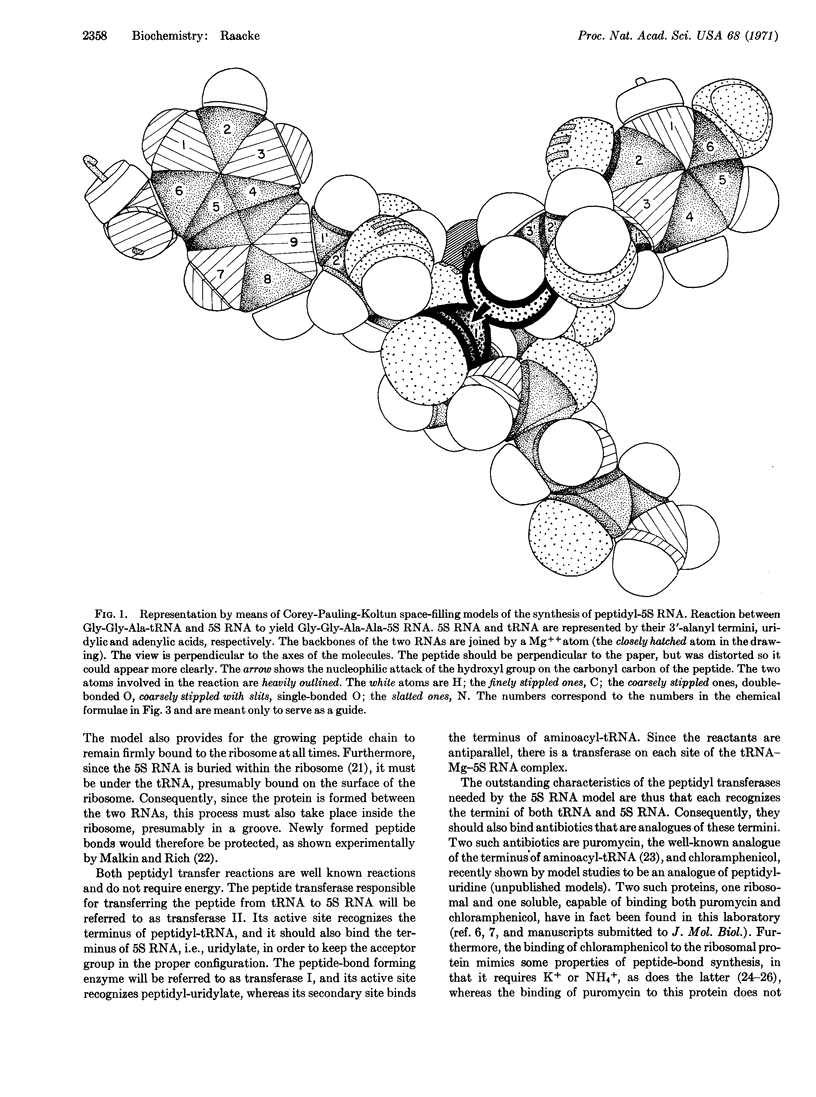
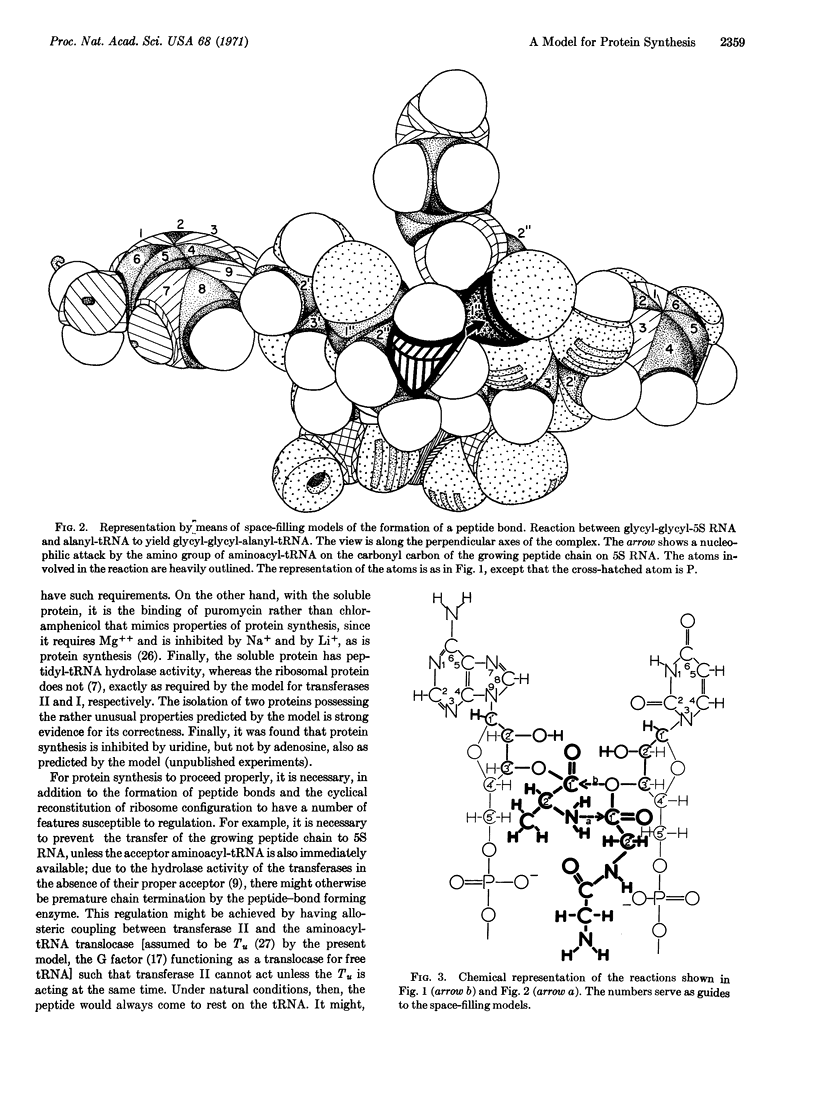

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnott S., Wilkins M. H., Fuller W., Langridge R. Molecular and crystal structures of double-helical RNA. 3. An 11-fold molecular model and comparison of the agreement between the observed and calculated three-dimensional diffraction data for 10- and 11-fold models. J Mol Biol. 1967 Aug 14;27(3):535–548. doi: 10.1016/0022-2836(67)90057-5. [DOI] [PubMed] [Google Scholar]
- Barondes S. H., Nirenberg M. W. Fate of a Synthetic Polynucleotide Directing Cell-Free Protein Synthesis I. Characteristics of Degradation. Science. 1962 Nov 16;138(3542):810–813. doi: 10.1126/science.138.3542.810. [DOI] [PubMed] [Google Scholar]
- Bretscher M. S., Marcker K. A. Polypeptidyl-sigma-ribonucleic acid and amino-acyl-sigma-ribonucleic acid binding sites on ribosomes. Nature. 1966 Jul 23;211(5047):380–384. doi: 10.1038/211380a0. [DOI] [PubMed] [Google Scholar]
- Bretscher M. S. Translocation in protein synthesis: a hybrid structure model. Nature. 1968 May 18;218(5142):675–677. doi: 10.1038/218675a0. [DOI] [PubMed] [Google Scholar]
- CANNON M., KRUG R., GILBERT W. THE BINDING OF S-RNA BY ESCHERICHIA COLI RIBOSOMES. J Mol Biol. 1963 Oct;7:360–378. doi: 10.1016/s0022-2836(63)80030-3. [DOI] [PubMed] [Google Scholar]
- Chapeville F., Yot P., Paulin D. Enzymatic hydrolysis of N-acyl-aminoacyl transfer RNAs. Cold Spring Harb Symp Quant Biol. 1969;34:493–498. doi: 10.1101/sqb.1969.034.01.055. [DOI] [PubMed] [Google Scholar]
- Gottesman M. E. Reaction of ribosome-bound peptidyl transfer ribonucleic acid with aminoacyl transfer ribonucleic acid or puromycin. J Biol Chem. 1967 Dec 10;242(23):5564–5571. [PubMed] [Google Scholar]
- Koltun W. L. Precision space-filling atomic models. Biopolymers. 1965 Dec;3(6):665–679. doi: 10.1002/bip.360030606. [DOI] [PubMed] [Google Scholar]
- Kuriki Y., Fukuma I., Kaji A. The role of the adenosine terminus of transfer ribonucleic acid in the specific binding to ribosomes and their subunits. J Biol Chem. 1969 Mar 10;244(5):1365–1372. [PubMed] [Google Scholar]
- Kurland C. G. The requirements for specific sRNA binding by ribosomes. J Mol Biol. 1966 Jun;18(1):90–108. doi: 10.1016/s0022-2836(66)80079-7. [DOI] [PubMed] [Google Scholar]
- LENGYEL P., SPEYER J. F., OCHOA S. Synthetic polynucleotides and the amino acid code. Proc Natl Acad Sci U S A. 1961 Dec 15;47:1936–1942. doi: 10.1073/pnas.47.12.1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LUBIN M. A priming reaction in protein synthesis. Biochim Biophys Acta. 1963 Jun 25;72:345–348. [PubMed] [Google Scholar]
- Lipmann F. Polypeptide chain elongation in protein biosynthesis. Science. 1969 May 30;164(3883):1024–1031. doi: 10.1126/science.164.3883.1024. [DOI] [PubMed] [Google Scholar]
- Lucas-Lenard J., Lipmann F. Separation of three microbial amino acid polymerization factors. Proc Natl Acad Sci U S A. 1966 Jun;55(6):1562–1566. doi: 10.1073/pnas.55.6.1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malkin L. I., Rich A. Partial resistance of nascent polypeptide chains to proteolytic digestion due to ribosomal shielding. J Mol Biol. 1967 Jun 14;26(2):329–346. doi: 10.1016/0022-2836(67)90301-4. [DOI] [PubMed] [Google Scholar]
- Monier R., Feunteun J., Forget B., Jordan B., Reynier M., Varricchio F. 5 S RNA and the assembly of bacterial ribosomes. Cold Spring Harb Symp Quant Biol. 1969;34:139–148. doi: 10.1101/sqb.1969.034.01.020. [DOI] [PubMed] [Google Scholar]
- Monro R. E., Cerná J., Marcker K. A. Ribosome-catalyzed peptidyl transfer: substrate specificity at the P-site. Proc Natl Acad Sci U S A. 1968 Nov;61(3):1042–1049. doi: 10.1073/pnas.61.3.1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishizuka Y., Lipmann F. The interrelationship between guanosine triphosphatase and amino acid polymerization. Arch Biochem Biophys. 1966 Sep 26;116(1):344–351. doi: 10.1016/0003-9861(66)90040-3. [DOI] [PubMed] [Google Scholar]
- Raacke I. D. "Cloverleaf" conformation for 5S RNAs. Biochem Biophys Res Commun. 1968 May 23;31(4):528–533. doi: 10.1016/0006-291x(68)90509-3. [DOI] [PubMed] [Google Scholar]
- Raacke I. D. Stereochemistry of the puromycin reaction. Biochem Biophys Res Commun. 1971 Apr 2;43(1):168–173. doi: 10.1016/s0006-291x(71)80102-x. [DOI] [PubMed] [Google Scholar]
- Rychlík I. Release of lysine peptides by puromycin from polylysyl-transfer ribonucleic acid in the presence of ribosomes. Biochim Biophys Acta. 1966 Feb 21;114(2):425–427. doi: 10.1016/0005-2787(66)90327-3. [DOI] [PubMed] [Google Scholar]
- Staehelin T., Maglott D. M., Monro R. E. On the catalytic center of peptidyl transfer: a part of the 50 S ribosome structure. Cold Spring Harb Symp Quant Biol. 1969;34:39–48. doi: 10.1101/sqb.1969.034.01.008. [DOI] [PubMed] [Google Scholar]
- WATSON J. D. THE SYNTHESIS OF PROTEINS UPON RIBOSOMES. Bull Soc Chim Biol (Paris) 1964;46:1399–1425. [PubMed] [Google Scholar]
- Yarmolinsky M. B., Haba G. L. INHIBITION BY PUROMYCIN OF AMINO ACID INCORPORATION INTO PROTEIN. Proc Natl Acad Sci U S A. 1959 Dec;45(12):1721–1729. doi: 10.1073/pnas.45.12.1721. [DOI] [PMC free article] [PubMed] [Google Scholar]