Abstract
A silent transgene in Arabidopsis thaliana was reactivated in an outcross but not upon selfing of hemizygous plants. This result could only be explained by assuming a genetic difference between the transgene-free gametes of the wild-type and hemizygous transgenic plants, respectively, and led to the discovery of ploidy differences between the parental plants. To investigate whether a change of ploidy by itself can indeed influence gene expression, we performed crosses of diploid or tetraploid plants with a strain containing a single copy of a transgenic resistance gene in an active state. We observed reduced gene expression of the transgene in triploid compared with diploid hybrids. This led to loss of the resistant phenotype at various stages of seedling development in part of the population. The gene inactivation was reversible. Thus, an increased number of chromosomes can result in a new type of epigenetic gene inactivation, creating differences in gene expression patterns. We discuss the possible impact of this finding for genetic diploidization in the light of widespread, naturally occurring polyploidy and polysomaty in plants.
Full text
PDF

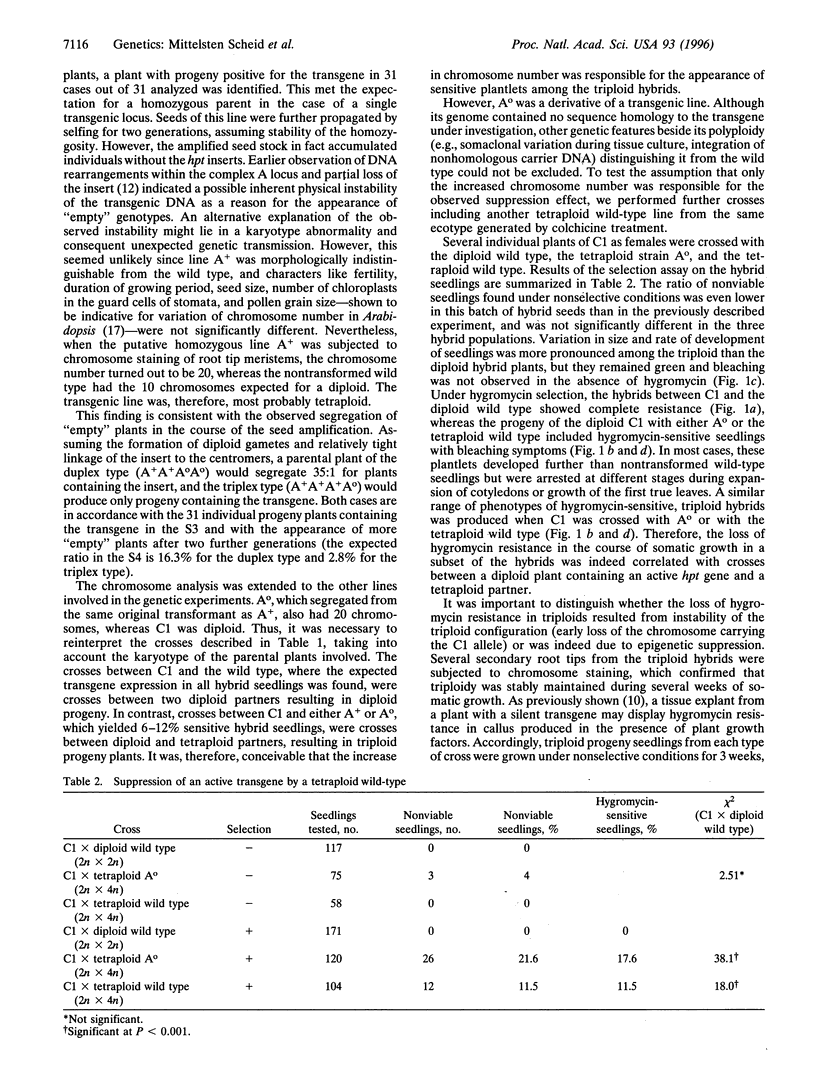
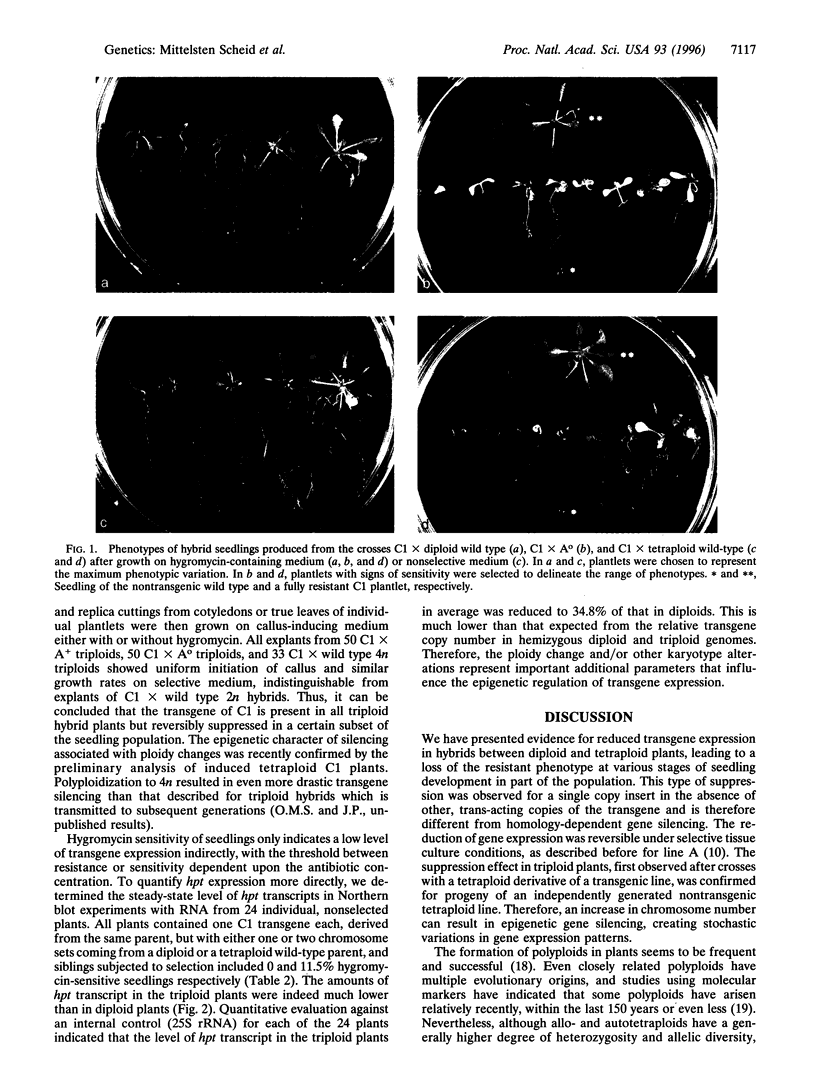
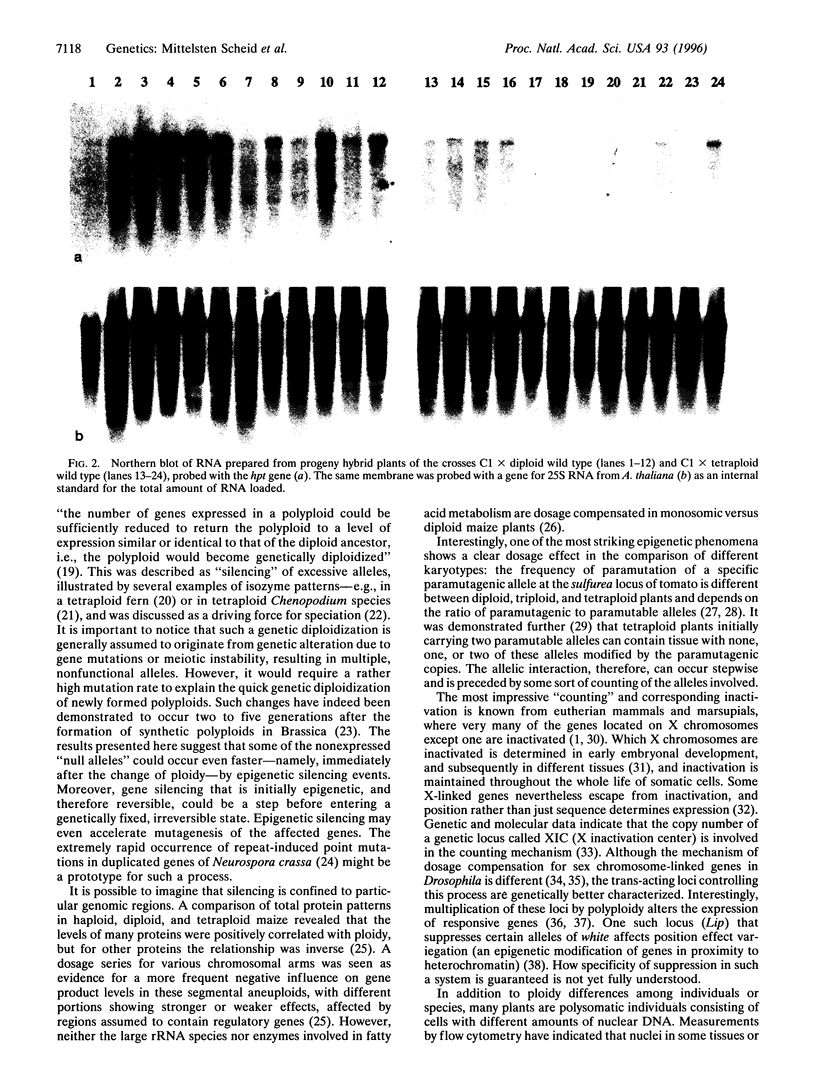

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birchler J. A., Hiebert J. C., Paigen K. Analysis of autosomal dosage compensation involving the alcohol dehydrogenase locus in Drosophila melanogaster. Genetics. 1990 Mar;124(3):679–686. [PMC free article] [PubMed] [Google Scholar]
- Birchler J. A., Newton K. J. Modulation of protein levels in chromosomal dosage series of maize: the biochemical basis of aneuploid syndromes. Genetics. 1981 Oct;99(2):247–266. doi: 10.1093/genetics/99.2.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brink R. A. Paramutation. Annu Rev Genet. 1973;7:129–152. doi: 10.1146/annurev.ge.07.120173.001021. [DOI] [PubMed] [Google Scholar]
- Csink A. K., Linsk R., Birchler J. A. The Lighten up (Lip) gene of Drosophila melanogaster, a modifier of retroelement expression, position effect variegation and white locus insertion alleles. Genetics. 1994 Sep;138(1):153–163. doi: 10.1093/genetics/138.1.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Rocher E. J., Harkins K. R., Galbraith D. W., Bohnert H. J. Developmentally regulated systemic endopolyploid in succulents with small genomes. Science. 1990 Oct 5;250(4977):99–101. doi: 10.1126/science.250.4977.99. [DOI] [PubMed] [Google Scholar]
- Flavell R. B. Inactivation of gene expression in plants as a consequence of specific sequence duplication. Proc Natl Acad Sci U S A. 1994 Apr 26;91(9):3490–3496. doi: 10.1073/pnas.91.9.3490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galbraith D. W., Harkins K. R., Knapp S. Systemic Endopolyploidy in Arabidopsis thaliana. Plant Physiol. 1991 Jul;96(3):985–989. doi: 10.1104/pp.96.3.985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gastony G. J. Gene silencing in a polyploid homosporous fern: paleopolyploidy revisited. Proc Natl Acad Sci U S A. 1991 Mar 1;88(5):1602–1605. doi: 10.1073/pnas.88.5.1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman M., Baker B. S. How flies make one equal two: dosage compensation in Drosophila. Trends Genet. 1994 Oct;10(10):376–380. doi: 10.1016/0168-9525(94)90135-x. [DOI] [PubMed] [Google Scholar]
- Hart C. M., Fischer B., Neuhaus J. M., Meins F., Jr Regulated inactivation of homologous gene expression in transgenic Nicotiana sylvestris plants containing a defense-related tobacco chitinase gene. Mol Gen Genet. 1992 Nov;235(2-3):179–188. doi: 10.1007/BF00279359. [DOI] [PubMed] [Google Scholar]
- Jorgensen R. A. Cosuppression, flower color patterns, and metastable gene expression States. Science. 1995 May 5;268(5211):686–691. doi: 10.1126/science.268.5211.686. [DOI] [PubMed] [Google Scholar]
- Klimyuk V. I., Carroll B. J., Thomas C. M., Jones J. D. Alkali treatment for rapid preparation of plant material for reliable PCR analysis. Plant J. 1993 Mar;3(3):493–494. doi: 10.1111/j.1365-313x.1993.tb00169.x. [DOI] [PubMed] [Google Scholar]
- Lyon M. F. Some milestones in the history of X-chromosome inactivation. Annu Rev Genet. 1992;26:16–28. doi: 10.1146/annurev.ge.26.120192.000313. [DOI] [PubMed] [Google Scholar]
- Matzke M. A., Matzke A. J. Homology-dependent gene silencing in transgenic plants: what does it really tell us? Trends Genet. 1995 Jan;11(1):1–3. doi: 10.1016/s0168-9525(00)88973-8. [DOI] [PubMed] [Google Scholar]
- Matzke M. A., Moscone E. A., Park Y. D., Papp I., Oberkofler H., Neuhuber F., Matzke A. J. Inheritance and expression of a transgene insert in an aneuploid tobacco line. Mol Gen Genet. 1994 Nov 15;245(4):471–485. doi: 10.1007/BF00302260. [DOI] [PubMed] [Google Scholar]
- Melaragno J. E., Mehrotra B., Coleman A. W. Relationship between Endopolyploidy and Cell Size in Epidermal Tissue of Arabidopsis. Plant Cell. 1993 Nov;5(11):1661–1668. doi: 10.1105/tpc.5.11.1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittelsten Scheid O., Afsar K., Paszkowski J. Gene inactivation in Arabidopsis thaliana is not accompanied by an accumulation of repeat-induced point mutations. Mol Gen Genet. 1994 Aug 2;244(3):325–330. doi: 10.1007/BF00285461. [DOI] [PubMed] [Google Scholar]
- Mittelsten Scheid O., Paszkowski J., Potrykus I. Reversible inactivation of a transgene in Arabidopsis thaliana. Mol Gen Genet. 1991 Aug;228(1-2):104–112. doi: 10.1007/BF00282454. [DOI] [PubMed] [Google Scholar]
- Parkhurst S. M., Meneely P. M. Sex determination and dosage compensation: lessons from flies and worms. Science. 1994 May 13;264(5161):924–932. doi: 10.1126/science.8178152. [DOI] [PubMed] [Google Scholar]
- Patterson G. I., Chandler V. L. Paramutation in maize and related allelic interactions. Curr Top Microbiol Immunol. 1995;197:121–141. doi: 10.1007/978-3-642-79145-1_9. [DOI] [PubMed] [Google Scholar]
- Rabinow L., Nguyen-Huynh A. T., Birchler J. A. A trans-acting regulatory gene that inversely affects the expression of the white, brown and scarlet loci in Drosophila. Genetics. 1991 Oct;129(2):463–480. doi: 10.1093/genetics/129.2.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riggs A. D., Pfeifer G. P. X-chromosome inactivation and cell memory. Trends Genet. 1992 May;8(5):169–174. doi: 10.1016/0168-9525(92)90219-t. [DOI] [PubMed] [Google Scholar]
- Schiebel K., Weiss B., Wöhrle D., Rappold G. A human pseudoautosomal gene, ADP/ATP translocase, escapes X-inactivation whereas a homologue on Xq is subject to X-inactivation. Nat Genet. 1993 Jan;3(1):82–87. doi: 10.1038/ng0193-82. [DOI] [PubMed] [Google Scholar]
- Selker E. U. Premeiotic instability of repeated sequences in Neurospora crassa. Annu Rev Genet. 1990;24:579–613. doi: 10.1146/annurev.ge.24.120190.003051. [DOI] [PubMed] [Google Scholar]
- Song K., Lu P., Tang K., Osborn T. C. Rapid genome change in synthetic polyploids of Brassica and its implications for polyploid evolution. Proc Natl Acad Sci U S A. 1995 Aug 15;92(17):7719–7723. doi: 10.1073/pnas.92.17.7719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan S. S., Williams E. A., Tam P. P. X-chromosome inactivation occurs at different times in different tissues of the post-implantation mouse embryo. Nat Genet. 1993 Feb;3(2):170–174. doi: 10.1038/ng0293-170. [DOI] [PubMed] [Google Scholar]
- Willard H. F., Brown C. J., Carrel L., Hendrich B., Miller A. P. Epigenetic and chromosomal control of gene expression: molecular and genetic analysis of X chromosome inactivation. Cold Spring Harb Symp Quant Biol. 1993;58:315–322. doi: 10.1101/sqb.1993.058.01.037. [DOI] [PubMed] [Google Scholar]




