Abstract
Objective: Accurate real-time continuous glucose measurements may improve glucose control in the critical care unit. We evaluated the accuracy of the FreeStyle® Navigator® (Abbott Diabetes Care, Alameda, CA) subcutaneous continuous glucose monitoring (CGM) device in critically ill adults using two methods of calibration.
Subjects and Methods: In a randomized trial, paired CGM and reference glucose (hourly arterial blood glucose [ABG]) were collected over a 48-h period from 24 adults with critical illness (mean±SD age, 60±14 years; mean±SD body mass index, 29.6±9.3 kg/m2; mean±SD Acute Physiology and Chronic Health Evaluation score, 12±4 [range, 6–19]) and hyperglycemia. In 12 subjects, the CGM device was calibrated at variable intervals of 1–6 h using ABG. In the other 12 subjects, the sensor was calibrated according to the manufacturer's instructions (1, 2, 10, and 24 h) using arterial blood and the built-in point-of-care glucometer.
Results: In total, 1,060 CGM–ABG pairs were analyzed over the glucose range from 4.3 to 18.8 mmol/L. Using enhanced calibration median (interquartile range) every 169 (122–213) min, the absolute relative deviation was lower (7.0% [3.5, 13.0] vs. 12.8% [6.3, 21.8], P<0.001), and the percentage of points in the Clarke error grid Zone A was higher (87.8% vs. 70.2%).
Conclusions: Accuracy of the Navigator CGM device during critical illness was comparable to that observed in non–critical care settings. Further significant improvements in accuracy may be obtained by frequent calibrations with ABG measurements.
Introduction
Abnormalities of glucose metabolism are common during critical illness and are associated with adverse outcomes.1 Studies aimed at intensive glucose control have shown contradictory results with increased rates of hypoglycemia.2 Current guidelines suggest frequent glucose measurements for safe implementation of insulin therapy, but this increases the workload of the nursing staff.3 Even regular glucose measurements may fail to identify hypo- or hyperglycemia during periods of rapid glucose change, and the use of accurate continuous glucose monitoring (CGM) may aid safer insulin therapy during critical illness by identifying real-time fluctuations in glucose level, prompting treatment to avoid hypo- and hyperglycemia.
Minimally invasive subcutaneous CGM systems are increasingly used in the management of type 1 diabetes, improving glycemic control4 and reducing the burden of hypoglycemia.5,6 Placed in the subcutaneous tissue, a miniature probe containing a glucose oxidase layer generates electrical current proportional to the glucose concentration in the interstitial fluid. Calibration is through finger-stick glucose measurements taken at 12–24-h intervals. Subcutaneous CGM devices measure glucose in the interstitial compartment with a physiological lag time. In contrast to outpatient use, patients with critical illness may have disturbed microcirculation owing to a range of factors such as tissue edema, hemodynamic compromise, and treatment with vasopressors. These factors, in theory, could affect the accuracy as well as lag time of subcutaneous CGM, especially at times of rapid glucose change. An alternative to subcutaneous CGM is intravascular CGM, but this approach requires dedicated arterial/venous placement free of potential contaminants with the risk of thrombus in the venous periphery or medical risks associated with arterial placement of the sensing element.
We hypothesized that accuracy of subcutaneous CGM in intensive care settings may be improved through enhanced calibration and evaluated the accuracy of the FreeStyle® Navigator® (Abbott Diabetes Care, Alameda, CA) subcutaneous CGM system using standard and enhanced methods of calibration. An abstract containing some of the data presented in this article was presented at the 33rd International Symposium on Intensive Care and Emergency Medicine, held March 2013, in Brussels, Belgium.7
Research Design and Methods
Patients and study design
The data were collected during a clinical investigation assessing the efficacy of closed-loop insulin delivery in critically ill adults8 using a prospective, randomized, parallel-group design. The study was performed in the 24-bed Neurosciences Critical Care Unit (NCCU) at Addenbrooke's Hospital, Cambridge, United Kingdom, a tertiary-care trauma and neurosurgical referral center in the east of England. The Cambridge Central Research Ethics Committee approved the study.
Inclusion criteria were 18 years of age and older, expected stay at the NCCU of at least 48 h, and arterial glucose level greater than 10.0 mmol/L or already on insulin treatment. Exclusion criteria included diabetic ketoacidosis, therapeutic hypothermia, fatal organ failures, and pregnancy.
Written informed consent/assent was obtained prior to enrollment from either the patient or the next of kin. Patients were randomized to glucose control by an automated closed-loop glucose control system with enhanced sensor calibration or to a standard paper-based intravenous insulin administration protocol with sensor calibration according to the manufacturer's instructions.
Enhanced calibration protocol
The FreeStyle Navigator sensor with a 1-h warm-up time9 was calibrated at variable intervals ranging from 30 min to 6 h using arterial blood glucose (ABG) measured by the blood gas analyzer. The calibrations were more frequent when larger deviation between sensor and ABG were observed and less frequent when sensor and ABG agreed. The enhanced calibration protocol was optimized on a validated simulation environment.10 Subjects were treated by a closed-loop glucose control system.
Standard calibration protocol
The FreeStyle Navigator CGM device with a 1-h warm-up time9 was calibrated according to the manufacturer's instructions (1, 2, 10, and 24 h after insertion) using arterial blood and the built-in point-of-care glucose meter of the Navigator receiver. Each participant's glucose was controlled according to the standard paper-based intravenous insulin administration protocol used in the NCCU.
Common study procedures
Apart from glucose control and the calibration protocol, other aspects of patient care including nutritional management and treatment of hypoglycemia and hyperglycemia were carried out according to standard treatment protocols in the NCCU and were identical between treatments. All study-related activities were carried out for a maximum period of 48 h or until the end of the NCCU stay, whichever came first.
Reference glucose measurements
ABG measurements, made using an on-site blood gas analyzer (cobas b 221; Roche Diagnostics, Burgess Hill, United Kingdom) at hourly intervals, were used as the reference standard for the calculation of sensor accuracy metrics.
Statistical analysis
Numerical accuracy was assessed by absolute difference, absolute relative difference, and International Organization for Standardization criteria. Clinical accuracy was evaluated using the Clarke error grid.11 A repeated-measures linear regression model with an autoregressive first-order covariance structure was fitted to compare numerical accuracy metrics of the two calibration protocols. The lag time was derived by calculating the maximum correlation coefficient between sensor glucose and ABG at a pure time lag of 0, 1, 2, …, 24, and 25 min. This was carried out by delaying sensor glucose in 1-min intervals. The accuracy metrics were calculated by GStat software (version 1.3; University of Cambridge, Cambridge, United Kingdom), and statistical tests were carried out using SPSS software (version 19; IBM Software, Hampshire, United Kingdom). Data are given as mean (SD) or median (interquartile range) values.
Results
Study participants
Twenty-four subjects completed the study (12 enhanced calibration and 12 standard calibration). In total, 1,060 ABG–sensor pairs were obtained (enhanced calibration, 516 pairs; standard calibration, 544 pairs). The baseline characteristics of the treatment groups were similar (Supplementary Table S1; Supplementary Data are available online at www.liebertonline.com/dia) with comparable age and disease severity. The most common reason for NCCU admission was trauma followed by post-neurosurgery. The mean Acute Physiology and Chronic Health Evaluation (APACHE) II scores (mean±SD) in patients using the enhanced calibration and standard calibration protocols were 12.9±5.0 and 11.2±3.4. The amounts of carbohydrate and total calories as well as the numbers of feeding interruptions were similar between the two groups. The proportion of subjects receiving inotropes (42% vs. 33%) and corticosteroids (42% vs. 25%) was higher using enhanced calibration.
Numerical and clinical accuracy and lag time
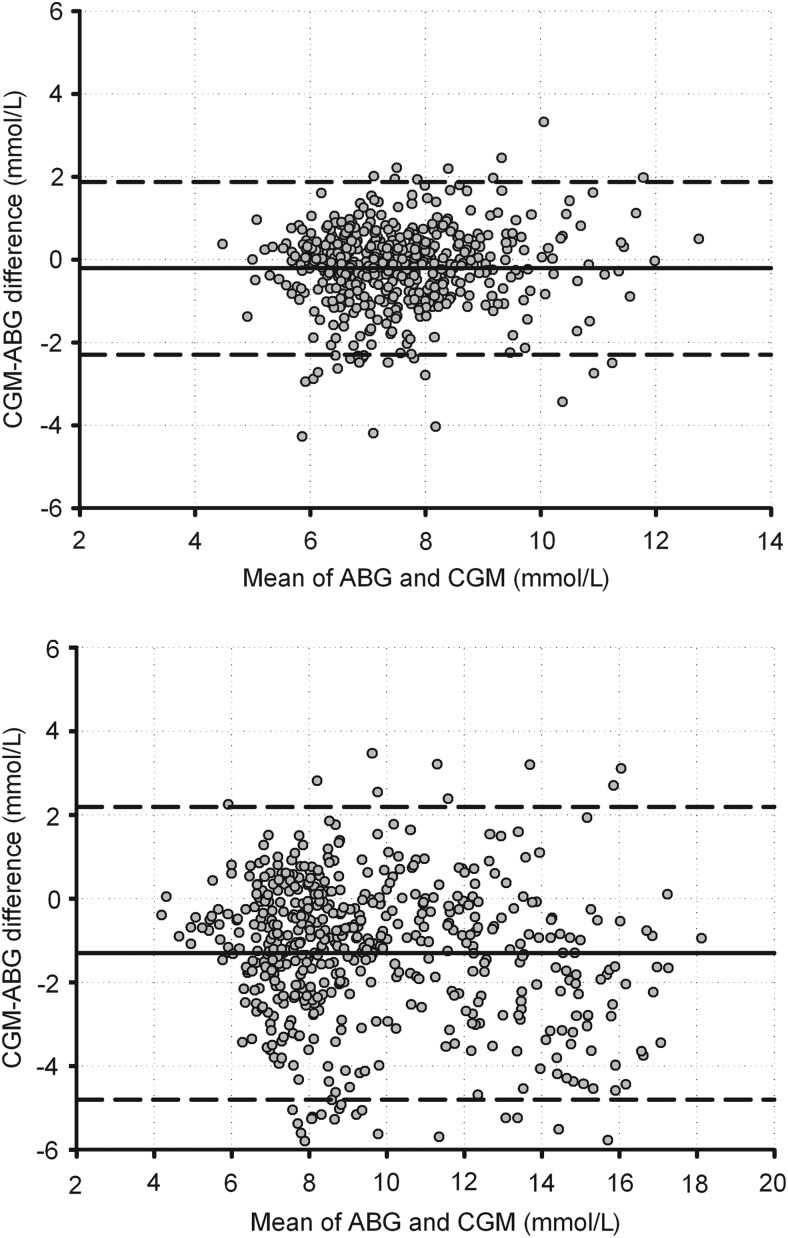
Numerical and clinical accuracy was significantly improved using the enhanced calibration protocol compared with the standard calibration protocol (Table 1). There were no data points in the Clarke error grid Zones C–E with enhanced calibration. Bland–Altman plots (Fig. 1) and Clarke error grids (Supplementary Fig. S1) of reference and sensor glucose confirmed the improved performance of enhanced calibration across the glucose range. There were no data points in the hypoglycemic range in either protocol. The lag time was 14 and 12 min for the enhanced and standard calibration protocols, respectively.
Table 1.
Numerical and Clinical Accuracy Using the Enhanced Calibration Protocol and the Standard Calibration Protocol
| Enhanced calibration (n=12) | Standard calibration (n=12) | P value | |
|---|---|---|---|
| Number of paired points | 516 | 544 | |
| Mean plasma glucose (mmol/L) | 7.6 (1.4) | 10.3 (3.2) | <0.001 |
| ISO (%) | 87.8 | 70.2 | |
| Clarke error grid (%) | |||
| Zone A | 87.8 | 70.2 | |
| Zone B | 12.2 | 29.0 | |
| Zone C | 0.0 | 0.0 | |
| Zone D | 0.0 | 0.8 | |
| Zone E | 0.0 | 0.0 | |
| Median bias (IQR) (mmol/L) | −0.1 (−0.7, 0.4) | −1.1 (−2.3, −0.1) | <0.001 |
| Whole range (4.3–18.8 mmol/L) | |||
| Median AD (mmol/L) | 0.5 (0.3, 1.0) | 1.2 (0.6, 2.4) | <0.001 |
| Median ARD (%) | 7.0 (3.5, 13.0) | 12.8 (6.3, 21.8) | <0.001 |
| Mean AD (mmol/L) | 0.8±0.8 | 1.7±1.5 | <0.001 |
| Mean ARD (%) | 9.6±8.9 | 15.6±12.0 | <0.001 |
| Euglycemia (3.9–8 mmol/L) | |||
| Number of paired points (%) | 345 (66.8%) | 148 (27.2%) | |
| Median ARD (%) | 6.3 (3.4, 12.4) | 11.2 (6.0, 18.4) | <0.001 |
| Hyperglycemia (>8 mmol/L) | |||
| Number of paired points (%) | 171 (33.2%) | 396 (72.8%) | |
| Median ARD (%) | 8.7 (3.6, 14.4) | 13.5 (6.5, 24.6) | <0.001 |
Data shown are mean±SD or median (interquartile range) values. No measurements were observed in the hypoglycemia range.
AD, absolute deviation; ARD, absolute relative deviation; ISO, International Organization for Standardization.
FIG. 1.
Bland–Altman plot of reference and sensor arterial blood glucose (ABG) using (top panel) the enhanced calibration protocol and (bottom panel) the standard calibration protocol (manufacturer's instructions). The solid black line represents the mean difference; the dashed lines indicate 1.96×SD of the difference.
Calibration frequency
The number of calibrations with the enhanced calibration protocol during the first and second 24-h periods was 9.5 (9, 14) and 7 (4, 8), respectively. This translates to a calibration interval of 152 (105, 160) and 205 (180, 360) min during the first and second 24-h period, respectively. With standard calibration, four calibrations were performed during the first 24 h (1, 2, 10, and 24 h after insertion) and none during the second 24 h.
Adverse events
There were no adverse events associated with sensor usage in either calibration protocol.
Discussion
We improved subcutaneous CGM accuracy by more frequent calibrations with reference glucose measurements. Accuracy observed with the enhanced calibration method exceeded our previously reported observations in type 1 diabetes with standard calibration (for enhanced calibration vs. type 1 diabetes12: median absolute relative deviation, 7.0% vs. 9.9%; Clarke error grid Zone A, 87.8% vs. 78.4%). During the first 24 h, calibration was performed on average every 2.5 h and during the second 24 h every 3.5 h. It is important that the Navigator sensor lag time observed in our study was similar between the two methods of calibration (14 vs. 12 min for the enhanced and standard calibration protocols) and comparable to that recorded in type 1 diabetes.13 This suggests that lag time is not prolonged in critical illness. We consider the main reason for the negative −1.1 mmol bias in the standard calibration arm to be calibration bias due to biased built-in glucometer readings. This calibration bias then propagated into sensor bias.
During the present study, the proportion of sensor glucose values within 20% of reference glucose was 88% with the enhanced protocol, compared with reported values between 63% and 75%14–16 other than for Brunner et al.,17 who reported a higher percentage of 92%. Most intensive care unit studies evaluated Medtronic (Northridge, CA) sensors,14,16–18 and only one study evaluated Navigator.19 Further improvements in accuracy may be achievable through the intravenous sampling route eliminating or significantly reducing the lag time of 6–15 min reported with subcutaneous sensors.13,20–22 Preliminary studies using the intravenous microdialysis technique have shown high accuracy (mean absolute relative deviation, 5.6%; data points in Clarke error grid Zone A, 97%).23 Advantages of subcutaneous glucose monitoring compared with intravenous measurements include reduced invasiveness obviating the need for dedicated venous placement and a risk of contamination from dextrose or other medications that may interfere with glucose measurements. The risk of infection and thrombosis is lower with the subcutaneous measurement route. In the present study, the subcutaneous sensor placement was not associated with any complications, and the high accuracy obtained with frequent calibrations supports the use of subcutaneous sensing in certain groups of critically ill patients.
The strengths of our study include the randomized controlled study design, the use of hourly ABG to assess outcomes, comparability of the patient group's nutrition, and treatment modalities. Study limitations include the small sample size in a single center and the parallel study design involving a subspecialized patient population with relatively lower mean APACHE scores. The range and mean glucose level using the enhanced calibration protocol were lower than with the standard calibration protocol.
In conclusion, we achieved a high degree of subcutaneous CGM accuracy by additional calibrations using reference glucose. Accurate subcutaneous CGM levels may facilitate safe and efficacious intensive insulin therapy in critically ill patients. Larger studies in more diverse patient populations are warranted to assess the accuracy of CGM and utility of CGM-based closed-loop systems to achieve safer glucose control. Furthermore, a head-to-head comparison of subcutaneous and intravenous sensors may allow identification of patient characteristics associated with differential performance of subcutaneous and intravascular routes.
Supplementary Material
Acknowledgments
We are indebted to patients and family members for participating in/consenting to the study. We thank all the staff at the Neurosciences Critical Care Unit at Addenbrooke's Hospital, Cambridge, United Kingdom. This study was supported by the University of Cambridge National Institute for Health Research Biomedical Research Centre, Cambridge Medical Research Council Centre for Obesity and Related Metabolic Diseases, University of Cambridge Confidence in Concepts, JDRF, and Diabetes UK. We thank Drs. Tonny Veenith and Ari Ercole for their help with recruitment. Abbott Diabetes Care provided technical support but did not play any role in clinical studies or data analysis.
Author Disclosure Statement
R.H. reports having received speaker honoraria from Minimed Medtronic, LifeScan, Eli Lilly, and Novo Nordisk, serving on an advisory panel for Animas and Minimed Medtronic, receiving license fees from BBraun, and having served as a consultant to BBraun and Profil. M.L.E. reports having received speaker honoraria/travel support from Abbott Diabetes Care, Animas, Medtronic, and Eli Lilly and serving on advisory boards for Medtronic, Roche, and Cellnovo. L.L., S.W.E., H.T., K.C., J.M.A., K.K., M.E.W., M.N., A.H., and R.B. declare no conflicts of interest exist.
R.H. conceptualized the study and is the guarantor and had full access to all the data in the study. R.H., L.L., R.B., S.W.E., and M.L.E. co-designed the study. L.L., H.T., S.W.E., K.C., and J.M.A. were responsible for patient screening and enrollment and informed consent. L.L., H.T., K.C., J.M.A., and K.K. provided patient care and contributed to acquisition of data. R.H. designed and implemented the control algorithm. R.H., M.N., and M.E.W. developed and validated the closed-loop system, including the conduct of simulation studies. L.L., A.H., and M.N. carried out the data and statistical analyses. L.L. and R.H. drafted the manuscript. All authors critically revised the manuscript and have seen and approved the final version of the report.
References
- 1.Badawi O, Waite MD, Fuhrman SA, Zuckerman IH: Association between intensive care unit-acquired dysglycemia and in-hospital mortality. Crit Care Med 2012;40:3180–3188 [DOI] [PubMed] [Google Scholar]
- 2.Van den Berghe G: Intensive insulin therapy in the ICU—reconciling the evidence. Nat Rev Endocrinol 2012;8:374–378 [DOI] [PubMed] [Google Scholar]
- 3.Aragon D: Evaluation of nursing work effort and perceptions about blood glucose testing in tight glycemic control. Am J Crit Care 2006;15:370–377 [PubMed] [Google Scholar]
- 4.Tamborlane WV, Beck RW, Bode BW, Buckingham B, Chase HP, Clemons R, Fiallo-Scharer R, Fox LA, Gilliam LK, Hirsch IB, Huang ES, Kollman C, Kowalski AJ, Laffel L, Lawrence JM, Lee J, Mauras N, O'Grady M, Ruedy KJ, Tansey M, Tsalikian E, Weinzimer S, Wilson DM, Wolpert H, Wysocki T, Xing D; Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study Group: Continuous glucose monitoring and intensive treatment of type 1 diabetes. N Engl J Med 2008;359:1464–1476 [DOI] [PubMed] [Google Scholar]
- 5.Battelino T, Phillip M, Bratina N, Nimri R, Oskarsson P, Bolinder J: Effect of continuous glucose monitoring on hypoglycemia in type 1 diabetes. Diabetes Care 2011;34:795–800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beck RW, Hirsch IB, Laffel L, Tamborlane WV, Bode BW, Buckingham B, Chase P, Clemons R, Fiallo-Scharer R, Fox LA, Gilliam LK, Huang ES, Kollman C, Kowalski AJ, Lawrence JM, Lee J, Mauras N, O'Grady M, Ruedy KJ, Tansey M, Tsalikian E, Weinzimer SA, Wilson DM, Wolpert H, Wysocki T, Xing D; Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study Group: The effect of continuous glucose monitoring in well-controlled type 1 diabetes. Diabetes Care 2009;32:1378–1383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leelarathna L, English SW, Thabit H, Caldwell K, Allen JM, Kumareswaran K, Wilinska ME, Nodale M, Mangat J, Evans ML, Burnstein R, Hovorka R: Continuous glucose monitoring in critically ill adults: comparison of two different calibration protocols [abstract]. Crit Care 2013;17(Suppl 2):P459 [Google Scholar]
- 8.Leelarathna L, English SW, Thabit H, Caldwell K, Allen JM, Kumareswaran K, Wilinska ME, Nodale M, Mangat J, Evans ML, Burnstein R, Hovorka R: Feasibility of fully automated closed-loop glucose control utilizing continuous subcutaneous glucose measurements in critical illness: a randomised controlled trial. Crit Care 2013;17:R159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Geoffrey M, Brazg R, Richard W: FreeStyle Navigator continuous glucose monitoring system with TRUstart algorithm, a 1-hour warm-up time. J Diabetes Sci Technol 2011;5:99–106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wilinska ME, Blaha J, Chassin LJ, Cordingley JJ, Dormand NC, Ellmerer M, Haluzik M, Plank J, Vlasselaers D, Wouters PJ, Hovorka R: Evaluating glycemic control algorithms by computer simulations. Diabetes Technol Ther 2011;13:713–722 [DOI] [PubMed] [Google Scholar]
- 11.Clarke W, Cox D, Gonder-Frederick L, Carter W, Pohl S: Evaluating clinical accuracy of systems for self-monitoring of blood glucose. Diabetes Care 1987;10:622–628 [DOI] [PubMed] [Google Scholar]
- 12.Leelarathna L, Nodale M, Allen JM, Elleri D, Kumareswaran K, Haidar A, Caldwell K, Wilinska ME, Acerini CL, Evans ML, Murphy HR, Dunger DB, Hovorka R: Evaluating the accuracy and large inaccuracy of two continuous glucose monitoring systems. Diabetes Technol Ther 2013;15:143–149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garg SK, Voelmle M, Gottlieb PA: Time lag characterization of two continuous glucose monitoring systems. Diabetes Res Clin Pract 2010;87:348–353 [DOI] [PubMed] [Google Scholar]
- 14.Lorencio C, Leal Y, Bonet A, Bondia J, Palerm CC, Tache A, Sirvent JM, Vehi J: Real-time continuous glucose monitoring in an intensive care unit: better accuracy in patients with septic shock. Diabetes Technol Ther 2012;14:568–575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rabiee A, Andreasik RN, Abu-Hamdah R, Galiatsatos BS, Khouri Z, Gibson BR, Andersen DK, Elahi D. Numerical and clinical accuracy of a continuous glucose monitoring system during intravenous insulin therapy in the surgical and burn intensive care units. J Diabetes Sci Technol 2009;3:951–959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jacobs B, Phan K, Bertheau L, Dogbey G, Schwartz F, Shubrook J: Continuous glucose monitoring system in a rural intensive care unit: a pilot study evaluating accuracy and acceptance. J Diabetes Sci Technol 2010;4:636–644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brunner R, Kitzberger R, Miehsler W, Herkner H, Madl C, Holzinger U: Accuracy and reliability of a subcutaneous continuous glucose-monitoring system in critically ill patients. Crit Care Med 2011;39:659–664 [DOI] [PubMed] [Google Scholar]
- 18.Piper HG, Alexander JL, Shukla A, Pigula F, Costello JM, Laussen PC, Jaksic T, Agus MS: Real-time continuous glucose monitoring in pediatric patients during and after cardiac surgery. Pediatrics 2006;118:1176–1184 [DOI] [PubMed] [Google Scholar]
- 19.Siegelaar SE, Barwari T, Hermanides J, Stooker W, van der Voort PH, DeVries JH: Accuracy and reliability of continuous glucose monitoring in the intensive care unit: a head-to-head comparison of two subcutaneous glucose sensors in cardiac surgery patients. Diabetes Care 2011;34:e31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kamath A, Mahalingam A, Brauker J: Analysis of time lags and other sources of error of the DexCom SEVEN continuous glucose monitor. Diabetes Technol Ther 2009;11:689–695 [DOI] [PubMed] [Google Scholar]
- 21.Wei C, Lunn DJ, Acerini CL, Allen JM, Larsen AM, Wilinska ME, Dunger DB, Hovorka R: Measurement delay associated with the Guardian RT continuous glucose monitoring system. Diabet Med 2010;27:117–122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Keenan DB, Mastrototaro JJ, Voskanyan G, Steil GM: Delays in minimally invasive continuous glucose monitoring devices: a review of current technology. J Diabetes Sci Technol 2009;3:1207–1214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schierenbeck F, Owall A, Franco-Cereceda A, Liska J: Evaluation of a continuous blood glucose monitoring system using a central venous catheter with an integrated microdialysis function. Diabetes Technol Ther 2013;15:26–31 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.