Abstract
Parathyroid hormone-related protein (PTHrP) is a polyhormone secretory protein that plays fundamental roles in the development and function of various tissues. Transforming growth factor (TGF)-β is an important tumor suppressor that induces cell cycle arrest and apoptosis. Increased PTHrP expression has been implicated in TGF-β-induced growth inhibition in human hepatocellular carcinoma cells. However, whether PTHrP is involved in TGF-β-induced apoptosis remains unknown. Using Hep3B and HuH-7, two human hepatocellular carcinoma cell lines, the current study examined the hypothesis that TGF-β-induced apoptosis is mediated by the induction of PTHrP expression. We found that (1) TGF-β induces PTHrP mRNA expression, protein expression and secretion in a time-dependent fashion; (2) knockdown of PTHrP gene expression or neutralization of secreted PTHrP isoforms blocks TGF-β-induced apoptosis; and (3) TGF-β-induced PTHrP expression is Smad3-dependent. Thus, we have identified PTHrP as a novel mediator for TGF-β-induced apoptosis in Hep3B cells. Our findings provide further insights into the mechanisms through which TGF-β conveys tumor suppression activity.
Keywords: Transforming growth factor-β, Parathyroid hormone-related protein Apoptosis Small interfering RNA, Apoptosis, Small interfering RNA
1. Introduction
Transforming growth factor (TGF)-β is a multifunctional protein with a broad spectrum of cellular activities ranging from regulation of target gene activity to the control of cell growth and apoptosis [1,2]. TGF-β signals by binding to a cell surface receptor complex that phosphorylates the intracellular signaling proteins, Smad2 and Smad3 [3]. The phosphorylated Smad2 and Smad3 form a heteromeric complex with Smad4, translocate into the nucleus, and regulate transcription of target genes [1,4]. TGF-β-induced apoptosis is critical in development and tissue homeostasis, and is also important for its tumor suppressor activity. Although the mechanisms of TGF-β-induced apoptosis vary among different cell types, the intracellular signaling protein Smad3 functions as a key mediator in TGF-β-induced apoptosis. For example, TGF-β induces apoptosis in heptocytes through Smad3-dependent cleavage of BAD [5], or through Smad2, Smad3 and Smad4-mediated expression of DAP kinase [6]. TGF-β induces apoptosis in hepatocytes and B-lymphocytes through Smad3-dependent transcription of the MAPK phosphatase MKP2, which enhances the proapoptotic effect of the Bcl-2 family member Bim [7]. Our group has previously reported that Smad3 plays an essential role in TGF-β-induced apoptosis in hepatocytes and lung epithelial cells, and Akt interacts with Smad3 to inhibit Smad3-mediated transcription and apoptosis [8].
Parathyroid hormone-related protein (PTHrP) is a polyhormone secretory protein that plays a critical role in a number of biological processes by acting via paracrine, autocrine and intracrine pathways [9]. PTHrP was identified as a tumor-derived humoral factor that causes humoral hypercalcemia of malignancy [10–12]. There are three isoforms of human PTHrP peptides ranging in length from 139 to 173 residues [13], all of which are subjected to extensive post-translational processing to generate multiple secretory isoforms of mature peptides representing the N-terminal, mid-region, and C-terminal portions of the protein. The N-terminal 1–13 amino acids of PTHrP are highly homologous with parathyroid hormone (PTH). The mid-region of PTHrP contains a bipartite nuclear/nucleolar localization signal [14–17]. PTH and PTHrP act through a common receptor, the type I PTH receptor (PTHR1), which is a class B G protein-coupled receptor. The N-terminal 1–34 amino acids of PTH and PTHrP are sufficient for receptor activation. However, each region of PTHrP exhibits unique biological activities, likely acting through its own receptors [17–19]. Although PTHrP circulates in some cancer patients and interacts with PTH/PTHrP receptors in bones and kidneys to cause hypercalcemia, the peptide does not normally circulate in an appreciable amount [13,17]. Therefore, PTHrP is considered a local regulatory factor near its site of production rather than a classical circulating hormone [20]. The peptide and its mRNA are ubiquitously expressed in many mature and developing tissues. The various isoforms have been shown to regulate many cellular functions such as myorelaxation, calcium transport, cell growth, and differentiation [14], indicating that PTHrP plays fundamental roles in the development and function of many tissues [18].
PTHrP gene expression is induced rapidly and can be altered by a number of factors such as TGF-β and serum. TGF-β stimulates the expression of PTHrP in hepatocytes and inhibits cell proliferation through a PTHrP-dependent mechanism [21]. Overexpression of PTHrP enhances apoptosis in intestinal epithelial cells following serum depletion; mutation of the nuclear localization signal abolishes its ability to promote apoptosis by serum withdrawal [20]. Functional linkage has been established between TGF-β and PTHrP during bone formation and bone metastasis by certain cancers. For example, TGF-β stimulates PTHrP expression in bone culture and inhibits endochondral bone formation including condrocyte proliferation, hypertrophic differentiation, and matrix mineralization [22]. The majority of breast cancers metastasizing to bone secrete PTHrP, which induces local osteolysis that leads to activation of bone matrix-derived TGF-β. In turn, TGF-β stimulates PTHrP expression, thereby accelerating bone destruction [23–25]. These data suggest that PTHrP mediates certain biological effects of TGF-β.
In this study, we examined whether PTHrP is a downstream mediator for TGF-β-induced apoptosis in human hepatocytes. We found that (1) TGF-β induces PTHrP mRNA expression, protein expression and secretion; (2) TGF-β-induced apoptosis is blocked by knockdown of PTHrP expression and by the antisera against PTHrP isoforms; (3) Smad3 mediates TGF-β-induced PTHrP expression. Thus we have identified PTHrP as a novel mediator for TGF-β-induced apoptosis in human hepatocytes.
2. Material and methods
2.1. Cell line
Human hepatocellular carcinoma cell lines, Hep3B and HuH-7, were cultured in Minimum Essential Medium Eagle (MEM, Mediatech Inc., VA) supplemented with 10% fetal bovine serum (FBS, Invitrogen, CA), 1× non-essential amino acid (NEAA, Sigma-Aldrich, Inc., MO), and 1× sodium pyruvate (Sigma-Aldrich, Inc., MO).
2.2. Real-time quantitative PCR
Total RNA was isolated from cells using RNAqueous (Ambion, TX) and converted into cDNA using the RETROscript RT-PCR system (Ambion, TX) following the manufacturer’s instruction. Real time quantitative PCR was performed as previously described [26]. For detection of specific human mRNA transcripts, the following inventoried Taqman gene expression assays (Applied Biosystems, Foster City, CA) were used: PTHrP (Hs00174969_ml) and 18S rRNA (Hs00153153_m1). Duplicate CT (threshold cycle) values of each sample were analyzed using the comparative CT method as described by the manufacturer (Applied Biosystems, Foster City, CA), normalized to 18S, and expressed as mean ± SD.
2.3. Immunoassay for secreted PTHrP
The amount of PTHrP secreted into the culture medium was measured using an immunoradiometric sandwich assay kit (Diagnostics Systems Laboratories, Webster, TX). The culture medium was collected and frozen at −80 °C for future use. The cell number was determined using a Z1 Coulter Counter (Beckman Coulter, Inc., Miami, FL) for normalization of PTHrP secretion. Prior to the assay, the aliquots of 1 ml culture medium were concentrated to 0.2 ml using Centricon centrifugal filter devices Ultracel YM-10 (Millipore Co., Billerica, MA). The assay was carried out according to manufacturer’s instruction [27].
2.4. Western blotting analysis
Cells were lysed in 1× cell lysis buffer (Cell Signaling Technology, Inc., MA). Protein concentration of cell lysates was quantified using a protein assay dye (Bio-Rad, Hercules, CA). Western blotting was performed as previously described [26]. Antibodies against PTHrP from Santa Cruz Biotechnology Inc. (Santa Cruz, CA), Smad2 and Smad3 were purchased from Invitrogen (Carlsbad, CA), β-actin from Sigma-Aldrich Co. (St. Louis, MO).
2.5. Specific gene silencing using siRNA
The siGENOME ON-TARGETplus SMARTpool reagents of human PTHrP siRNA (L-003698-00), Smad2 siRNA (L-003561-00), and Smad3 siRNA (L-020067-00) were purchased from Dharmacon Inc. (Lafayette, CO). Non-targeting siRNA (D-001206-13, Dharmacon Inc.) was used as a nonspecific siRNA control. The siRNA was transfected using the transfection reagent DharmaFECT 2 (Dharmacon Inc.) according to manufacturer’s instruction as previously described [26].
2.6. Apoptosis assays
DNA fragmentation was quantified by cell death detection ELISA assay (Roche Molecular Biochemicals, CA) according to manufacturer’s instruction as previously described [26]. The apoptotic cells were visualized by Hoechst 33258 staining. Digital images were acquired using MetaMorph software (Molecular Devices Co., Downingtown, PA) under a Nikon TE-2000 inverted microscope (Nikon Inc., Melville, NY).
2.7. Growth study
Forty-eight hours after siRNA transfection, the cells were treated with TGF-β or vehicle control for 48 h. The cells were trypsinized and counted as described before [26].
2.8. Statistical analysis
Data were expressed as means ± SEM. Differences between groups were analyzed by ANOVA with Tukey-Kramer multiple comparisons test, a p value < 0.05 is considered significant.
3. Results
3.1. TGF-β induces PTHrP gene expression and secretion
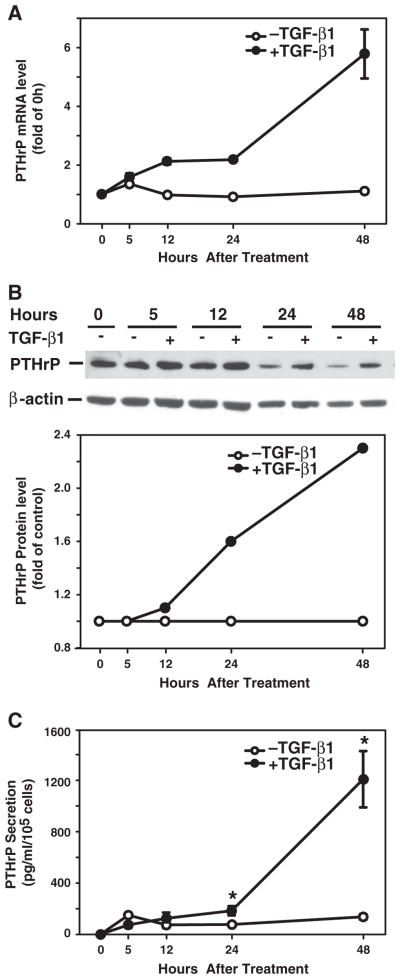
TGF-β has been shown to stimulate PTHrP expression in normal and malignant cells [21,23,24], and PTHrP mediates TGF-β-induced growth inhibition [21]. In this study, we used a human hepatocellular carcinoma cell line, Hep3B that is sensitive to TGF-β-induced apoptosis, to examine the role of PTHrP in TGF-β-induced apoptosis. First we determined whether TGF-β induced PTHrP mRNA expression in these cells. As shown in Fig. 1, TGF-β induced PTHrP mRNA expression beginning at 12 h, increasing over 48 h time course, with 4.5-fold increase at 48 h, compared to the time-matched control (Fig. 1A). Consistent with PTHrP mRNA level, TGF-β induced PTHrP protein expression with 1.6-fold increase at 24 h and 2.3-fold increase at 48 h (Fig. 1B), TGF-β also induced PTHrP peptide secretion with 2.4-fold increase at 24 h and 8.8-fold increase at 48 h (Fig. 1C).
Fig. 1.
TGF-β induces PTHrP expression and secretion. Hep3B cells were cultured in the presence or absence of TGF-β1 (40 pmol/L) over a 48 h time course. A. Total RNA was prepared at 0, 5, 12, 24, and 48 h after TGF-β1 treatment, and converted to cDNA. PTHrP mRNA expression was detected by real-time quantitative PCR. PTHrP mRNA level was expressed as fold of the value at time 0 h. B. Cell lysates were prepared for Western blotting using specific antibody against PTHrP. β-actin served as a protein loading control. Western blotting images are presented on the top panel and PTHrP protein levels were normalized to β-actin and presented as folds of time-matched controls without TGF-β1 on the bottom panel. C. Cell culture supernatants were collected for measurement of PTHrP peptide secretion. The concentration of PTHrP from triplicate wells is expressed as pg/ml and normalized by cell number. Results from triplicate wells are expressed as mean ± SEM. *p < 0.05 compared to the group in the absence of TGF-β1.
3.2. PTHrP mediates TGF-β-induced apoptosis
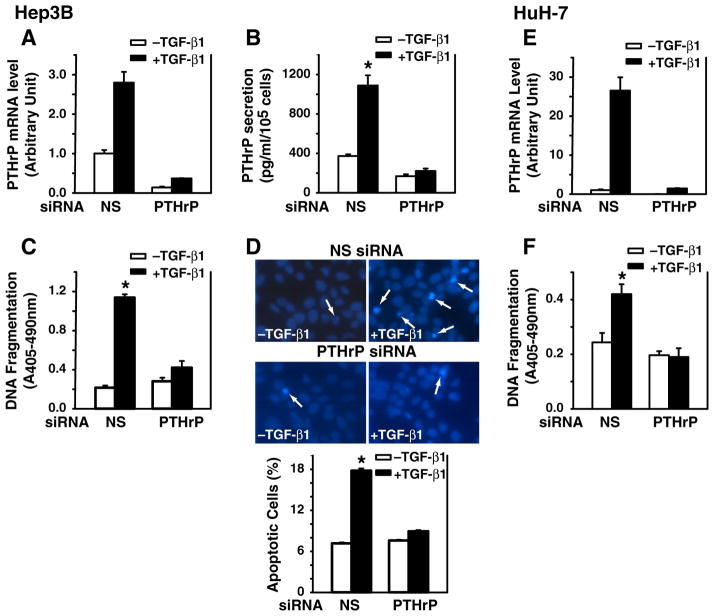
PTHrP mediates the growth inhibitory effect of TGF-β in HepG2 cells, and overexpression of PTHrP enhances apoptosis in intestinal epithelial cells following serum depletion [20,21]. Furthermore, TGF-β induces capspase-3 activity in Hep3B cells as reported [6] and PARP cleavage in our study (Supplemental Fig. 1). Here, we asked whether PTHrP is involved in TGF-β-induced apoptosis by knockdown of PTHrP gene expression using specific siRNA. We report that transfection with the PTHrP siRNA blocked PTHrP mRNA expression in both TGF-β-treated and -untreated Hep3B cells (Fig. 2A). These results were also reflected in the PTHrP secretion pattern in that TGF-β induced PTHrP secretion in non-specific siRNA group, while PTHrP siRNA transfection blocked TGF-β-induced PTHrP secretion (Fig. 2B). In addition, Western blotting was performed for the validation of the siRNA knockdown of PTHrP protein levels. PTHrP siRNA knocked down PTHrP protein in both cell lines from 41 to 47% (Supplemental Fig. 2). Furthermore, TGF-β induced DNA fragmentation by 5.3-fold in the nonspecific siRNA group, whereas TGF-β did not induce DNA fragmentation in PTHrP siRNA-transfected group, compared to vehicle control (Fig. 2C). These findings in Hep3B cells were confirmed by using another human hepatocellular carcinoma cell line HuH-7 (Fig. 2E and F). Similar results were obtained from both cell lines. Consistent with the DNA fragmentation results in Hep3B cells, TGF-β increased the number of apoptotic cells by 10.6% in the nonspecific siRNA group, whereas TGF-β did not increase the number of apoptotic cells in PTHrP siRNA-transfected group (Fig. 2D). These data demonstrate that knockdown of PTHrP gene expression by specific PTHrP siRNA blocked TGF-β-induced apoptosis, suggesting that PTHrP mediates TGF-β-induced apoptosis.
Fig. 2.
Silencing PTHrP gene expression blocks TGF-β-induced apoptosis. Hep3B and HuH-7 cells were transfected with human PTHrP siRNA (100 nmol/L) using DharmaFECT2 and then replated 24 h after siRNA transfection. The cells were then cultured in the presence or absence of TGF-β1 (40 pmol/L) in MEM supplemented with 0.1% FBS for 48 h. Panels of Hep3B cells: A. Total RNA was prepared and converted to cDNA for real-time quantitative PCR analysis of PTHrP mRNA expression. B. The cell culture supernatants were collected and concentrated for measurement of PTHrP peptide secretion. The concentration of PTHrP from triplicate wells is expressed as pg/ml and normalized by cell number. Results from triplicate wells are expressed as mean ± SEM. C. DNA fragmentation was quantified by cell death detection assay. Results from triplicate wells are expressed as mean ± SEM. D. Nuclear morphology of Hep3B cells was examined using Hoechst 33258 staining. Arrows indicate apoptotic cells. Apoptotic cells are expressed as percentage of apoptotic cells versus total cells. Panels of HuH-7 cells: E. Total RNA was prepared and converted to cDNA for real-time quantitative PCR analysis of PTHrP mRNA expression. F. DNA fragmentation was quantified by cell death detection assay. Results from triplicate wells are expressed as mean ± SEM. *p < 0.05 compared to the group in the absence of TGF-β1.
Furthermore, we determined whether antibodies against secreted PTHrP isoforms block TGF-β-induced apoptosis. Two rabbit antisera were generated against PTHrP(1 to 34) and PTHrP(−5 to 139) peptides (kindly provided by Dr. CW Cooper at The University of Texas Medical Branch). The cells were treated with the antisera (5%) in the presence or absence of TGF-β. The preimmune normal sera (5%) were used as a control of the antisera. As shown in Fig. 3, TGF-β induced apoptosis in the presence of normal sera as expected. However, TGF-β did not induce apoptosis in the presence of the antisera against PTHrP (1 to 34) (Fig. 3A) or PTHrP(−5 to 139) (Fig. 3B). These results demonstrate that the antisera against PTHrP isoforms blocked TGF-β-induced apoptosis, and further support our hypothesis that PTHrP mediates TGF-β-induced apoptosis in Hep3B cells. In addition, PTHrP knockdown attenuated TGF-β’s inhibitory effects on cell proliferation in both Hep3B and HuH-7 cells (Fig. 4), indicating that PTHrP also mediates TGF-β’s growth inhibitory effects at least in part.
Fig. 3.
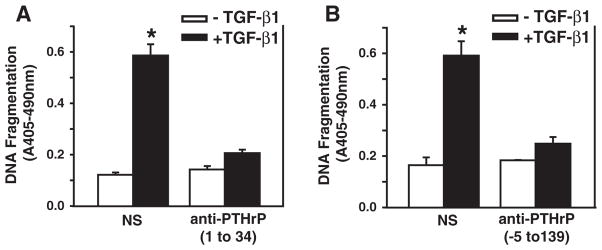
Antisera against PTHrP(1 to 34) and PTHrP(−5 to 139) blocks TGF-β-induced apoptosis. Hep3B cells were treated with 5% of preimmune normal antisera, antisera against PTHrP(1 to 34) (A) or against PTHrP(−5 to 139) (B) in MEM in the presence or absence of TGF-β1 at 40 pmol/L. The cells were harvested at 48 h after treatment and DNA fragmentation was quantified by cell death detection assay. Results from triplicate wells are expressed as mean ± SEM. *p < 0.05 compared to the group in the absence of TGF-β1.
Fig. 4.
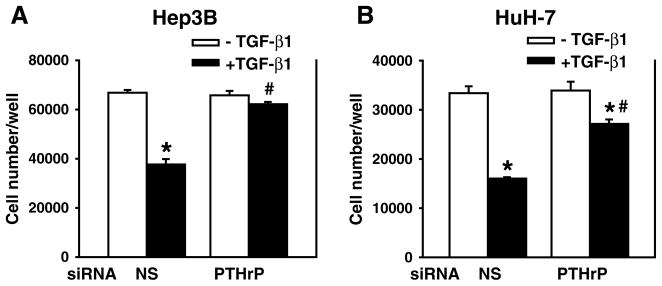
Silencing PTHrP gene expression attenuates TGF-β-induced growth inhibition. Hep3B and HuH-7 cells were transfected with human PTHrP siRNA (100 nmol/L) using DharmaFECT2 and then replated 24 h after siRNA transfection. The cells were then cultured in the presence or absence of TGF-β1 (40 pmol/L) in MEM supplemented with 0.1% FBS for 48 h. Cell numbers were counted. Results from triplicate wells are expressed as mean ± SEM. *p < 0.05 compared to the group in the absence of TGF-β1. #p < 0.05 compared to the group in the presence of TGF-β1 and NS.
3.3. Smad3 mediates TGF-β-induced PTHrP expression for apoptosis induction
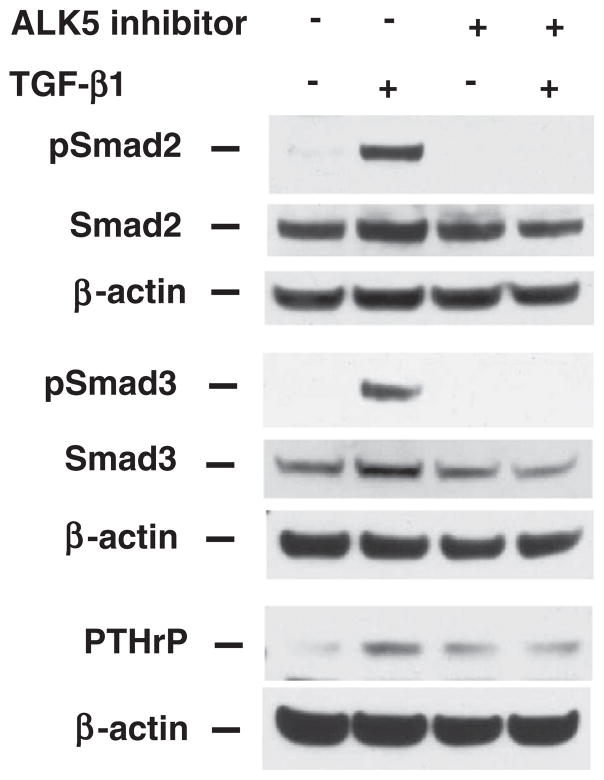
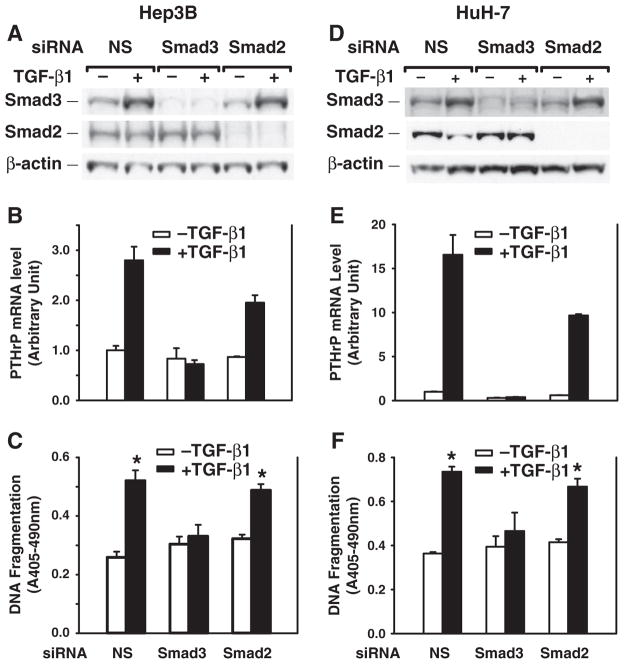
Smad2 and Smad3 proteins are key intracellular mediators of TGF-β signaling pathway [1,3]. Inhibition of TGF-β receptor signaling by a TGF-β RI kinase inhibitor (ALK5 inhibitor) abolished TGF-β-induced phosphorylation of Smad2, Smad3 and PTHrP protein expression (Fig. 5), although ALK5 inhibitor alone induced PTHrP expression. To further investigate the role of Smad2 and Smad3 in TGF-β-induced PTHrP expression, specific siRNAs were used to knockdown Smad2 or Smad3 gene expression. In the nonspecific siRNA transfection group, TGF-β induced Smad3 protein expression, but not Smad2 protein expression. Transfection of Smad3 siRNA suppressed Smad3 expression, blocked TGF-β-induced Smad3 expression, blocked PTHrP expression, but had no effect on Smad2 expression. Conversely transfection of Smad2 siRNA suppressed Smad2 expression, did not block TGF-β-induced PTHrP expression, and had no effect on Smad3 expression (Fig. 6A, B). Moreover, Smad3 siRNA transfection blocked TGF-β-induced apoptosis; in contrast, Smad2 siRNA transfection had no effect on TGF-β-induced apoptosis (Fig. 6C). Similar results were obtained using HuH-7 cells (Fig. 6D, E, F). Taken together, these results support the specific role of Smad3 in mediating TGF-β-induced PTHrP expression for the apoptosis induction.
Fig. 5.
Blocking ALK5 abolishes TGF-β-induced Smad2 and Smad3 activation and PTHrP protein expression. Hep3B cells were treated with ALK5 inhibitor (3 μM) for 45 m followed by treatment with TGF-β1 (40 pmol/L) or vehicle control (0.1% BSA and 4 mM HCl). Cell lysates were harvested at 30 m for Western blotting using antibodies against phospho-Smad2 and -Smad3, Smad2 and Smad3. Cell lysates were harvested at 48 h for Western blotting using antibody against PTHrP. β-actin served as a protein loading control.
Fig. 6.
Silencing Smad3 gene expression blocks TGF-β-induced PTHrP expression and apoptosis induction. Hep3B and HuH-7 cells were transfected with human Smad3 or Smad2 siRNA (100 nmol/L) using DharmaFECT2 and then replated 24 h after siRNA transfection. Cells were then cultured in the presence or absence of TGF-β1 (40 pmol/L) in MEM supplemented with 0.1% FBS for 48 h. A, D. Whole cell lysates were prepared for Western blotting of Smad3 and Smad2. B, E. Total RNAs were prepared and converted to cDNAs for real-time quantitative PCR analysis of PTHrP mRNA expression. C, F. DNA fragmentation was quantified by cell death detection assay. Results from triplicate wells are expressed as mean ± SEM. *p < 0.05 compared to the group in the absence of TGF-β1.
4. Discussion
We have previously shown that Smad3 is the key mediator of TGF-β-induced apoptosis [8]. However, multiple steps along the apoptosis pathway of TGF-β signaling that link between Smad3 to the apoptotic machinery remain to be explored. The main findings of this study are that TGF-β induces apoptosis by increasing PTHrP expression and secretion; knockdown of PTHrP gene expression using siRNA or blocking PTHrP action using antisera against secreted PTHrP isoforms blocks TGF-β-induced apoptosis; knockdown of Smad3 blocks TGF-β-induced PTHrP expression. These data suggest that PTHrP is a novel mediator in TGF-β/Smad3 signaling pathway for apoptosis induction.
Hep3B cells are widely used human hepatocellular carcinoma cells for study of TGF-β-induced cell cycle arrest and apoptosis, the major tumor suppressor functions of TGF-β [6,28,29]. In our case, PTHrP-mediated apoptosis could be one means by which the populations of the hepatocytes are regulated by TGF-β/Smad3. Similar effects of PTHrP on apoptosis induction have also been noted in intestinal epithelial cells [20]. However, in the other cell lines of non-gastrointestinal origin, PTHrP has been shown to protect against induction of apoptosis. For example, PTHrP inhibits TNF-α-induced apoptosis in human embryonic kidney cell line [30], and protects chondrocytes against apoptosis induced by serum depletion [31]. These differences are most likely due to the different cell models used.
PTHrP carries out its physiological functions locally through paracrine, autocrine and intracrine mechanisms [9]. In this study, we demonstrate that knockdown of PTHrP gene blocked PTHrP mRNA expression as well as PTHrP protein secretion. Furthermore, the antisera against PTHrP(1 to 34) and PTHrP(−5 to 139) isoforms blocked TGF-β-induced apoptosis. These results imply that TGF-β induces apoptosis in Hep3B cells via induction of PTHrP secretion, which may function through autocrine or paracrine mechanisms for mediating apoptosis. In addition, PTHrP also mediates TGF-β’s growth inhibitory effects in part because knockdown of PTHrP attenuated TGF-β’s effects on cell proliferation.
As a key mediator in TGF-β signaling pathway, Smad3 plays an essential role in TGF-β-induced apoptosis. To study the role of Smad3 in PTHrP expression, we used siRNAs to specifically knockdown Smad3, or Smad2 gene expression. We found that knockdown of Smad3 gene expression blocked TGF-β-induced PTHrP mRNA expression without affecting basal level of PTHrP mRNA expression, indicating that TGF-β-induced PTHrP expression is Smad3-dependent. Furthermore, knockdown of Smad3 blocked TGF-β-induced apoptosis. However, knockdown of Smad2, a close member of Smad3 in TGF-β signaling pathway, did not affect TGF-β-induced PTHrP expression or TGF-β-induced apoptosis. These findings were consistently observed in two human hepatocellular carcinoma cell lines Hep3B and HuH-7. Thus the differential effect of Smad3 and Smad2 on TGF-β-induced apoptosis once again supports our previous finding that Smad3 is the key mediator for TGF-β-induced apoptosis [8].
In conclusion, TGF-β/Smad3 induces apoptosis in human hepatocellular carcinoma cells through increase of PTHrP expression and secretion. Thus, we have identified PTHrP as a novel mediator for TGF-β-induced apoptosis, which further advances our understanding of critical elements in the apoptotic pathway of TGF-β.
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.regpep.2013.03.024.
Supplementary Material
Acknowledgments
The authors thank E. Figueroa and S. Schuenke from the Department of Surgery, the University of Texas Medical Branch for manuscript and figure preparation and submission, and G. He from the Department of Surgery, the University of Texas Health Science Center at Houston for technical support. This study is supported by grants to T.C.K. from NIH (R01 DK60105), to T.C.K, C.M.T. and M.R.H from NIH (P01 DK35608).
Abbreviations
- PTHrP
parathyroid hormone-related protein
- (TGF)-β
transforming growth factor
- siRNA
small interfering RNA
- real-time quantitative PCR
real-time quantitative polymerase chain reaction
References
- 1.Heldin CH, Miyazono K, ten Dijke P. TGF-beta signalling from cell membrane to nucleus through SMAD proteins. Nature. 1997;390:465–71. doi: 10.1038/37284. [DOI] [PubMed] [Google Scholar]
- 2.Hsing AY, Kadomatsu K, Bonham MJ, Danielpour D. Regulation of apoptosis induced by transforming growth factor-beta1 in nontumorigenic rat prostatic epithelial cell lines. Cancer Res. 1996;56:5146–9. [PubMed] [Google Scholar]
- 3.Zhang Y, Derynck R. Regulation of Smad signalling by protein associations and signalling crosstalk. Trends Cell Biol. 1999;9:274–9. doi: 10.1016/s0962-8924(99)01579-2. [DOI] [PubMed] [Google Scholar]
- 4.Massague J. How cells read TGF-beta signals. Nat Rev Mol Cell Biol. 2000;1:169–78. doi: 10.1038/35043051. [DOI] [PubMed] [Google Scholar]
- 5.Kim BC, Mamura M, Choi KS, Calabretta B, Kim SJ. Transforming growth factor beta 1 induces apoptosis through cleavage of BAD in a Smad3-dependent mechanism in FaO hepatoma cells. Mol Cell Biol. 2002;22:1369–78. doi: 10.1128/mcb.22.5.1369-1378.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jang CW, Chen CH, Chen CC, Chen JY, Su YH, Chen RH. TGF-beta induces apoptosis through Smad-mediated expression of DAP-kinase. Nat Cell Biol. 2002;4:51–8. doi: 10.1038/ncb731. [DOI] [PubMed] [Google Scholar]
- 7.Ramesh S, Qi XJ, Wildey GM, Robinson J, Molkentin J, Letterio J, Howe PH. TGF beta-mediated BIM expression and apoptosis are regulated through SMAD3-dependent expression of the MAPK phosphatase MKP2. EMBO Rep. 2008;9:990–7. doi: 10.1038/embor.2008.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Conery AR, Cao Y, Thompson EA, Townsend CM, Jr, Ko TC, Luo K. Akt interacts directly with Smad3 to regulate the sensitivity to TGF-beta induced apoptosis. Nat Cell Biol. 2004;6:366–72. doi: 10.1038/ncb1117. [DOI] [PubMed] [Google Scholar]
- 9.Strewler GJ. The parathyroid hormone-related protein. Endocrinol Metab Clin North Am. 2000;29:629–45. doi: 10.1016/s0889-8529(05)70154-7. [DOI] [PubMed] [Google Scholar]
- 10.Moseley JM, Kubota M, Diefenbach-Jagger H, Wettenhall RE, Kemp BE, Suva LJ, Rodda CP, Ebeling PR, Hudson PJ, Zajac JD, et al. Parathyroid hormone-related protein purified from a human lung cancer cell line. Proc Natl Acad Sci USA. 1987;84:5048–52. doi: 10.1073/pnas.84.14.5048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burtis WJ, Wu T, Bunch C, Wysolmerski JJ, Insogna KL, Weir EC, Broadus AE, Stewart AF. Identification of a novel 17,000-dalton parathyroid hormone-like adenylate cyclase-stimulating protein from a tumor associated with humoral hypercalcemia of malignancy. J Biol Chem. 1987;262:7151–6. [PubMed] [Google Scholar]
- 12.Strewler GJ, Stern PH, Jacobs JW, Eveloff J, Klein RF, Leung SC, Rosenblatt M, Nissenson RA. Parathyroid hormonelike protein from human renal carcinoma cells. Structural and functional homology with parathyroid hormone. J Clin Invest. 1987;80:1803–7. doi: 10.1172/JCI113275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burtis WJ. Parathyroid hormone-related protein: structure, function, and measurement. Clin Chem. 1992;38:2171–83. [PubMed] [Google Scholar]
- 14.Sourbier C, Massfelder T. Parathyroid hormone-related protein in human renal cell carcinoma. Cancer Lett. 2006;240:170–82. doi: 10.1016/j.canlet.2005.08.020. [DOI] [PubMed] [Google Scholar]
- 15.Clemens TL, Cormier S, Eichinger A, Endlich K, Fiaschi-Taesch N, Fischer E, Friedman PA, Karaplis AC, Massfelder T, Rossert J, Schluter KD, Silve C, Stewart AF, Takane K, Helwig JJ. Parathyroid hormone-related protein and its receptors: nuclear functions and roles in the renal and cardiovascular systems, the placental trophoblasts and the pancreatic islets. Br J Pharmacol. 2001;134:1113–36. doi: 10.1038/sj.bjp.0704378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fiaschi-Taesch NM, Stewart AF. Minireview: parathyroid hormone-related protein as an intracrine factor—trafficking mechanisms and functional consequences. Endocrinology. 2003;144:407–11. doi: 10.1210/en.2002-220818. [DOI] [PubMed] [Google Scholar]
- 17.Orloff JJ, Reddy D, de Papp AE, Yang KH, Soifer NE, Stewart AF. Parathyroid hormone-related protein as a prohormone: posttranslational processing and receptor interactions. Endocr Rev. 1994;15:40–60. doi: 10.1210/edrv-15-1-40. [DOI] [PubMed] [Google Scholar]
- 18.Gensure RC, Gardella TJ, Juppner H. Parathyroid hormone and parathyroid hormone-related peptide, and their receptors. Biochem Biophys Res Commun. 2005;328:666–78. doi: 10.1016/j.bbrc.2004.11.069. [DOI] [PubMed] [Google Scholar]
- 19.Juppner H, Abou-Samra AB, Freeman M, Kong XF, Schipani E, Richards J, Kolakowski LF, Jr, Hock J, Potts JT, Jr, Kronenberg HM, et al. A G protein-linked receptor for parathyroid hormone and parathyroid hormone-related peptide. Science. 1991;254:1024–6. doi: 10.1126/science.1658941. [DOI] [PubMed] [Google Scholar]
- 20.Ye Y, Wang C, Du P, Falzon M, Seitz PK, Cooper CW. Overexpression of parathyroid hormone-related protein enhances apoptosis in the rat intestinal cell line, IEC-6. Endocrinology. 2001;142:1906–14. doi: 10.1210/endo.142.5.8121. [DOI] [PubMed] [Google Scholar]
- 21.Li H, Seitz PK, Selvanayagam P, Rajaraman S, Cooper CW. Effect of endogenously produced parathyroid hormone-related peptide on growth of a human hepatoma cell line (Hep G2) Endocrinology. 1996;137:2367–74. doi: 10.1210/endo.137.6.8641188. [DOI] [PubMed] [Google Scholar]
- 22.Serra R, Karaplis A, Sohn P. Parathyroid hormone-related peptide (PTHrP)-dependent and -independent effects of transforming growth factor beta (TGF-beta) on endochondral bone formation. J Cell Biol. 1999;145:783–94. doi: 10.1083/jcb.145.4.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yin JJ, Selander K, Chirgwin JM, Dallas M, Grubbs BG, Wieser R, Massague J, Mundy GR, Guise TA. TGF-beta signaling blockade inhibits PTHrP secretion by breast cancer cells and bone metastases development. J Clin Invest. 1999;103:197–206. doi: 10.1172/JCI3523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guise TA. Parathyroid hormone-related protein and bone metastases. Cancer. 1997;80:1572–80. doi: 10.1002/(sici)1097-0142(19971015)80:8+<1572::aid-cncr7>3.3.co;2-d. [DOI] [PubMed] [Google Scholar]
- 25.Johnson RW, Nguyen MP, Padalecki SS, Grubbs BG, Merkel AR, Oyajobi BO, Matrisian LM, Mundy GR, Sterling JA. TGF-beta promotion of Gli2-induced expression of parathyroid hormone-related protein, an important osteolytic factor in bone metastasis, is independent of canonical Hedgehog signaling. Cancer Res. 2011;71:822–31. doi: 10.1158/0008-5472.CAN-10-2993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cao Y, Deng C, Townsend CM, Jr, Ko TC. TGF-beta inhibits Akt-induced transformation in intestinal epithelial cells. Surgery. 2006;140:322–9. doi: 10.1016/j.surg.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 27.Falzon M, Zong J. The noncalcemic vitamin D analogs EB1089 and 22-oxacalcitriol suppress serum-induced parathyroid hormone-related peptide gene expression in a lung cancer cell line. Endocrinology. 1998;139:1046–53. doi: 10.1210/endo.139.3.5774. [DOI] [PubMed] [Google Scholar]
- 28.Yang YA, Zhang GM, Feigenbaum L, Zhang YE. Smad3 reduces susceptibility to hepatocarcinoma by sensitizing hepatocytes to apoptosis through downregulation of Bcl-2. Cancer Cell. 2006;9:445–57. doi: 10.1016/j.ccr.2006.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen RH, Chang TY. Involvement of caspase family proteases in transforming growth factor-beta-induced apoptosis. Cell Growth Differ. 1997;8:821–7. [PubMed] [Google Scholar]
- 30.Okoumassoun L, Averill-Bates D, Denizeau F, Henderson JE. Parathyroid hormone related protein (PTHrP) inhibits TNFalpha-induced apoptosis by blocking the extrinsic and intrinsic pathways. J Cell Physiol. 2007;210:507–16. doi: 10.1002/jcp.20892. [DOI] [PubMed] [Google Scholar]
- 31.Aarts MM, Davidson D, Corluka A, Petroulakis E, Guo J, Bringhurst FR, Galipeau J, Henderson JE. Parathyroid hormone-related protein promotes quiescence and survival of serum-deprived chondrocytes by inhibiting rRNA synthesis. J Biol Chem. 2001;276:37934–43. doi: 10.1074/jbc.M105510200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.