Abstract
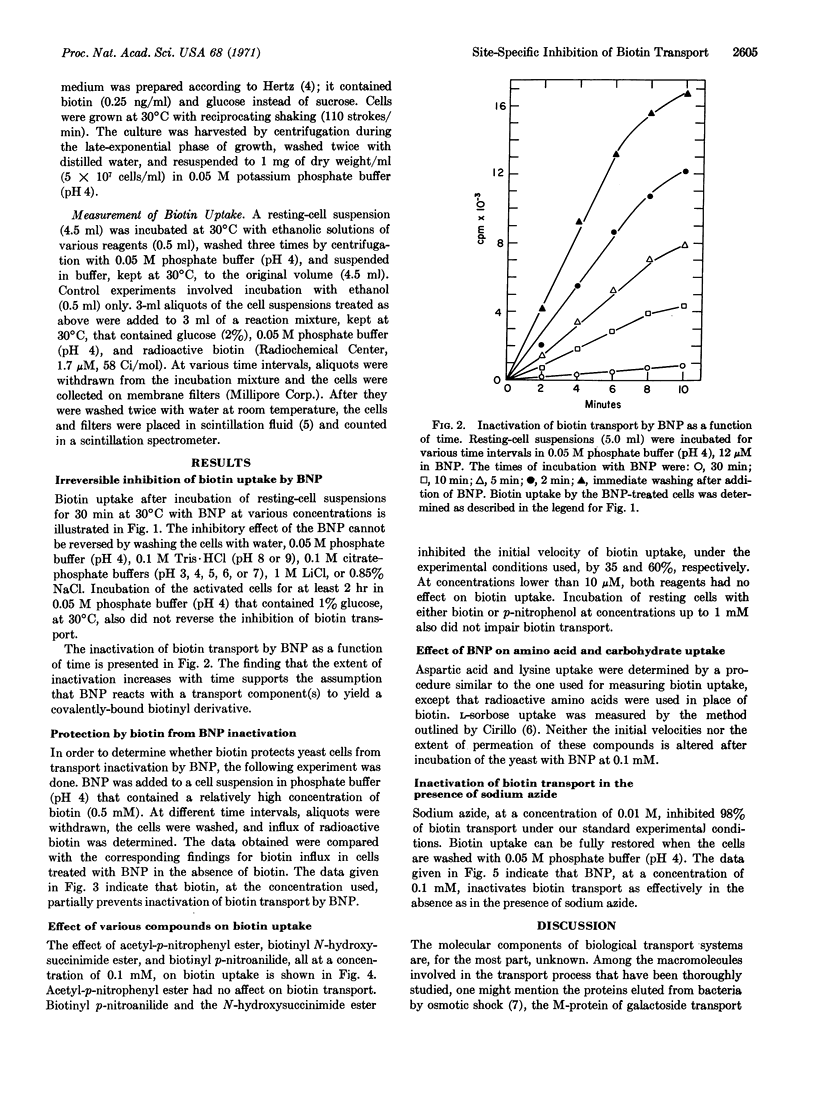
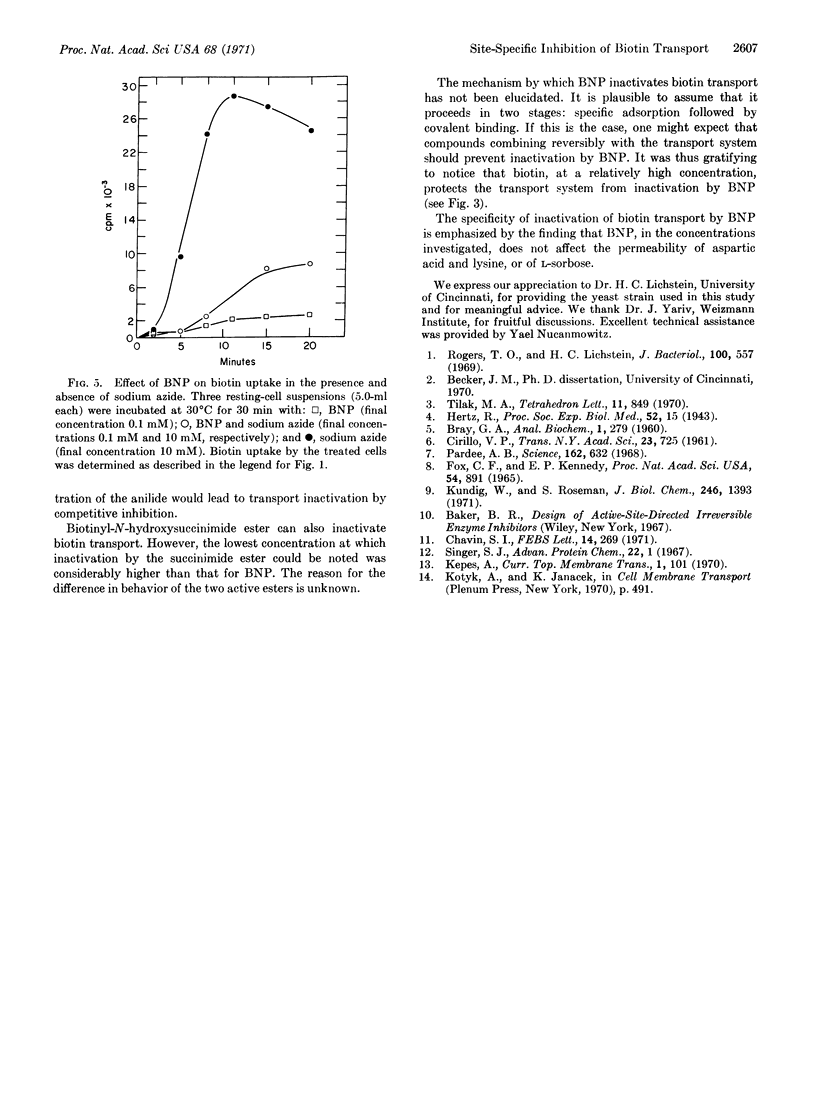
Biotinyl-p-nitrophenyl ester (BNP), an active-ester derivative of biotin, irreversibly inactivates biotin transport in the yeast Saccharomyces cerevisiae. Transport inactivation is progressive with time and occurs at concentrations of the ester as low as 10-7 M. In the presence of sodium azide, a reagent known to block biotin accumulation in yeast, the derivative is still effective. The specificity of inactivation by the ester is revealed by the following findings: (a) Biotinyl-p-nitroanilide and acetyl-p-nitrophenyl ester do not affect biotin transport; (b) the nitrophenyl ester does not affect the transport of lysine and aspartic acid, or that of L-sorbose; (c) inactivation of biotin transport by the ester is partially prevented when the cells are incubated with it in the presence of relatively high concentrations of biotin.
Keywords: affinity label, biotinyl-p-nitroanilide, acetyl-p-nitrophenyl ester, sorbose transport, amino-acid transport
Full text
PDF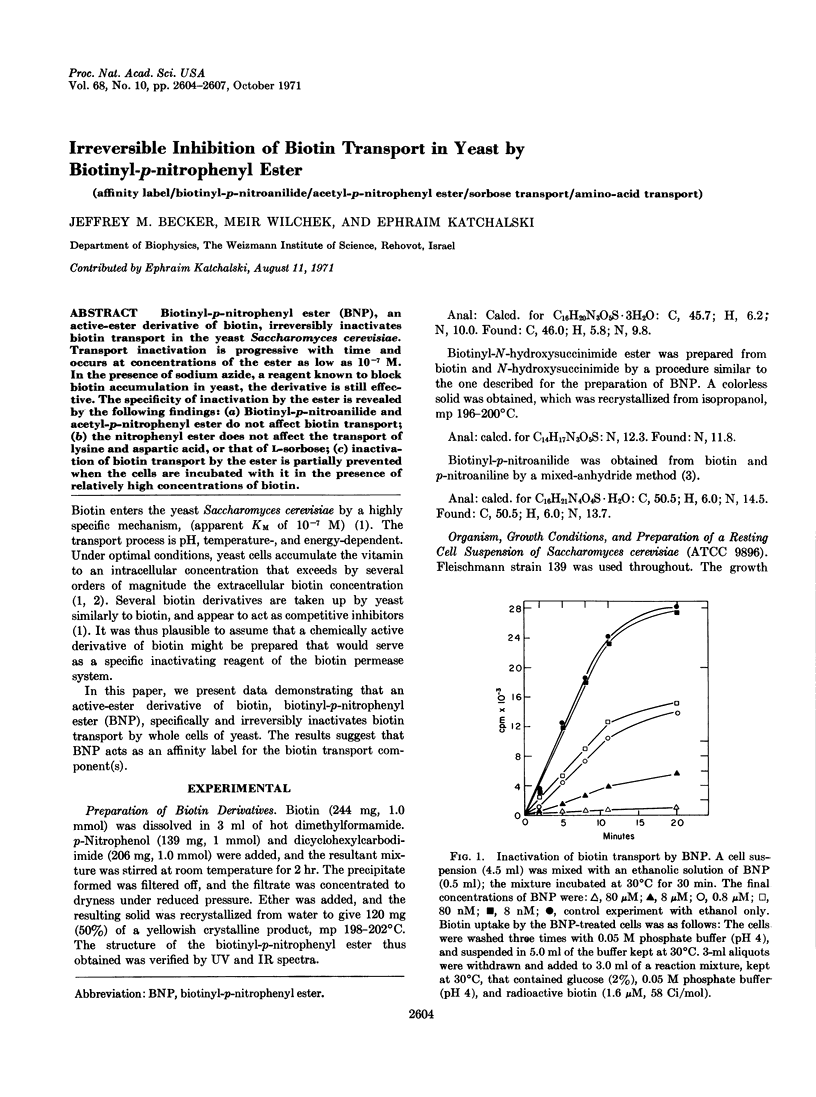

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chavin S. I. Isolation and study of functional membrane proteins Present status and future prospects. FEBS Lett. 1971 May 20;14(5):269–282. doi: 10.1016/0014-5793(71)80278-8. [DOI] [PubMed] [Google Scholar]
- Fox C. F., Kennedy E. P. Specific labeling and partial purification of the M protein, a component of the beta-galactoside transport system of Escherichia coli. Proc Natl Acad Sci U S A. 1965 Sep;54(3):891–899. doi: 10.1073/pnas.54.3.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kundig W., Roseman S. Sugar transport. I. Isolation of a phosphotransferase system from Escherichia coli. J Biol Chem. 1971 Mar 10;246(5):1393–1406. [PubMed] [Google Scholar]
- Pardee A. B. Membrane transport proteins. Proteins that appear to be parts of membrane transport systems are being isolated and characterized. Science. 1968 Nov 8;162(3854):632–637. doi: 10.1126/science.162.3854.632. [DOI] [PubMed] [Google Scholar]
- Rogers T. O., Lichstein H. C. Characterization of the biotin transport system in Saccharomyces cerevisiae. J Bacteriol. 1969 Nov;100(2):557–564. doi: 10.1128/jb.100.2.557-564.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer S. J. Covalent labeling of active sites. Adv Protein Chem. 1967;22:1–54. doi: 10.1016/s0065-3233(08)60040-6. [DOI] [PubMed] [Google Scholar]


