Abstract
Mother-to-child-transmission of HIV by breast-feeding remains a major obstacle in the eradication of HIV infection. Compared to adults, HIV-infected infants have more rapid disease and show higher susceptibility to co-infections like tuberculosis (TB). Although the Bacille Calmette-Guérin vaccine can be administered at birth to protect against TB, BCG can disseminate in HIV-infected infants and increase mortality. Thus, a pediatric combination vaccine to stop both HIV and TB infection in infants is urgently needed.
Towards the goal of developing a pediatric combination HIV-TB vaccine to prevent both oral HIV acquisition by breast-feeding and TB infection, we tested and optimized an immunization regimen using a novel live attenuated Mycobacterium tuberculosis vaccine engineered to express simian immunodeficiency (SIV) antigens followed by heterologous MVA-SIV boosting in the infant macaque model. A single oral dose of the attenuated Mtb-SIV vaccine strain mc26435 during the first week of life was sufficient to induce persistent TB-specific immune responses. SIV-specific immunity was induced at low but comparable magnitudes after oral or intradermal priming, and was enhanced following MVA-SIV boosts. T cell responses were most pronounced in intestinal tissues and oral lymph nodes. Importantly, in addition to plasma SIV-specific IgG and IgA antibodies, infant macaques developed mucosal SIV-specific IgA in saliva and intestinal IgA and IgG. While future SIV and Mtb challenge studies will be needed to determine the protective efficacy of the Mtb-SIV / MVA-SIV vaccine, infants at high risk for oral HIV acquisition by breast-feeding and TB infection could profoundly benefit from an effective combination vaccine.
INTRODUCTION
The coverage of antiretroviral therapy (ART) for HIV-infected mothers has increased substantially, yet many resource-poor countries are still afflicted by rising rates of HIV mother-to-child-transmission (MTCT) (1). Infant ART coverage remains below 30% and ART prophylaxis does not span the entire breast-feeding period (1). The majority of pediatric HIV infections occur in sub-Saharan Africa where tuberculosis (TB) burden is also high. HIV-infected infants face a higher risk of TB infection (2), and pregnant women co-infected with HIV and TB are more likely to transmit both HIV and TB to their infants (2, 3). The Bacille Calmette-Guérin (BCG) vaccine for prevention of TB infection can disseminate in HIV-infected infants with a case fatality of 75% (4). The high morbidity and mortality associated with HIV and TB disease in infants underscore the urgent need for a safe neonatal vaccine to prevent pediatric HIV and TB infections.
Currently, BCG is the only live attenuated vaccine approved for administration in neonates at birth. BCG-inherent adjuvant properties likely enhance pediatric immune responses because a single BCG dose induces robust cellular immunity comparable to responses in adults. These compelling facts provided the rationale for the development of combination HIV-TB vaccines. In fact, based on murine TB efficacy data with a recombinant auxothroph BCG vaccine expressing an African consensus HIV-1 clade A Gag immunogen (rBCG.HIVA) (5–8), phase I clinical trials have been initiated in African neonates. The restriction of preclinical BCG-HIV immunogenicity and safety studies to murine models or adult macaques (5, 7–12), however, is problematic. Substantial differences in i) infant and adult immune function, ii) immune development between neonatal mice and human newborns, and iii) inherent limitations of BCG-derived vaccines (importantly the safety risk for immunocompromised individuals and lack of relevant protective TB antigens), argue for the tandem pursuit of alternative regimens.
We hypothesized that a live attenuated human-adapted Mycobacterium tuberculosis (Mtb) vaccine similar to BCG but with an improved safety profile could be safe and protective, even in immunosuppressed infants. We chose human-adapted Mtb H37Rv in lieu of M. bovis-derived BCG because Mtb contains known immunodominant epitopes for humans that are absent in BCG (13, 14). Secondly, we intentionally deleted specific H37Rv genes important for replication and immune evasion, whereas BCG was attenuated only through serial passaging. Rhesus macaques are an ideal and validated animal model in which to evaluate our combination vaccine due to their extremely sensitivity to Mtb and to simian immunodeficiency virus (SIV) (16–18), and the shared immunological, developmental and physiological similarities between human infants and neonatal macaques (19–21). The translational potential of our vaccine for application in human infants at risk for HIV is supported by our data demonstrating that neonatal SIV-infected macaques could be safely vaccinated with the live attenuated auxotroph Mtb vaccine mc26435 (15).
Towards the long-term goal of developing a pediatric HIV-TB vaccine, the current study tested whether this highly attenuated recombinant Mtb-SIV vaccine can efficiently prime both Mtb and SIV-specific immunity. To establish proof-of concept, we deemed the expression of only a single SIV antigen, SIV Gag, by mc26435 sufficient. SIV Gag contains numerous immunogenic T cell epitopes, and several vaccine studies support the importance of SIV Gag-specific T cell responses in the control of viral replication (22–24). Indeed, our data demonstrate that (i) a single dose of mc26435 induced both Mtb- and SIV-specific immune responses in infant macaques, (ii) vaccine-induced SIV immunity was enhanced and broadened by heterologous MVA-SIV Gag, Pol and Env boosts, and (iii) the combined oral Mtb-SIV Gag prime/ intramuscular MVA-SIV regimen effectively induced mucosal and systemic immune responses.
MATERIALS & METHODS
Animals
Infant rhesus macaques (Macaca mulatta), obtained from the SIV-negative and type D retrovirus-free CNPRC colony (UC Davis, CA), were nursery-reared and housed according to the “Guide for Care and Use of Laboratory Animals” and standards by the AAALAC. Animal protocols were approved by the UC Davis Institutional Animal Care and Use Committee. For all interventions, animals were immobilized by 10mg/kg body weight of ketamine-HCl (Parke-Davis, Morris Plains, NC), injected intramuscularly (IM).
Vaccine strains and immunization regimens
The construction, attenuations and safety profiles of the Mtb H37Rv-derived vaccine strains mc26435 and mc26020 have been described previously (15, 25–30). Briefly, in strain mc26020, the panCD and lysA loci were deleted to generate a highly replication-attenuated double-auxotroph strain. The double-auxotroph strain mc26435 (ΔpanCDΔleuCD) was further modified by deleting the secA2 locus, and engineered to co-expresses a mycobacterial codon-usage-optimized full length SIVmac239 Gag cassette. SIV Gag expression from vaccine cultures was confirmed by immunoblot using a V5 antibody-HRP.
Infant macaques (n=8) were orally (PO) vaccinated with mc26435 at birth (3–7 days old = week 0), IM boosted at 3 and 6 weeks with MVA-SIV expressing SIV Gag, Pol, and Env (kindly provided Dr. B. Moss and Dr. P. Earl, NIAID, NIH, Bethesda, MD) (31–33), and followed for 18 weeks (Group A). Age-matched control animals (n=3) were mock-vaccinated with saline (Group B). To test how replication-attenuation affects Mtb-specific immune responses, three infants were vaccinated both PO and intradermally (ID) with strain mc26020 without boosting, and followed for 24 weeks (Group C).
Sample processing
Blood and saliva samples were collected prior to all interventions and throughout the study period. Plasma was stored at −80° C for antibody measurement. At the time of euthanasia, tonsils, lymph nodes (LN: axillary, mesenteric, retropharyngeal and submandibular), and intestinal tissues (colon and ileum) were collected, and mononuclear cell suspensions (MNC) were prepared as described (34). Saliva was collected using Weck-Cel sponges (Beaver Visitec), immediately snap frozen, and later eluted as described At the terminal time point, 2–3 grams of fresh stool were added to 0.5% BSA in 5ml sterile PBS supplemented with 1/100 protease inhibitor cocktail (Sigma, P8340) and snap-frozen. Fecal extracts were prepared by vortexing the thawed contents until complete homogenization, removing debris by ultracentrifugation (4°C, 10 min), filtration (0.45μm), and concentration to 0.5ml using an Amicon Ultra-4 50K centrifugal filter unit (Millipore).
SIV-specific antibodies and immunoglobulin (Ig) measurements
Anti-SIV IgG and IgA antibodies in plasma and fecal extracts, and salivary IgA were measured by ELISA as described (34). Briefly, plates were coated overnight with 100ng SIVmac251 Env rgp120 (Immune Technology, New York, NY) or 100μl 1/400 SIVmac239 lysate (Advanced Biotechnologies, Columbia, MD) per well in PBS. Antibodies against the lysate are termed “Gag, Pol”-specific because Env protein is undetectable at the dilution used. Total IgA or IgG in saliva or fecal extracts were measured by capture ELISA (15, 35) using goat anti-monkey IgA (AlphaDiagnostics, San Antonio, TX) or goat anti-monkey IgG (MP Biomedicals), respectively, to capture Ig. The following day, plates were washed, blocked and loaded with diluted samples and a monkey serum standard containing known amounts of Ig, SIV-specific IgA or SIV-specific IgG. After overnight incubation (4°C), the plates were developed with biotinylated goat anti-monkey IgA or anti-human IgG antibody (SouthernBiotech, Birmingham, AL), and avidin-labeled peroxidase (Sigma). The SIV Env or Gag, Pol-specific IgA or IgG antibody concentrations in each secretion were normalized to total IgA or IgG concentration. The specific activity (ng IgA or IgG antibody per μg total IgA or IgG) was deemed significant if it was greater than the mean + 3SD of negative controls.
Antigen-specific T cell responses
1x106 blood or tissue MNC were treated with 0.5μg anti-CD28 and anti-CD49d (BD Biosciences, San Jose, CA) in 1ml supplemented RPMI plus i) no stimulant, ii) 5ng/ml PMA /500ng/ml Ionomycin (Sigma, St. Louis, MO) iii) 10 μg SIVmac239 p27 Gag peptide pool, iv) 10 μg SIVmac239 Env peptide pool (both: AIDS Research and Reference Reagent Program), v) 25 μg purified protein derivative (Tuberculin PPD, Statens Serum Institute, Copenhagen, Denmark), vi) 10 μl BCG vaccine (105 CFU; Statens Serum Institute), vii) 50 μg Mtb H37Rv recombinant culture filtrate protein-10 reference standard (rCFP), viii) 50 μg Mtb H37Rv whole strain lysate, or ix) 25 μg Antigen 85b reference standard (Ag85b; all from BEI Resources Manassas, VA). Cells were cultured for 6 hrs (37°C, 5% CO2) with 1x Brefeldin A (eBioscience, San Diego, CA) treatment after the first hour. Cells were stained using a fixable live/dead discriminator (Invitrogen) and antibodies against rhesus macaque CD3, CD4, IL-2, TNF-α, IFN-γ, (BD Biosciences, San Jose, CA) and IL-17A (eBioscience). When cell counts permitted, unstimulated MNC suspensions were stained for markers of T cell memory (CD45RA, CCR7) and mucosal homing (CD103). All panels included fluorescence-minus-one controls. Paraformaldehyde-fixed samples were acquired on a LSRII instrument and analyzed using FlowJo software with Boolean gating (TreeStar, Ashland, OR). Antigen-specific T cell responses were corrected for background responses in unstimulated controls and are reported as percentage of CD4+ or CD8+ T cells.
Statistical analysis
Data were analyzed using Prism (GraphPad, Inc., La Jolla, CA). Correlations between IgA in various compartments (plasma, saliva, intestine) were analyzed using the Spearman rank test to determine the effect of antibody transudation versus mucosal antibody production. Frequencies of CD103 positive T cell populations were compared by applying a two-tailed Student’s t-test with Welch’s correction. P values ≤0.05 were considered significant.
RESULTS
TB-specific immunity after a single prime with attenuated Mtb
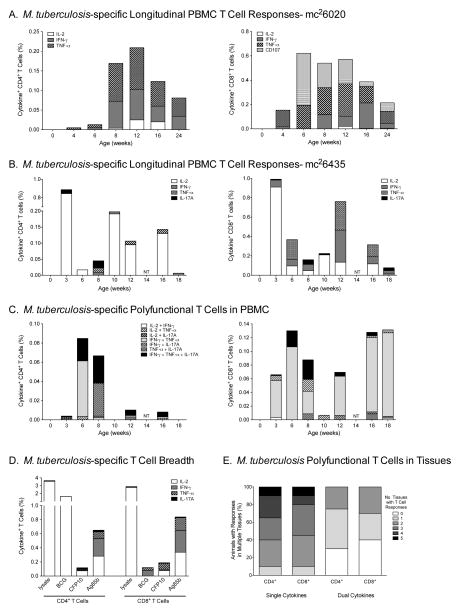
We have previously shown that mc26435 induces plasma IgG antibodies to the mycobacterial antigen PSTS-1, albeit at significantly lower levels than the more replication-competent strain mc26020 (15). In contrast, independent of replication capacity, the magnitude and persistence of Mtb-specific peripheral blood T cell responses were similar in mc26020 and mc26435 vaccinated infant macaques (Figure 1A/B, Table 1). Mtb-specific T cell responses consisted primarily of cells producing IL-2, IFN-γ, TNF-α or IL-17 (Figure 1C, Table 1), but dual and triple polyfunctional T cells were detected at multiple time points (Figure 1D). Although only PPD and Ag85b-specific responses were consistently evaluated due to limited infant blood volumes, mc26435 vaccinated animals also developed rCFP10-specific CD4+ and CD8+ T cell responses, indicating that mc26435 elicited responses to human immunodominant epitopes that are absent in BCG (Figure 1D). Broad polyfunctional Mtb-specific T cell responses were also elicited in multiple tissues (Figure 1E). Consistent with the oral route of immunization, Mtb-specific T cell responses were induced in tonsils and LN draining the oropharynx (Table 1), with the highest Mtb-specific T cell responses developing in ileum and colon (Table 1).
Figure 1. Mtb-specific T cell responses in PBMC.
Panel A and Panel B: CD4+ and CD8+ T cytokine responses after in vitro BCG or PPD stimulation of longitudinally collected blood samples from a representative animal vaccinated with strain mc26020 (Panel B) or with strain mc26435 (Panel C), respectively. Note, only single-functional responses are shown. Panel C: Polyfunctional T cell responses in a representative animal in longitudinally collected blood samples. Panel D: T cell responses are induced against a number of Mtb-specific antigens (PBMC data from a representative animal). Panel E: The percentage of animals with vaccine-induced single and polyfunctional PPD-specific T cell responses in tissues. T cells were isolated from selected tissues (tonsil, submandibular LN, ileum, colon and axillary LN) from all animals in Group B. Using a gray scale gradient, the percentage of vaccinated animals that have PPD-specific cytokine producing T cells in all 5 tissues (indicated as ‘5’) is indicated in black, whereas animals lacking PPD-specific responses in all 5 tissues (indicated as ‘0’) are shown in white. Data are stratified by CD4+ and CD8* T cells. CFP, Mtb culture filtrate protein; lysate, Mtb whole cell lysate; BCG, bacille Calmette-Guérin vaccine strain; Ag85b, antigen 85b.
Table 1.
Mtb-specific single cytokine T cell responses
| Tissue | Time (wks) | PPD | Ag85b | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| IL-2a | IFN-γ | IL-17A | TNF-α | Animal No. (%)b | IL-2 | IFN-γ | IL-17A | TNF-α | Animal No. (%) | ||
| PBMC | |||||||||||
| CD4+T | 3 | 0.028–0.844 | 0.014c | 0.038–0.050 | 0.016 | 6/6 (100) | NTd | NT | NT | NT | |
| 6 | 0.014–0.022 | 0.013 | NS | 0.021 | 6/7 (86) | 0.168–16.09 | NSe | NS | NS | 2/2 (100) | |
| 8 | 0.012–0.084 | 0.010–0.012 | 0.039 | 0.012–0.013 | 6/8 (75) | 0.158–0.239 | 0.023–0.051 | 0.037–0.046 | 1.440–2.220 | 5/5 (100) | |
| 16–18 | 0.017–0.130 | NS | 0.061–0.148 | 0.013 | 3/8 (38) | 0.148–0.373 | NS | 0.038–0.191 | NS | 5/8 (63) | |
| CD8+T | 3 | 0.238–0.911 | 0.033–0.296 | NS | 0.047–0.307 | 6/6 (100) | NT | NT | NT | NT | |
| 6 | NS | 0.038–0.139 | NS | 0.041–0.204 | 4/7 (57) | 0.163–15.19 | 0.493 | NS | NS | 2/2 (100) | |
| 8 | NS | 0.013–0.066 | NS | 0.048–0.053 | 6/8 (75) | 0.130–0.386 | 0.121–0.603 | 0.046–0.137 | 0.399–1.146 | 5/5 (100) | |
| 16–18 | NS | 0.025–0.074 | 0.141 | 0.053–0.123 | 5/8 (63) | 0.152–0.212 | 0.043–0.208 | 0.093 | 0.148 | 3/8 (38) | |
| Tonsil | |||||||||||
| CD4+T | 16–18 | 0.011–0.033 | NS | 0.158 | 0.013 | 3/8 (38) | NT | NT | NT | NT | |
| CD8+T | 16–18 | 0.034–0.131 | NS | NS | 0.088 | 3/8 (38) | NT | NT | NT | NT | |
| Subm. LN | |||||||||||
| CD4+T | 16–18 | 0.085–0.968 | NS | 0.066–0.081 | 4.258 | 4/8 (50) | 0.041 | 0.011 | NS | 0.022 | 3/7 (43) |
| CD8+T | 16–18 | 0.440–0.812 | NS | 0.136 | 0.040–0.677 | 4/8 (50) | NS | NS | NS | NS | 0/7 (0) |
| Ileum | |||||||||||
| CD4+T | 16–18 | 0.043–0.077 | 0.039–0.044 | 0.275–0.800 | 0.057–0.166 | 4/6 (67) | 0.023–0.062 | NS | 0.597 | 0.037–0.633 | 6/7 (86) |
| CD8+T | 16–18 | 0.294 | 0.171 | 0.305 | 0.080–1.068 | 4/6 (67) | 0.014–0.097 | 0.020–0.095 | 0.239–0.298 | 0.454–1.328 | 6/7 (86) |
| Colon | |||||||||||
| CD4+T | 16–18 | 0.068–0.363 | 0.020–0.450 | 0.182–0.320 | 0.055–0.166 | 6/8 (75) | 0.066–0.591 | 0.019–0.600 | NS | 0.025–0.156 | 7/8 (88) |
| CD8+T | 16–18 | 0.535 | 1.360 | NS | 0.055–0.212 | 4/8 (50) | 0.187–0.525 | 0.023–1.550 | 0.204–0.360 | 0.180–0.259 | 7/8 (88) |
| Mes. LN | |||||||||||
| CD4+T | 16–18 | NS | NS | 0.048 | 0.011–0.166 | 4/8 (50) | NS | 0.067 | NS | NS | 1/8 (13) |
| CD8+T | 16–18 | NS | NS | 0.018 | 0.024–0.097 | 6/8 (75) | 0.021–0.029 | 0.033 | NS | 0.125 | 7/8 (88) |
| Ax. LN | |||||||||||
| CD4+T | 16–18 | 0.040 | NS | NS | 0.012 | 2/8 (25) | 0.025 | NS | NS | NS | 1/8 (13) |
| CD8+T | 16–18 | NS | 0.049 | NS | 0.068–0.138 | 2/8 (25) | NS | NS | 0.014 | 0.036–0.080 | 5/8 (63) |
Shown are the ranges of the percentage of cytokine positive CD4 or CD8 T cells.
Only a single animal had a positive response
NT= not tested
NS= not significant; responses were not different from media only controls
Depending on cell numbers, not all animals were tested at all time points for antigen-specific responses. Shown are the number of responding animals / total animals tested. In parentheses we report the the percentages of animals with positive responses.
SIV-specific T cell immune responses in peripheral blood and tissues
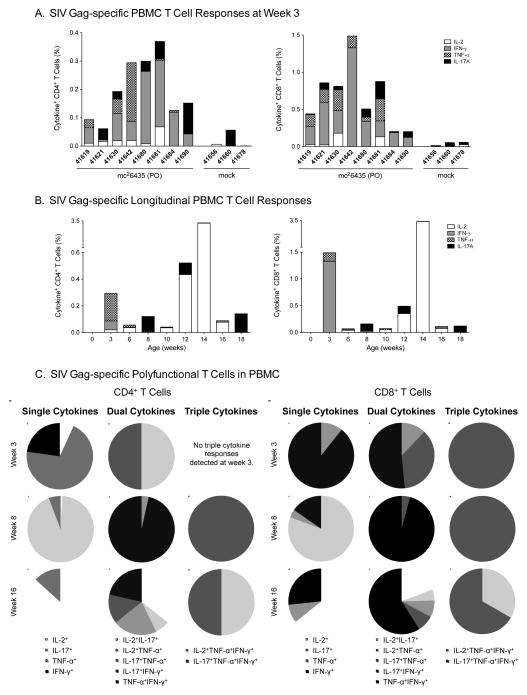
A single oral immunization with mc26435 was sufficient to induce SIV Gag-specific T cell responses by 3 weeks of age (Figure 2A), prior to MVA-SIV boosting. SIV Gag-specific T cell frequencies did not increase after MVA-SIV boosts (Figure 2B), but dual and triple cytokine-producing T cells increased in frequency and diversity (Figure 2C), suggesting improved quality of SIV Gag responses. In addition, SIV–specific T cell immunity increased in breadth through induction of Env-specific T cells. SIV Gag and SIV Env-specific T cell responses were observed at multiple time points indicative of induction of SIV-specific T cell memory in blood and tissues (Table 2).
Figure 2. SIV-specific peripheral blood T cell responses.
Panel A: Cytokine production following SIV Gag stimulation in peripheral T cells at week 3 for all mc26435-primed animals in Group B. Panel B: CD4+ (left panel) and CD8+ (right panel) T cell cytokine production in response to in vitro SIV Gag stimulation in longitudinally collected blood samples of a representative animal after vaccination. Panel C: SIV-specific single, dual and triple positive cytokine responses in peripheral blood CD4+ (left panel) and CD8+ (right panel) T cells at weeks 3 (post-prime, pre-boost), 8 (post-boosts) and 16. Distinct cytokines or combinations thereof are indicated by different colors in the graph legend.
Table 2.
SIV-specific single cytokine T cell responses
| Tissue | Time (wks) | SIV Gag | SIV Env | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| IL-2 | IFN-γ | IL-17A | TNF-α | Animal No. (%) | IL-2 | IFN-γ | IL-17A | TNF-α | Animal No. (%) | ||
| PBMC | |||||||||||
| CD4+T | 3 | 0.068 | 0.043–0.255 | 0.061–0.109 | 0.028–0.207 | 7/8 (88) | NT | NT | NT | NT | |
| 6 | 0.040–0.217 | NS | 0.056–0.072 | 0.011–0.016 | 6/8 (75) | 0.030 | NS | 0.081 | 0.023 | 3/4 (75) | |
| 8 | 0.175 | NS | 0.113 | NS | 2/8 (25) | 0.038 | NS | 0.051 | 0.014 | 3/6 (50) | |
| 16–18 | 0.050 | NS | 0.062–0.136 | 0.010–0.015 | 5/8 (63) | 0.016–0.096 | 0.014–0.025 | 0.045–0.202 | 0.013–0.019 | 6/8 (75) | |
| CD8+T | 3 | 0.131–0.181 | 0.125–1.329 | 0.077–0.233 | 0.056–0.281 | 8/8 (100) | NT | NT | NT | NT | |
| 6 | 0.224 | 0.050–0.100 | 0.181 | 0.022–0.096 | 5/8 (63) | NS | 0.063 | 0.069 | 0.039–0.044 | 3/4 (75) | |
| 8 | 0.219 | 0.065 | 0.129 | 0.022–0.049 | 5/8 (63) | NS | 0.012–0.174 | 0.041 | 0.030 | 4/6 (67) | |
| 16–18 | 0.048–0.062 | 0.038–0.063 | 0.104–0.124 | 0.080 | 6/8 (75) | 0.139 | 0.018–0.049 | 0.089–0.240 | 0.025–0.120 | 7/8 (88) | |
| Tonsil | |||||||||||
| CD4+T | 16–18 | 0.167 | NS | 0.196 | NS | 3/8 (38) | 0.015–0.126 | NS | 0.155 | 0.019–0.028 | 5/5 (100) |
| CD8+T | 16–18 | 0.013–0.213 | 0.027–0.027 | 0.176–0.572 | 0.095–0.105 | 6/8 (75) | 0.042–0.227 | NS | 0.412 | 0.053–0.190 | 5/5 (100) |
| Subm. LN | |||||||||||
| CD4+T | 16–18 | NS | NS | NS | NS | 0/8 (0) | 0.011–0.013 | NS | NS | 0.018 | 3/8 (38) |
| CD8+T | 16–18 | 0.010–0.018 | 0.011–0.016 | NS | NS | 4/8 (50) | 0.035–0.048 | 0.012 | NS | 0.010–0.030 | 7/8 (88) |
| Ileum | |||||||||||
| CD4+T | 16–18 | 0.058–0.174 | 0.021–0.144 | 0.165–0.900 | 0.144–0.396 | 6/7 (86) | 0.0221–0.075 | 0.024–0.242 | 0.315–0.395 | 0.241–0.499 | 7/7 (100) |
| CD8+T | 16–18 | 0.023–0.294 | 0.011–0.077 | 0.426 | 0.026–0.878 | 7/7 (100) | 0.064 | NS | 0.090–1.065 | 0.032–1.008 | 7/7 (100) |
| Colon | |||||||||||
| CD4+T | 16–18 | 0.333 | 0.290 | 0.118 | 0.029 | 3/8 (38) | 0.228–0.711 | 0.033–0.920 | 0.010–0.130 | 0.011–0.019 | 6/8 (75) |
| CD8+T | 16–18 | 0.143–0.625 | 0.043–1.130 | NS | 0.069–0.237 | 4/8 (50) | 0.022–0.505 | 0.015–0.820 | 0.050–0.161 | 0.156–0.193 | 6/8 (75) |
| Mes. LN | |||||||||||
| CD4+T | 16–18 | NS | 0.010 | 0.025–0.464 | 0.016–0.033 | 6/8 (75) | 0.010 | 0.014 | 0.101 | 0.033 | 5/8 (63) |
| CD8+T | 16–18 | 0.022–0.029 | NS | 0.024–0.568 | 0.062–0.121 | 7/8 (88) | 0.012–0.048 | 0.027 | 0.075 | 0.049–0.073 | 6/8 (75) |
| Ax. LN | |||||||||||
| CD4+T | 16–18 | NS | NS | NS | NS | 0/8 (0) | NS | NS | NS | 0.016 | 1/8 (13) |
| CD8+T | 16–18 | NS | 0.052 | NS | 0.053–0.19 | 5/5 (100) | 0.047–0.062 | NS | NS | 0.073–0.083 | 3/8 (38) |
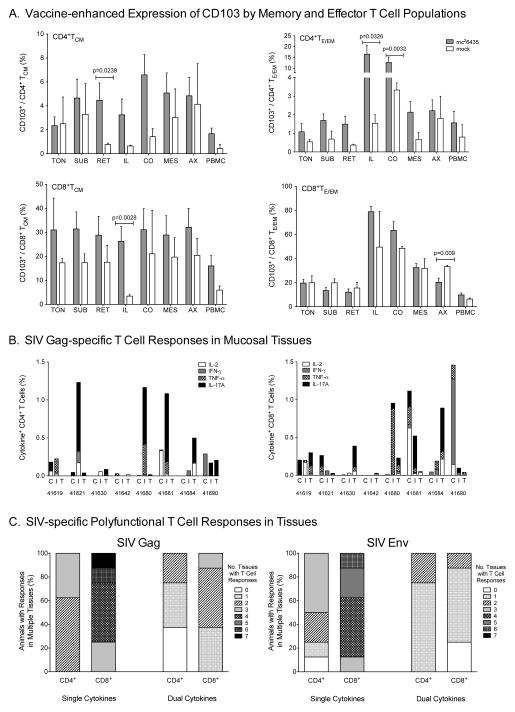
Consistent with oral priming, frequencies of CD103 positive central memory (CD3+CD4+CD45RA−CCR7+, TCM) and effector/effector memory (CD3+CD4+CD45RA+/−CCR7−, TE/EM) CD4+ T cell populations were higher in multiple tissues of vaccinated animals compared to mock animals, including the ileum (CD4+TE/EM: p=0.0326), colon (CD4+TE/EM: p=0.0032), and the retropharyngeal LN (CD4+TCM: p=0.0239) draining the oropharynx (Figure 3A). Similarly, a higher percentage of ileal CD8+ TCM expressed CD103 compared to mock animals (p=0.0028). The biological meaning of decreased CD103+CD8+TE/EM cells in axillary LN is questionable because CD103 is a mucosal homing marker, and CD103+CD8+TE/EM frequencies were similar in all other tissues (Figure 3A). Due to limited cell numbers, memory and homing markers were only evaluated on total CD4+ and CD8+ T cell populations. However, tissues with higher CD103+ T cell frequencies generally also had higher numbers of SIV-specific T cells. Thus, SIV-specific T cells were most pronounced in intestinal tissues (Figure 3B), but also detectable in at least one other tissue of each vaccinated animal (Figure 3C). Responses were directed to SIV Gag and SIV Env and consisted of single and polyfunctional T cells with CD8+T cells being induced at higher frequencies and in more tissues than SIV-specific CD4+T cells (Figure 3C).
Figure 3. Homing and SIV-specific T cell responses in tissues.
Panel A: Animals primed with strain mc26435 and boosted with MVA-SIV (gray bars) have higher percentages of memory and effector T cells expressing CD103 compared to unvaccinated animals (white bars). Memory and effector/effector memory cells were defined within the CD4+ or CD8+ T cell populations as CD45RA−CCR7− (TCM) or CD45RA+/−CCR7− (TE/EM), respectively, before measuring CD103 expression. Statistical significance using a one-tailed t-test was calculated using log-transformed values. Shown are mean values ± SEM. Tissues are abbreviated as follows: tonsil (TON), submandibular lymph node (SUB), retropharyngeal lymph node (RET), ileum (ILE), colon (CO), mesenteric lymph node (MES), axillary lymph node (AX), and PBMC. Panel B: CD4+ (left panel) and CD8+ (right panel) T cell single cytokine production in response to in vitro SIV Gag stimulation of tissue cell populations. Each bar represents the sum of all single cytokine responses in the relevant tissue (see legend for cytokine color coding). Shown are data for all animals that were vaccinated orally with strain mc26435 and boosted with MVA-SIV. The three bars for each animal represent from left to right: colon (C), ileum (I), and tonsil (T). Panel C: The percentage of animals with vaccine-induced single and polyfunctional T cell responses to SIV Gag (left panel) or SIV Env (right panel). T cells were isolated from tonsil, submandibular LN, retropharyngeal LN, ileum, colon, mesenteric LN, and axillary LN of all vaccinated animals. Data are stratified for single and dual cytokine producing CD4+ and CD8* T cells. Using a gray scale gradient, animals with T cells from all seven tissues producing no cytokine(s) in response to SIV are indicated by ‘0’ (white) and animals with all seven tissues producing cytokine(s) are shown as ‘7’ (black).
Induction of systemic and mucosal SIV-specific antibodies
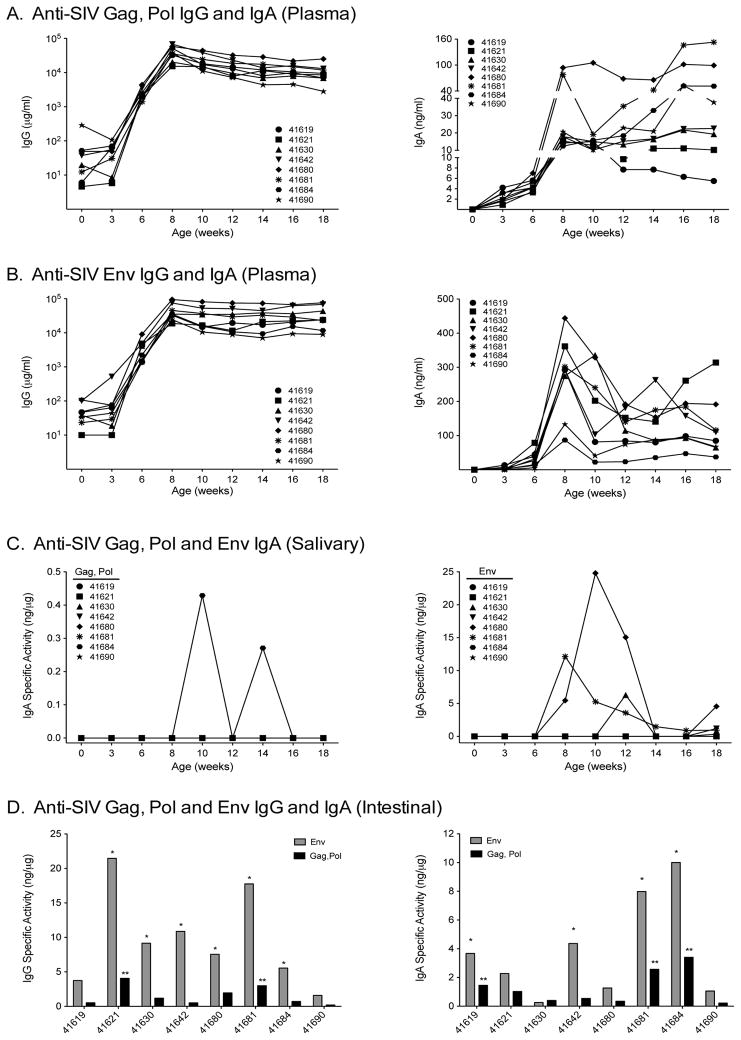
In contrast to SIV-specific T cell responses, anti-SIV Gag, Pol plasma IgG antibodies were rarely detected prior to boosting, but ultimately developed in all animals after the MVA-SIV boosts. Anti-SIV Gag, Pol- and anti-SIV Env-specific plasma IgG responses peaked at week 8 and were maintained at high levels (Figure 4A/B). Plasma SIV Env specific IgA antibodies also peaked by 8 weeks, but subsequently declined, while SIV Gag-specific IgA antibodies showed a more variable pattern (Figure 4A/B).
Figure 4. SIV-specific plasma and mucosal IgG and IgA antibodies.
Panel A and Panel B: Anti-SIV Gag, Pol or anti-SIV Env IgG (left graphs) and IgA (right graphs) antibody development in plasma following oral priming with strain mc26435 plus MVA-SIV boosts. Note that IgG values are represented on a log scale and IgA values are plotted linearly. Panel C: SIV Gag, Pol (left panel) and Env (right panel) specific activity for IgA in saliva following priming with strain mc26435 plus MVA-SIV boosts. Panel D: The specific activity for intestinally-secreted anti-SIV Gag, Pol (black) and anti-SIV Env (gray) IgA (left) and IgG (right) antibodies following priming with strain mc26435 plus MVA-SIV boosts. Intestinal antibodies were only measured at the time of euthanasia. One (*) or two stars (**) above bars indicate that antibody measurements for anti-SIV Env or anti-SIV Gag, Pol, respectively, exceeded mean background levels ± 3SD.
Considering the potential importance of mucosal antibodies in preventing MTCT of HIV by breast-feeding, we measured anti-SIV Gag, Pol and anti-SIV Env-specific IgA in mucosal compartments of vaccinated animals. Even though SIV Gag was a component of both the mc26435 prime and the MVA-SIV boosts, anti-SIV Gag, Pol-specific IgA was present in saliva of only 1 of 8 vaccinated animals, whereas anti-SIV Env-specific salivary IgA antibodies developed in 4 vaccinees (Figure 4C). At euthanasia, we detected anti-SIV Env- or anti-SIV Gag, Pol-specific IgA and IgG intestinal antibodies in 4 of 8 and 6 of 8 animals, respectively. One of 8 animals (#41690) failed to develop SIV-specific intestinal IgG or IgA antibodies (Figure 4D), despite high plasma antibodies to SIV Env. In fact, plasma anti-Env IgA levels did not correlate with those in stool (p = 0.9768; R = −0.0238) or in saliva (p = 0.3333; R= −1.000), suggesting that anti-Env-specific IgA in the secretions was derived from local mucosal synthesis rather than passive serum transudation (data not shown).
DISCUSSION
Protective immunity against TB is thought to be primarily mediated by T cells (36–38), while prevention of HIV infection requires broadly-neutralizing antibodies (39) and T cell immunity to control early viral replication should HIV infection occur (24, 40). Towards the long-term goal of developing a pediatric HIV-TB vaccine, the main study objective was to provide proof of concept that a highly attenuated Mtb strain expressing SIV antigens could induce both SIV and Mtb–specific immune responses in infant macaques. To prevent TB and HIV infections, vaccine-induced memory responses need to be elicited and persist at sites of pathogen entry and replication, i.e. the lung TB, and the oropharynx and intestinal tract, respectively. Thus, a secondary goal was to prove that the oral mc26435 prime/ IM MVA-SIV boost regimen could effectively induce persistent mucosal and systemic dual immune responses.
Indeed, our results show that a single oral immunization with mc26435 is sufficient to induce broad and persistent Mtb and SIV-specific T cell responses in blood and tissues. Mtb-specific T cell responses were elicited to multiple TB antigens, including CFP, a known immunodominant antigen in humans that is absent in BCG (14). Although we did not include a BCG control group in this first study, evidence from mouse studies shows that strain mc26435 is as immunogenic as BCG (manuscript in preparation, Ranganathan et al.). Furthermore, PPD-specific CD4+ and CD8+T cell frequencies in infant macaques after a single oral mc26435 immunization are comparable to PPD-specific ELISPOT responses (3,000–5000 SPU/106 PBMC or 0.3–0.5% PBMC) elicited by an auxotrophic recombinant BCG-SIV vaccine and by standard BCG in adult macaques (9). Some mc26435-vaccinated animals had predominantly IL-17A-producing Mtb-specific T cells in tissues. The role of IL-17 in TB is still controversial, but some studies suggest a protective role of Th17 cells in the lung (38, 41–44). The current study lacked the analysis of Mtb-specific lung responses, but future studies will thoroughly assess the quantity, quality and breadth of the lung responses to determine the potential of mc26435 vaccination to protect against TB infection.
SIV-specific tissue T cell responses were measured in sites draining the oropharynx, in the intestinal tract as an important site for vaccine-induced SIV immunity to prevent CD4+ T cell depletion, and the axillary LN as representative distal site following oral priming. SIV-specific T cell responses were most consistently induced in intestinal tissues and the tonsils. Interestingly, intestinal tissues and lymph nodes draining the oral cavity also contained significantly higher frequencies of CD103+ memory CD4+ and CD8+ T cells compared to mock-immunized infants indicative of mucosal homing. Support for the successful induction of mucosal immunity was further provided by the presence of SIV-specific IgG and IgA antibodies in intestine and mucosal IgA in saliva. Because the SIV Env immunogen was only present in the MVA-SIV boosts and because the intestinal antibodies had specificity for SIV Env and SIV Gag, Pol antigens, likely the mucosal (PO) prime and systemic (IM) boosts contributed to the development of the intestinal antibody response. The role of IgA in protection against oral SIV infection remains to be demonstrated conclusively. In the HIV Thai vaccine trial, systemic HIV Env-specific IgA antibodies correlated with an increased risk of HIV acquisition (45). However, mucosally-induced SIV Env-induced antibodies persisting at mucosal sites might be important for protection against oral HIV infection in infants. Passive immunization studies demonstrated that systemic administration of antiviral IgG, which transudates into the saliva, could confer protection against oral infection by high in vitro neutralization and/or ADCVI activity (46–48). Experiments assessing different vaccine-induced antibody functions will be essential in future studies (49). Oral SIV challenge studies will be needed to determine the relative contribution of SIV-specific T cell responses, SIV-specific salivary and intestinal IgA, and intestinal IgG responses in preventing or controlling infection.
In conclusion, The results of the current study, in combination with our previous safety assessment of mc26435 in infant macaques, provide the basis for future development of auxotrophic Mtb strains candidates as feasible and safe platform for a pediatric combination HIV-TB vaccine. Further optimization of this approach will include the testing a cocktail of attenuated Mtb strains expressing SIV Env, Gag and Pol and boosting Mtb-specific responses. While an attenuated Mtb prime/ heterologous viral boost vaccine strategy may not be sufficient to render sterilizing immunity, this approach may reduce viral transmission rates or viral loads in infants that do become infected. Future SIV and Mtb challenge studies will evaluate the efficacy of this or other combination vaccines, but if successful, they could confer protection against both HIV and TB during the early period of life, and potentially through adolescence and into adulthood.
Auxotroph Mtb is a safe platform for a combination HIV-TB vaccine at birth.
An Mtb-SIV vaccine induces SIV and TB immune responses in newborn macaques.
The novel pediatric SIV-TB vaccine induces systemic and mucosal immune responses.
Anti-HIV salivary IgA, intestinal IgG and IgA may prevent oral HIV acquisition.
Acknowledgments
The studies were supported by NIH grants 1R01 DE019064 (to KA, ML, and GF), T32 AI007419 and T32 AI007001-36 (to KJ), and by the Louisiana Vaccine Center funded by the Louisiana Board of Regents (PAK). The animal studies at the CNPRC were supported by NIH grants RR00169 from the National Center for Research Resources (NCRR; NIH) and the Office of Research Infrastructure Programs/ OD P51 OD011107. Studies at UNC Chapel Hill were supported by the Center for AIDS Research (CFAR; NIH grant 2 P30 AI050410), and by the UNC Flow Cytometry Core that receives support from the Department of Microbiology and Immunology and the NCI Center Core Support Grant P30 CA06086 to the UNC Chapel Hill Lineberger Cancer Center. We are especially grateful to Dr. B. Moss and Dr. P. Earl for providing us with the MVA-SIV vaccine vectors and to Jeffrey Americo for growth and quality control of the vaccine stocks (Laboratory of Viral Diseases, National Institute of Allergy and Infectious Diseases, NIH, Bethesda, MD). The SIV Gag and Env peptide pools were provided by the NIH AIDS Research and Reference Reagent Program (Division of AIDS, NIAID, NIH). Mtb antigens were provided by BEI Resources. In addition, we thank the staff of Colony Research Services, Pathology, Primate Medicine, and Clinical Laboratory at the CNPRC, and Yongzhi Geng for technical assistance in the studies.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
BIBLIOGRAPHY
- 1.http://www.unaids.org, posting date. [Online.]
- 2.Adhikari M, Jeena P, Bobat R, Archary M, Naidoo K, Coutsoudis A, Singh R, Nair N. HIV-Associated Tuberculosis in the Newborn and Young Infant. Int J Pediatr. 2011;2011:354208. doi: 10.1155/2011/354208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nair SS. Ethical aspects of the Revised National Tuberculosis Control Programme. Indian J Med Ethics. 2011;8:102–106. doi: 10.20529/IJME.2011.038. [DOI] [PubMed] [Google Scholar]
- 4.Hesseling AC, Marais BJ, Gie RP, Schaaf HS, Fine PE, Godfrey-Faussett P, Beyers N. The risk of disseminated Bacille Calmette-Guerin (BCG) disease in HIV-infected children. Vaccine. 2007;25:14–18. doi: 10.1016/j.vaccine.2006.07.020. [DOI] [PubMed] [Google Scholar]
- 5.Saubi N, Mbewe-Mvula A, Gea-Mallorqui E, Rosario M, Gatell JM, Hanke T, Joseph J. Pre-clinical development of BCG.HIVA(CAT), an antibiotic-free selection strain, for HIV-TB pediatric vaccine vectored by lysine auxotroph of BCG. PLoS One. 2012;7:e42559. doi: 10.1371/journal.pone.0042559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hopkins R, Bridgeman A, Joseph J, Gilbert SC, McShane H, Hanke T. Dual neonate vaccine platform against HIV-1 and M. tuberculosis. PLoS One. 2011;6:e20067. doi: 10.1371/journal.pone.0020067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saubi N, Im EJ, Fernandez-Lloris R, Gil O, Cardona PJ, Gatell JM, Hanke T, Joseph J. Newborn mice vaccination with BCG.HIVA(2)(2)(2) + MVA.HIVA enhances HIV-1-specific immune responses: influence of age and immunization routes. Clin Dev Immunol. 2011;2011:516219. doi: 10.1155/2011/516219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rosario M, Hopkins R, Fulkerson J, Borthwick N, Quigley MF, Joseph J, Douek DC, Greenaway HY, Venturi V, Gostick E, Price DA, Both GW, Sadoff JC, Hanke T. Novel recombinant Mycobacterium bovis BCG, ovine atadenovirus, and modified vaccinia virus Ankara vaccines combine to induce robust human immunodeficiency virus-specific CD4 and CD8 T-cell responses in rhesus macaques. J Virol. 2010;84:5898–5908. doi: 10.1128/JVI.02607-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cayabyab MJ, Korioth-Schmitz B, Sun Y, Carville A, Balachandran H, Miura A, Carlson KR, Buzby AP, Haynes BF, Jacobs WR, Letvin NL. Recombinant Mycobacterium bovis BCG prime-recombinant adenovirus boost vaccination in rhesus monkeys elicits robust polyfunctional simian immunodeficiency virus-specific T-cell responses. J Virol. 2009;83:5505–5513. doi: 10.1128/JVI.02544-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hopkins R, Bridgeman A, Bourne C, Mbewe-Mvula A, Sadoff JC, Both GW, Joseph J, Fulkerson J, Hanke T. Optimizing HIV-1-specific CD8(+) T-cell induction by recombinant BCG in prime-boost regimens with heterologous viral vectors. Eur J Immunol. 2011;41:3542–3552. doi: 10.1002/eji.201141962. [DOI] [PubMed] [Google Scholar]
- 11.Im EJ, Saubi N, Virgili G, Sander C, Teoh D, Gatell JM, McShane H, Joseph J, Hanke T. Vaccine platform for prevention of tuberculosis and mother-to-child transmission of human immunodeficiency virus type 1 through breastfeeding. J Virol. 2007;81:9408–9418. doi: 10.1128/JVI.00707-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rosario M, Fulkerson J, Soneji S, Parker J, Im EJ, Borthwick N, Bridgeman A, Bourne C, Joseph J, Sadoff JC, Hanke T. Safety and immunogenicity of novel recombinant BCG and modified vaccinia virus Ankara vaccines in neonate rhesus macaques. J Virol. 2010;84:7815–7821. doi: 10.1128/JVI.00726-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brodin P, Majlessi L, Marsollier L, de Jonge MI, Bottai D, Demangel C, Hinds J, Neyrolles O, Butcher PD, Leclerc C, Cole ST, Brosch R. Dissection of ESAT-6 system 1 of Mycobacterium tuberculosis and impact on immunogenicity and virulence. Infect Immun. 2006;74:88–98. doi: 10.1128/IAI.74.1.88-98.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dillon DC, Alderson MR, Day CH, Bement T, Campos-Neto A, Skeiky YA, Vedvick T, Badaro R, Reed SG, Houghton R. Molecular and immunological characterization of Mycobacterium tuberculosis CFP-10, an immunodiagnostic antigen missing in Mycobacterium bovis BCG. J Clin Microbiol. 2000;38:3285–3290. doi: 10.1128/jcm.38.9.3285-3290.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jensen K, Ranganathan UD, Van Rompay KK, Canfield DR, Khan I, Ravindran R, Luciw PA, Jacobs WR, Jr, Fennelly G, Larsen MH, Abel K. A recombinant attenuated Mycobacterium tuberculosis vaccine strain is safe in immunosuppressed simian immunodeficiency virus-infected infant macaques. Clin Vacc Immunol. 2012;19:1170–1181. doi: 10.1128/CVI.00184-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sharpe SA, McShane H, Dennis MJ, Basaraba RJ, Gleeson F, Hall G, McIntyre A, Gooch K, Clark S, Beveridge NE, Nuth E, White A, Marriott A, Dowall S, Hill AV, Williams A, Marsh PD. Establishment of an aerosol challenge model of tuberculosis in rhesus macaques and an evaluation of endpoints for vaccine testing. Clin Vacc Immunol. 2010;17:1170–1182. doi: 10.1128/CVI.00079-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang J, Ye YQ, Wang Y, Mo PZ, Xian QY, Rao Y, Bao R, Dai M, Liu JY, Guo M, Wang X, Huang ZX, Sun LH, Tang ZJ, Ho WZ. M. tuberculosis H37Rv infection of Chinese rhesus macaques. J Neuroimmune Pharmacol. 2011;6:362–370. doi: 10.1007/s11481-010-9245-4. [DOI] [PubMed] [Google Scholar]
- 18.McMurray DN. A nonhuman primate model for preclinical testing of new tuberculosis vaccines. Clin Infect Dis. 2000;30(Suppl 3):S210–212. doi: 10.1086/313885. [DOI] [PubMed] [Google Scholar]
- 19.Mehra S, Golden NA, Dutta NK, Midkiff CC, Alvarez X, Doyle LA, Asher M, Russell-Lodrigue K, Monjure C, Roy CJ, Blanchard JL, Didier PJ, Veazey RS, Lackner AA, Kaushal D. Reactivation of latent tuberculosis in rhesus macaques by coinfection with simian immunodeficiency virus. J Med Primatol. 2011;40:233–243. doi: 10.1111/j.1600-0684.2011.00485.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Safi H, Gormus BJ, Didier PJ, Blanchard JL, Lakey DL, Martin LN, Murphey-Corb M, Vankayalapati R, Barnes PF. Spectrum of manifestations of Mycobacterium tuberculosis infection in primates infected with SIV. AIDS Res Hum Retroviruses. 2003;19:585–595. doi: 10.1089/088922203322230950. [DOI] [PubMed] [Google Scholar]
- 21.Shen Y, Zhou D, Chalifoux L, Shen L, Simon M, Zeng X, Lai X, Li Y, Sehgal P, Letvin NL, Chen ZW. Induction of an AIDS virus-related tuberculosis-like disease in macaques: a model of simian immunodeficiency virus- mycobacterium coinfection. Infect Immun. 2002;70:869–877. doi: 10.1128/IAI.70.2.869-877.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Price DA, Asher TE, Wilson NA, Nason MC, Brenchley JM, Metzler IS, Venturi V, Gostick E, Chattopadhyay PK, Roederer M, Davenport MP, Watkins DI, Douek DC. Public clonotype usage identifies protective Gag-specific CD8+ T cell responses in SIV infection. J Exp Med. 2009;206:923–936. doi: 10.1084/jem.20081127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sun C, Chen Z, Tang X, Zhang Y, Feng L, Du Y, Xiao L, Liu L, Zhu W, Chen L, Zhang L. Mucosal priming with a replicating-vaccinia virus-based vaccine elicits protective immunity to simian immunodeficiency virus challenge in rhesus monkeys. J Virol. 2013;87:5669–5677. doi: 10.1128/JVI.03247-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stephenson KE, Li H, Walker BD, Michael NL, Barouch DH. Gag-specific cellular immunity determines in vitro viral inhibition and in vivo virologic control following simian immunodeficiency virus challenges of vaccinated rhesus monkeys. J Virol. 2012;86:9583–9589. doi: 10.1128/JVI.00996-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ranganathan UD, Larsen MH, Kim J, Porcelli SA, Jacobs WR, Jr, Fennelly GJ. Recombinant pro-apoptotic Mycobacterium tuberculosis generates CD8+ T cell responses against human immunodeficiency virus type 1 Env and M. tuberculosis in neonatal mice. Vaccine. 2009;28:152–161. doi: 10.1016/j.vaccine.2009.09.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Larsen MH, Biermann K, Chen B, Hsu T, Sambandamurthy VK, Lackner AA, Aye PP, Didier P, Huang D, Shao L, Wei H, Letvin NL, Frothingham R, Haynes BF, Chen ZW, Jacobs WR., Jr Efficacy and safety of live attenuated persistent and rapidly cleared Mycobacterium tuberculosis vaccine candidates in non-human primates. Vaccine. 2009;27:4709–4717. doi: 10.1016/j.vaccine.2009.05.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sambandamurthy VK, Derrick SC, Hsu T, Chen B, Larsen MH, Jalapathy KV, Chen M, Kim J, Porcelli SA, Chan J, Morris SL, Jacobs WR., Jr Mycobacterium tuberculosis DeltaRD1 DeltapanCD: a safe and limited replicating mutant strain that protects immunocompetent and immunocompromised mice against experimental tuberculosis. Vaccine. 2006;24:6309–6320. doi: 10.1016/j.vaccine.2006.05.097. [DOI] [PubMed] [Google Scholar]
- 28.Sambandamurthy VK, Wang X, Chen B, Russell RG, Derrick S, Collins FM, Morris SL, Jacobs WR., Jr A pantothenate auxotroph of Mycobacterium tuberculosis is highly attenuated and protects mice against tuberculosis. Nat Med. 2002;8:1171–1174. doi: 10.1038/nm765. [DOI] [PubMed] [Google Scholar]
- 29.Waters WR, Palmer MV, Nonnecke BJ, Thacker TC, Scherer CF, Estes DM, Hewinson RG, Vordermeier HM, Barnes SW, Federe GC, Walker JR, Glynne RJ, Hsu T, Weinrick B, Biermann K, Larsen MH, Jacobs WR., Jr Efficacy and immunogenicity of Mycobacterium bovis DeltaRD1 against aerosol M. bovis infection in neonatal calves. Vaccine. 2009;27:1201–1209. doi: 10.1016/j.vaccine.2008.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zimmerman DM, Waters WR, Lyashchenko KP, Nonnecke BJ, Armstrong DL, Jacobs WR, Jr, Larsen MH, Egan E, Dean GA. Safety and immunogenicity of the Mycobacterium tuberculosis DeltalysA DeltapanCD vaccine in domestic cats infected with feline immunodeficiency virus. Clin Vacc Immunol. 2009;16:427–429. doi: 10.1128/CVI.00396-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Earl PL, Wyatt LS, Montefiori DC, Bilska M, Woodward R, Markham PD, Malley JD, Vogel TU, Allen TM, Watkins DI, Miller N, Moss B. Comparison of vaccine strategies using recombinant env-gag-pol MVA with or without an oligomeric Env protein boost in the SHIV rhesus macaque model. Virology. 2002;294:270–281. doi: 10.1006/viro.2001.1345. [DOI] [PubMed] [Google Scholar]
- 32.Van Rompay KK, Abel K, Lawson JR, Singh RP, Schmidt KA, Evans T, Earl P, Harvey D, Franchini G, Tartaglia J, Montefiori D, Hattangadi S, Moss B, Marthas ML. Attenuated poxvirus-based simian immunodeficiency virus (SIV) vaccines given in infancy partially protect infant and juvenile macaques against repeated oral challenge with virulent SIV. J Acquir Immune Defic Syndr. 2005;38:124–134. doi: 10.1097/00126334-200502010-00002. [DOI] [PubMed] [Google Scholar]
- 33.Winstone N, Wilson AJ, Morrow G, Boggiano C, Chiuchiolo MJ, Lopez M, Kemelman M, Ginsberg AA, Mullen K, Coleman JW, Wu CD, Narpala S, Ouellette I, Dean HJ, Lin F, Sardesai NY, Cassamasa H, McBride D, Felber BK, Pavlakis GN, Schultz A, Hudgens MG, King CR, Zamb TJ, Parks CL, McDermott AB. Enhanced control of pathogenic Simian immunodeficiency virus SIVmac239 replication in macaques immunized with an interleukin-12 plasmid and a DNA prime-viral vector boost vaccine regimen. J Virol. 2011;85:9578–9587. doi: 10.1128/JVI.05060-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marthas ML, Van Rompay KK, Abbott Z, Earl P, Buonocore-Buzzelli L, Moss B, Rose NF, Rose JK, Kozlowski PA, Abel K. Partial efficacy of a VSV-SIV/MVA-SIV vaccine regimen against oral SIV challenge in infant macaques. Vaccine. 2011;29:3124–3137. doi: 10.1016/j.vaccine.2011.02.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kozlowski PA, Lynch RM, Patterson RR, Cu-Uvin S, Flanigan TP, Neutra MR. Modified wick method using Weck-Cel sponges for collection of human rectal secretions and analysis of mucosal HIV antibody. J Acquir Immune Defic Syndr. 2000;24:297–309. doi: 10.1097/00126334-200008010-00001. [DOI] [PubMed] [Google Scholar]
- 36.Abebe F, Bjune G. The protective role of antibody responses during Mycobacterium tuberculosis infection. Clin Exp Immunol. 2009;157:235–243. doi: 10.1111/j.1365-2249.2009.03967.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cooper AM. Host versus pathogen: two sides of the same challenge in the TB world. Eur J Immunol. 2009;39:632–633. doi: 10.1002/eji.200990014. [DOI] [PubMed] [Google Scholar]
- 38.Zuniga J, Torres-Garcia D, Santos-Mendoza T, Rodriguez-Reyna TS, Granados J, Yunis EJ. Cellular and humoral mechanisms involved in the control of tuberculosis. Clin Dev Immunol. 2012;2012:193923. doi: 10.1155/2012/193923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mascola JR, Haynes BF. HIV-1 neutralizing antibodies: understanding nature’s pathways. Immunol Rev. 2013;254:225–244. doi: 10.1111/imr.12075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hansen SG, Ford JC, Lewis MS, Ventura AB, Hughes CM, Coyne-Johnson L, Whizin N, Oswald K, Shoemaker R, Swanson T, Legasse AW, Chiuchiolo MJ, Parks CL, Axthelm MK, Nelson JA, Jarvis MA, Piatak M, Jr, Lifson JD, Picker LJ. Profound early control of highly pathogenic SIV by an effector memory T-cell vaccine. Nature. 2011;473:523–527. doi: 10.1038/nature10003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kleinsteuber K, Heesch K, Schattling S, Sander-Juelch C, Mock U, Riecken K, Fehse B, Fleischer B, Jacobsen M. SOCS3 promotes interleukin-17 expression of human T cells. Blood. 2012;120:4374–4382. doi: 10.1182/blood-2011-11-392738. [DOI] [PubMed] [Google Scholar]
- 42.Wang X, Barnes PF, Huang F, Alvarez IB, Neuenschwander PF, Sherman DR, Samten B. Early secreted antigenic target of 6-kDa protein of Mycobacterium tuberculosis primes dendritic cells to stimulate Th17 and inhibit Th1 immune responses. J Immunol. 2012;189:3092–3103. doi: 10.4049/jimmunol.1200573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pitt JM, Stavropoulos E, Redford PS, Beebe AM, Bancroft GJ, Young DB, O’Garra A. Blockade of IL-10 signaling during bacillus Calmette-Guerin vaccination enhances and sustains Th1, Th17, and innate lymphoid IFN-gamma and IL-17 responses and increases protection to Mycobacterium tuberculosis infection. J Immunol. 2012;189:4079–4087. doi: 10.4049/jimmunol.1201061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gopal R, Rangel-Moreno J, Slight S, Lin Y, Nawar HF, Fallert Junecko BA, Reinhart TA, Kolls J, Randall TD, Connell TD, Khader SA. Interleukin-17-dependent CXCL13 mediates mucosal vaccine-induced immunity against tuberculosis. Mucosal Immunol. 2013 doi: 10.1038/mi.2012.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Haynes BF, Gilbert PB, McElrath MJ, Zolla-Pazner S, Tomaras GD, Alam SM, Evans DT, Montefiori DC, Karnasuta C, Sutthent R, Liao HX, DeVico AL, Lewis GK, Williams C, Pinter A, Fong Y, Janes H, DeCamp A, Huang Y, Rao M, Billings E, Karasavvas N, Robb ML, Ngauy V, de Souza MS, Paris R, Ferrari G, Bailer RT, Soderberg KA, Andrews C, Berman PW, Frahm N, De Rosa SC, Alpert MD, Yates NL, Shen X, Koup RA, Pitisuttithum P, Kaewkungwal J, Nitayaphan S, Rerks-Ngarm S, Michael NL, Kim JH. Immune-correlates analysis of an HIV-1 vaccine efficacy trial. N Engl J Med. 2012;366:1275–1286. doi: 10.1056/NEJMoa1113425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Forthal DN, Landucci G, Gorny MK, Zolla-Pazner S, Robinson WE., Jr Functional activities of 20 human immunodeficiency virus type 1 (HIV-1)-specific human monoclonal antibodies. AIDS Res Hum Retroviruses. 1995;11:1095–1099. doi: 10.1089/aid.1995.11.1095. [DOI] [PubMed] [Google Scholar]
- 47.Ruprecht RM, Ferrantelli F, Kitabwalla M, Xu W, McClure HM. Antibody protection: passive immunization of neonates against oral AIDS virus challenge. Vaccine. 2003;21:3370–3373. doi: 10.1016/s0264-410x(03)00335-9. [DOI] [PubMed] [Google Scholar]
- 48.Van Rompay KK, Berardi CJ, Dillard-Telm S, Tarara RP, Canfield DR, Valverde CR, Montefiori DC, Cole KS, Montelaro RC, Miller CJ, Marthas ML. Passive immunization of newborn rhesus macaques prevents oral simian immunodeficiency virus infection. J Infect Dis. 1998;177:1247–1259. doi: 10.1086/515270. [DOI] [PubMed] [Google Scholar]
- 49.Forthal D, Hope TJ, Alter G. New paradigms for functional HIV-specific nonneutralizing antibodies. Curr Opin HIV AIDS. 2013;8:392–400. doi: 10.1097/COH.0b013e328363d486. [DOI] [PMC free article] [PubMed] [Google Scholar]