Abstract
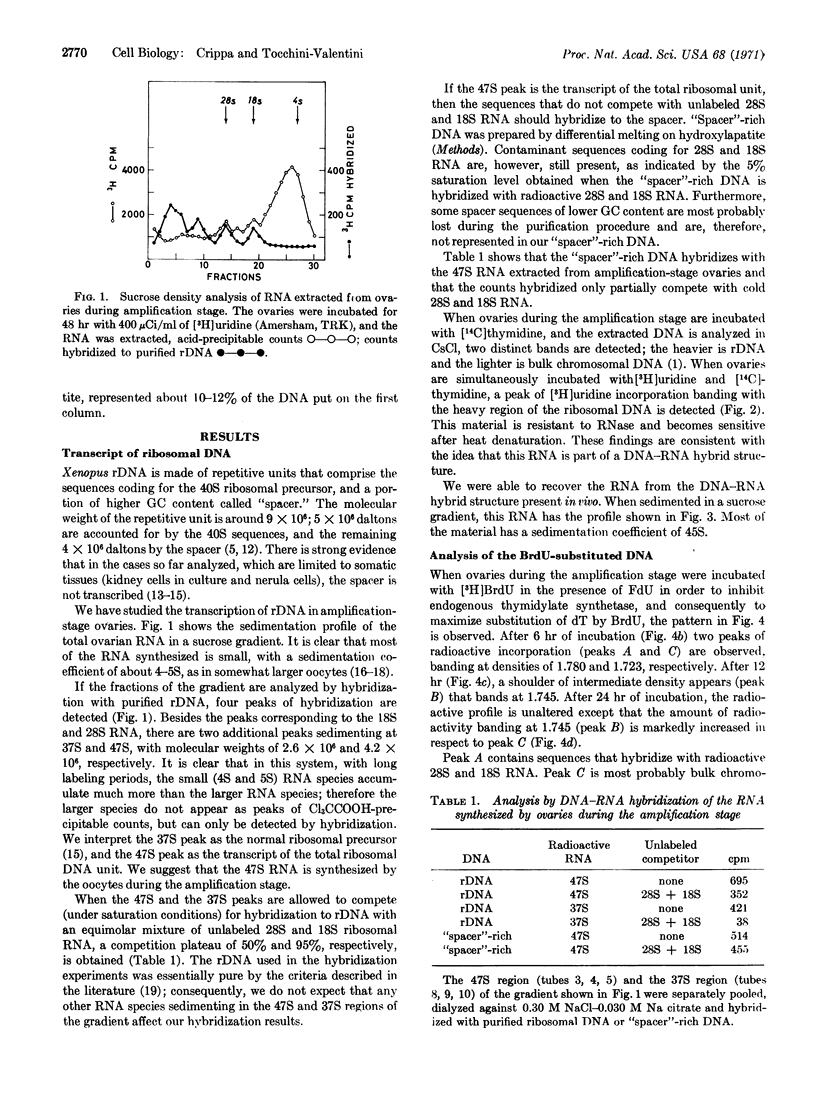
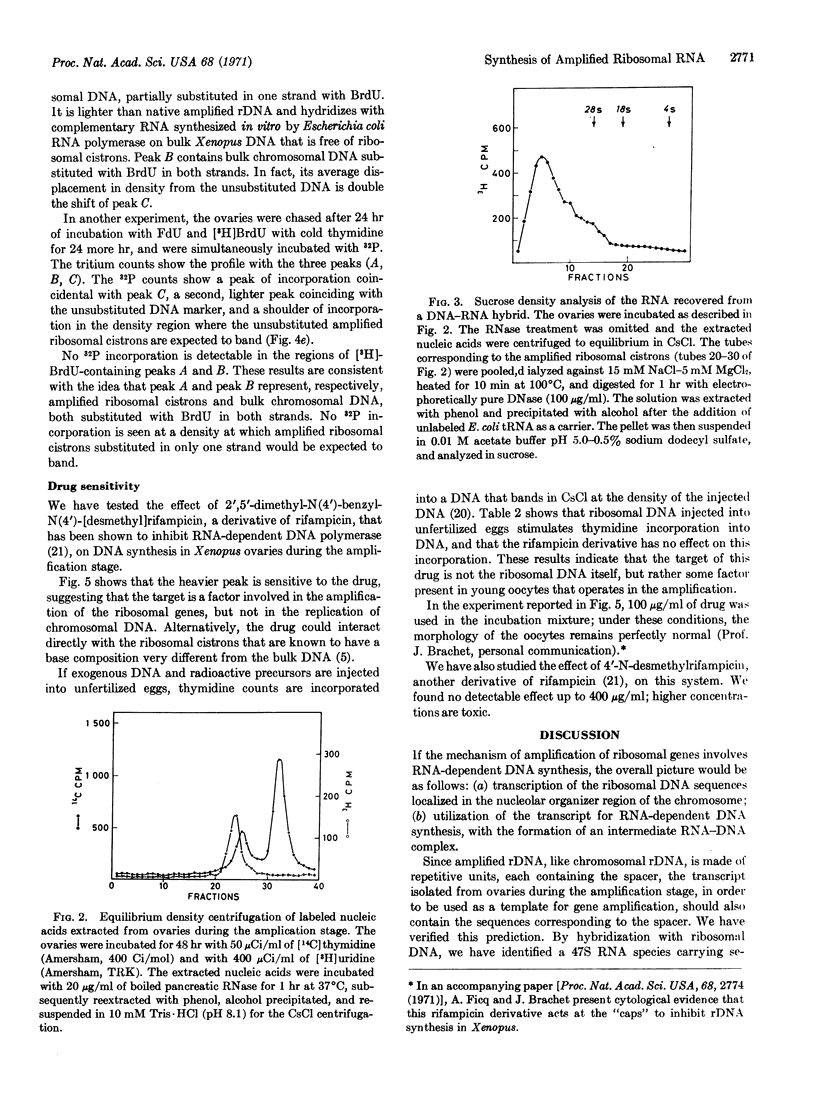
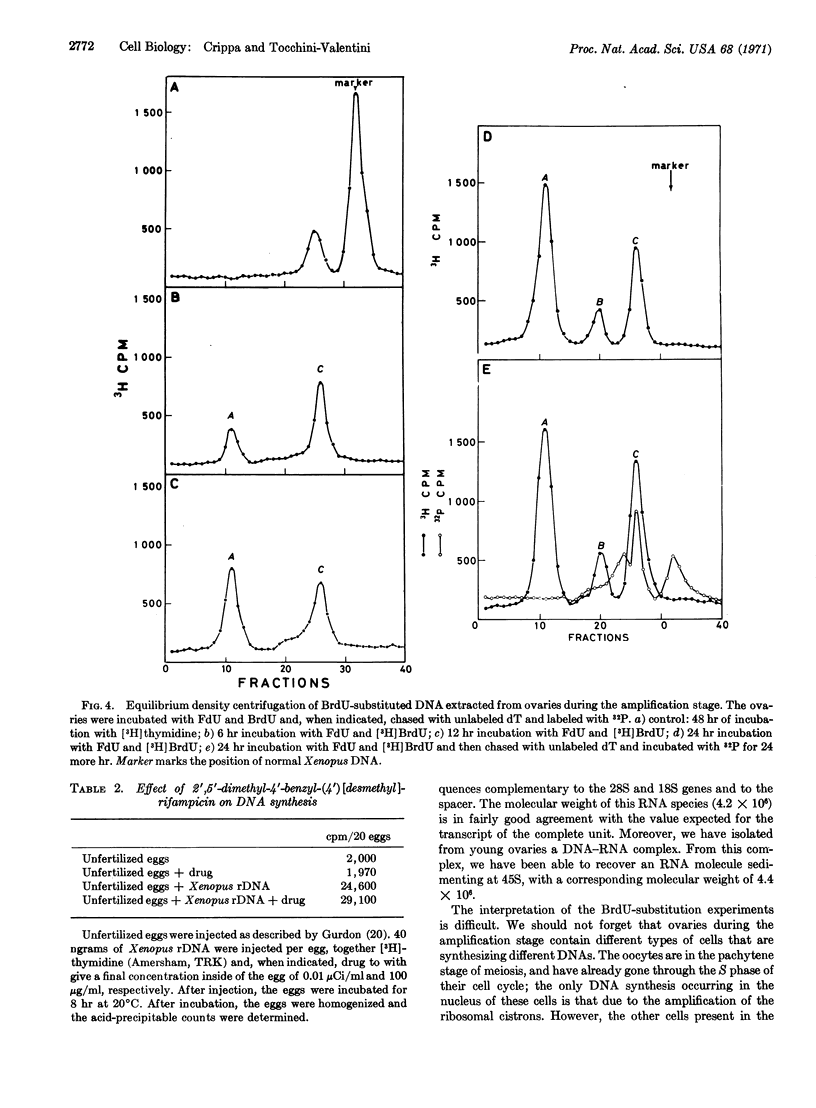
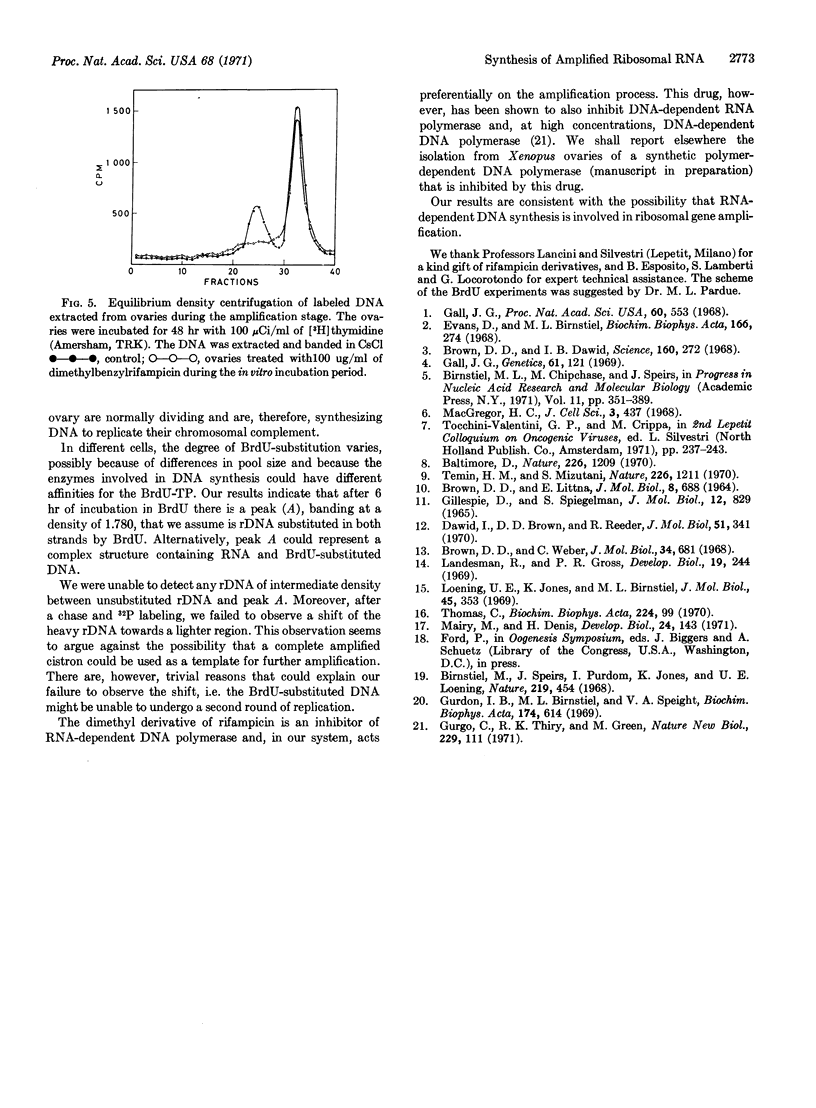
During the amplification stage in ovaries, the complete repetitive unit of the DNA that codes for ribosomal RNA in Xenopus appears to be transcribed. This large RNA transcript is found in a complex with DNA. Substitution experiments with 5-bromodeoxyuridine do not show any evidence that a complete amplified cistron is used as a template for further amplification. A derivative of rifampicin, 2′,5′-dimethyl-N(4′)benzyl-N(4′)[desmethyl] rifampicin, preferentially inhibits the DNA synthesis responsible for ribosomal gene amplification. These results are consistent with the hypothesis that RNA-dependent DNA synthesis is involved in gene amplification.
Keywords: density gradient centrifugation; 2′,5′-dimethyl-N(4′)benzyl-N(4′)[desmethyl] rifampicin; extrachromosomal DNA; Xenopus laevis; ovaries
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BROWN D. D., LITTNA E. VARIATIONS IN THE SYNTHESIS OF STABLE RNA'S DURING OOGENESIS AND DEVELOPMENT OF XENOPUS LAEVIS. J Mol Biol. 1964 May;8:688–695. doi: 10.1016/s0022-2836(64)80117-0. [DOI] [PubMed] [Google Scholar]
- Baltimore D. RNA-dependent DNA polymerase in virions of RNA tumour viruses. Nature. 1970 Jun 27;226(5252):1209–1211. doi: 10.1038/2261209a0. [DOI] [PubMed] [Google Scholar]
- Birnstiel M. L., Chipchase M., Speirs J. The ribosomal RNA cistrons. Prog Nucleic Acid Res Mol Biol. 1971;11:351–389. doi: 10.1016/s0079-6603(08)60332-3. [DOI] [PubMed] [Google Scholar]
- Birnstiel M., Speirs J., Purdom I., Jones K., Loening U. E. Properties and composition of the isolated ribosomal DNA satellite of Xenopus laevis. Nature. 1968 Aug 3;219(5153):454–463. doi: 10.1038/219454a0. [DOI] [PubMed] [Google Scholar]
- Brown D. D., Dawid I. B. Specific gene amplification in oocytes. Oocyte nuclei contain extrachromosomal replicas of the genes for ribosomal RNA. Science. 1968 Apr 19;160(3825):272–280. doi: 10.1126/science.160.3825.272. [DOI] [PubMed] [Google Scholar]
- Brown D. D., Weber C. S. Gene linkage by RNA-DNA hybridization. II. Arrangement of the redundant gene sequences for 28 s and 18 s ribosomal RNA. J Mol Biol. 1968 Jun 28;34(3):681–697. doi: 10.1016/0022-2836(68)90189-7. [DOI] [PubMed] [Google Scholar]
- Dawid I. B., Brown D. D., Reeder R. H. Composition and structure of chromosomal and amplified ribosomal DNA's of Xenopus laevis. J Mol Biol. 1970 Jul 28;51(2):341–360. doi: 10.1016/0022-2836(70)90147-6. [DOI] [PubMed] [Google Scholar]
- Evans D., Birnstiel M. L. Localization of amplified ribosomal DNA in the oocyte of Xenopus laevis. Biochim Biophys Acta. 1968 Aug 23;166(1):274–276. doi: 10.1016/0005-2787(68)90517-0. [DOI] [PubMed] [Google Scholar]
- Gall J. G. Differential synthesis of the genes for ribosomal RNA during amphibian oögenesis. Proc Natl Acad Sci U S A. 1968 Jun;60(2):553–560. doi: 10.1073/pnas.60.2.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gall J. G. The genes for ribosomal RNA during oögenesis. Genetics. 1969;61(1 Suppl):121–132. [PubMed] [Google Scholar]
- Gillespie D., Spiegelman S. A quantitative assay for DNA-RNA hybrids with DNA immobilized on a membrane. J Mol Biol. 1965 Jul;12(3):829–842. doi: 10.1016/s0022-2836(65)80331-x. [DOI] [PubMed] [Google Scholar]
- Gurdon J. B., Birnstiel M. L., Speight V. A. The replication of purified DNA introduced into living egg cytoplasm. Biochim Biophys Acta. 1969 Feb 18;174(2):614–628. doi: 10.1016/0005-2787(69)90291-3. [DOI] [PubMed] [Google Scholar]
- Gurgo C., Ray R. K., Thiry L., Green M. Inhibitors of the RNA and DNA dependent polymerase activities of RNA tumour viruses. Nat New Biol. 1971 Jan 27;229(4):111–114. doi: 10.1038/newbio229111a0. [DOI] [PubMed] [Google Scholar]
- Landesman R., Gross P. R. Patterns of macromolecule synthesis during development of Xenopus laevis. II. Identification of the 40 S precursor to ribosomal RNA. Dev Biol. 1969 Mar;19(3):244–260. doi: 10.1016/0012-1606(69)90063-3. [DOI] [PubMed] [Google Scholar]
- Loening U. E., Jones K. W., Birnstiel M. L. Properties of the ribosomal RNA precursor in Xenopus laevis; comparison to the precursor in mammals and in plants. J Mol Biol. 1969 Oct 28;45(2):353–366. doi: 10.1016/0022-2836(69)90110-7. [DOI] [PubMed] [Google Scholar]
- Mairy M., Denis H. Recherches biochimiques sur l'oogenèse. I. Synthèse et accumulation du RNA pendant l'oogenèse du crapaud sud-africain Xenopus laevis. Dev Biol. 1971 Feb;24(2):143–165. doi: 10.1016/0012-1606(71)90092-3. [DOI] [PubMed] [Google Scholar]
- Temin H. M., Mizutani S. RNA-dependent DNA polymerase in virions of Rous sarcoma virus. Nature. 1970 Jun 27;226(5252):1211–1213. doi: 10.1038/2261211a0. [DOI] [PubMed] [Google Scholar]
- Thomas C. Ribonucleic acids and ribonucleoproteins from small oöcytes of Xenopus laevis. Biochim Biophys Acta. 1970 Nov 12;224(1):99–113. doi: 10.1016/0005-2787(70)90624-6. [DOI] [PubMed] [Google Scholar]


