Abstract
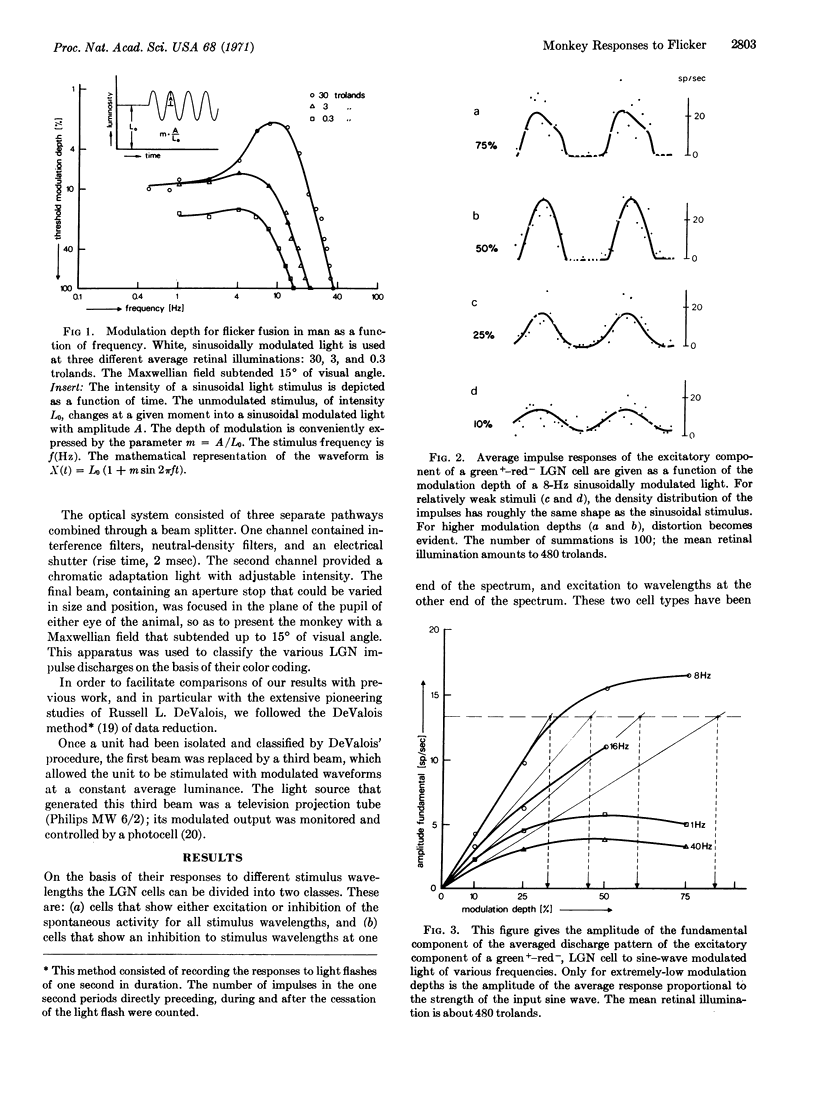
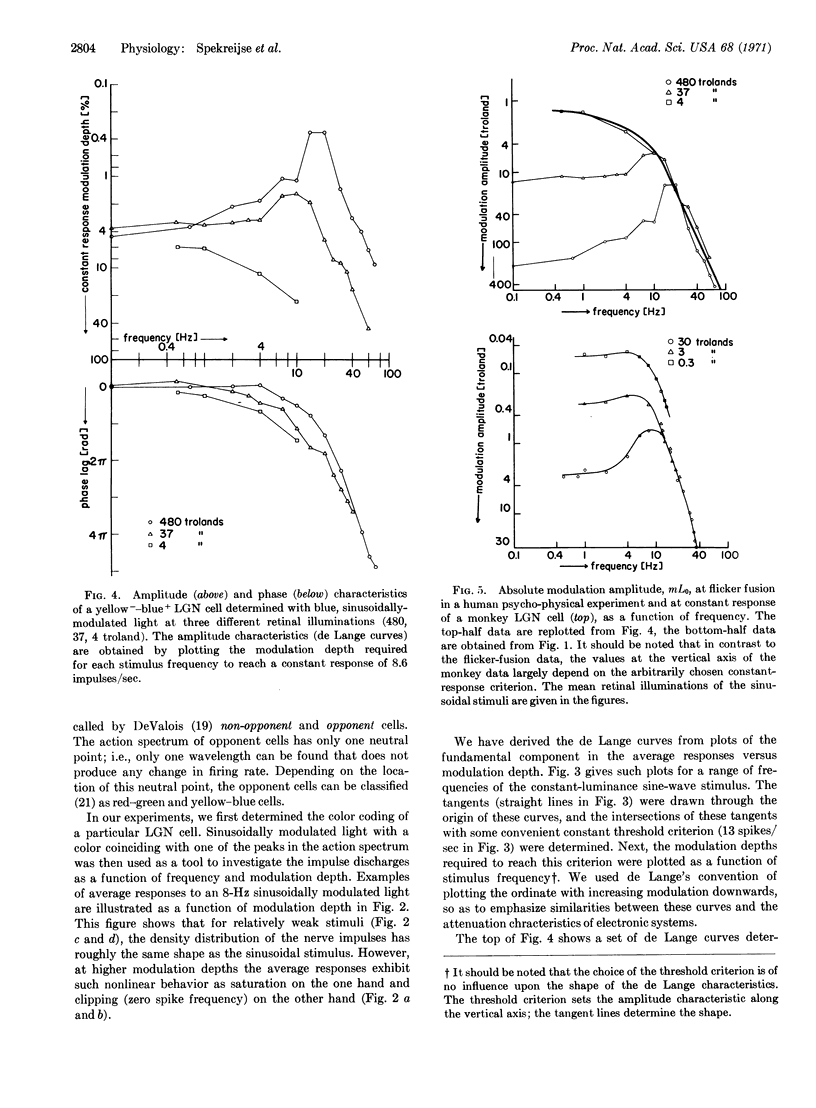
An analysis was made of the impulse discharge patterns—evoked by sinusoidal luminance modulation—of single cells in the lateral geniculate nucleus of the macaque monkey. The goal was to determine whether a correspondence could be observed between flicker detection by human subjects in psychophysical experiments and electrophysiological measurements of discharge patterns of single cells of the lateral geniculate nucleus. We found that the average discharge patterns of single cells exhibited the following behavior when mean retinal illumination was changed: In the low-frequency region (less than about 10 Hz) the response strength (impulses/sec) is independent of the mean luminance, in accordance with Weber's law; in the high-frequency region (above about 10 Hz) the response depends on the absolute modulation amplitude, in accordance with the Ferry-Porter law. Therefore the main features of human critical flicker frequency data are already present in the cellular (lateral geniculate nucleus) response of the macaque monkey. However, the steepness of high frequency fall-off in the response characteristics of these cells is much less than the corresponding fall-off in the human critical-flicker-frequency curves.
Keywords: de Lange characteristics, microelectrodes, macaques, single cells, critical flicker frequency
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CAMPBELL F. W., ROBSON J. G. THE ATTENUATION CHARACTERISTICS OF THE VISUAL SYSTEM DETERMINED BY MEASUREMENTS OF FLICKER THRESHOLD, BRIGHTNESS AND PUPILLOMOTOR EFFECT OF MODULATED LIGHT. Doc Ophthalmol. 1964;18:83–84. doi: 10.1007/BF00160565. [DOI] [PubMed] [Google Scholar]
- DE DZN H. L. Relationship between critical flicker-frequency and a set of low-frequency characteristics of the eye. J Opt Soc Am. 1954 May;44(5):380–389. doi: 10.1364/josa.44.000380. [DOI] [PubMed] [Google Scholar]
- DE LANGE DZN H. Research into the dynamic nature of the human fovea-cortex systems with intermittent and modulated light. I. Attenuation characteristics with white and colored light. J Opt Soc Am. 1958 Nov;48(11):777–784. doi: 10.1364/josa.48.000777. [DOI] [PubMed] [Google Scholar]
- DENIER VAN DER GON J. J., STRACKEE J., VAN DER TWEEL L. H. A source for modulated light. Phys Med Biol. 1958 Oct;3(2):164–173. doi: 10.1088/0031-9155/3/2/306. [DOI] [PubMed] [Google Scholar]
- DEVALOIS R. L., JACOBS G. H., ABRAMOV I. RESPONSES OF SINGLE CELLS IN VISUAL SYSTEM TO SHIFTS IN THE WAVELENGTH OF LIGHT. Science. 1964 Nov 27;146(3648):1184–1186. doi: 10.1126/science.146.3648.1184. [DOI] [PubMed] [Google Scholar]
- DEVOE R. D. LINEAR ELECTRICAL FLICKER RESPONSES FROM THE EYE OF THE WOLF SPIDER. Doc Ophthalmol. 1964;18:128–136. doi: 10.1007/BF00160569. [DOI] [PubMed] [Google Scholar]
- De Valois R. L., Abramov I., Jacobs G. H. Analysis of response patterns of LGN cells. J Opt Soc Am. 1966 Jul;56(7):966–977. doi: 10.1364/josa.56.000966. [DOI] [PubMed] [Google Scholar]
- De Valois R. L., Jacobs G. H. Primate color vision. Science. 1968 Nov 1;162(3853):533–540. doi: 10.1126/science.162.3853.533. [DOI] [PubMed] [Google Scholar]
- FUORTES M. G., HODGKIN A. L. CHANGES IN TIME SCALE AND SENSITIVITY IN THE OMMATIDIA OF LIMULUS. J Physiol. 1964 Aug;172:239–263. doi: 10.1113/jphysiol.1964.sp007415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KELLY D. H. Effects of sharp edges in a flickering field. J Opt Soc Am. 1959 Jul;49(7):730–732. doi: 10.1364/josa.49.000730. [DOI] [PubMed] [Google Scholar]
- KELLY D. H. SINE WAVES AND FLICKER FUSION. Doc Ophthalmol. 1964;18:16–35. doi: 10.1007/BF00160561. [DOI] [PubMed] [Google Scholar]
- LEVINSON J. Fusion of complex flicker. Science. 1959 Oct 9;130(3380):919–921. doi: 10.1126/science.130.3380.919. [DOI] [PubMed] [Google Scholar]
- LEVINSON J., HARMON L. D. Studies with artificial neurons. III. Mechanisms of flicker-fusion. Kybernetik. 1961 Dec;1:107–117. doi: 10.1007/BF00290181. [DOI] [PubMed] [Google Scholar]
- Marimont R. B. Numerical studies of the Fuortes-Hodgkin Limulus model. J Physiol. 1965 Aug;179(3):489–497. doi: 10.1113/jphysiol.1965.sp007675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinter R. B. Sinusoidal and delta function responses of visual cells of the Limulus eye. J Gen Physiol. 1966 Jan;49(3):565–593. doi: 10.1085/jgp.49.3.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SPERLING G. LINEAR THEORY AND THE PSYCHOPHYSICS OF FLICKER. Doc Ophthalmol. 1964;18:3–15. doi: 10.1007/BF00160560. [DOI] [PubMed] [Google Scholar]
- Sidley N. A., Sperling H. G. Photopic spectral sensitivity in the rhesus monkey. J Opt Soc Am. 1967 Jun;57(6):816–818. doi: 10.1364/josa.57.000816. [DOI] [PubMed] [Google Scholar]


