Abstract
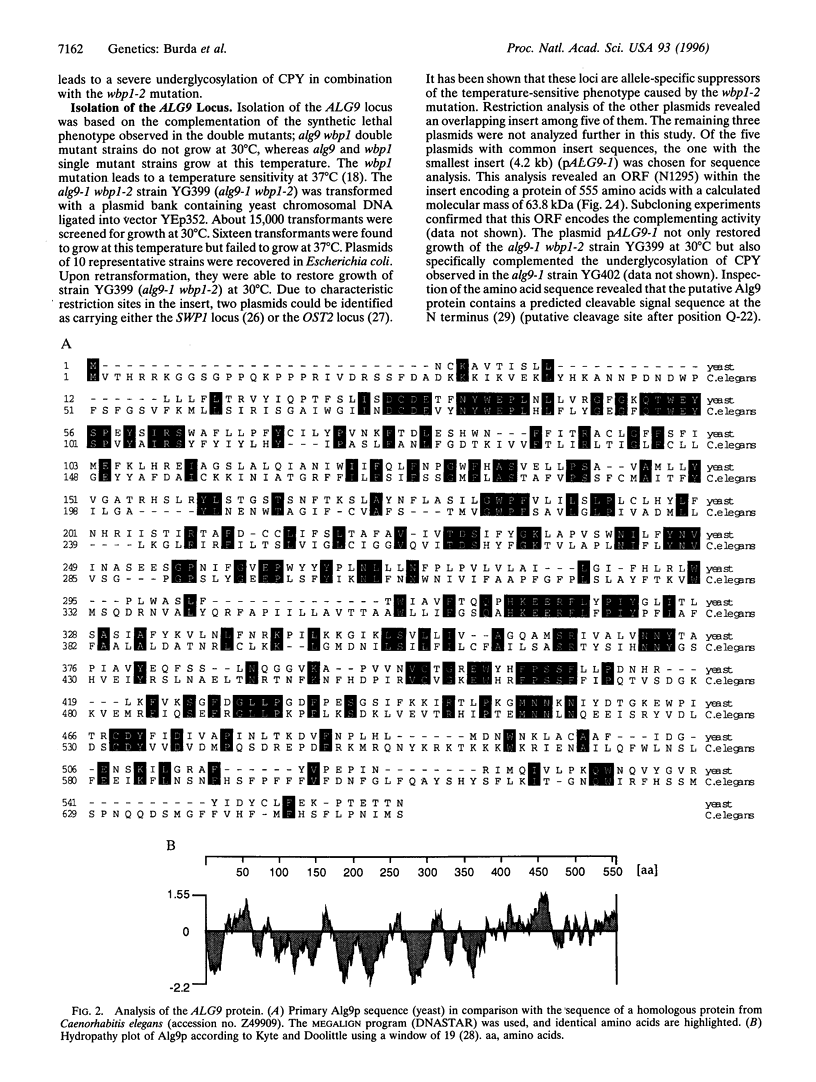
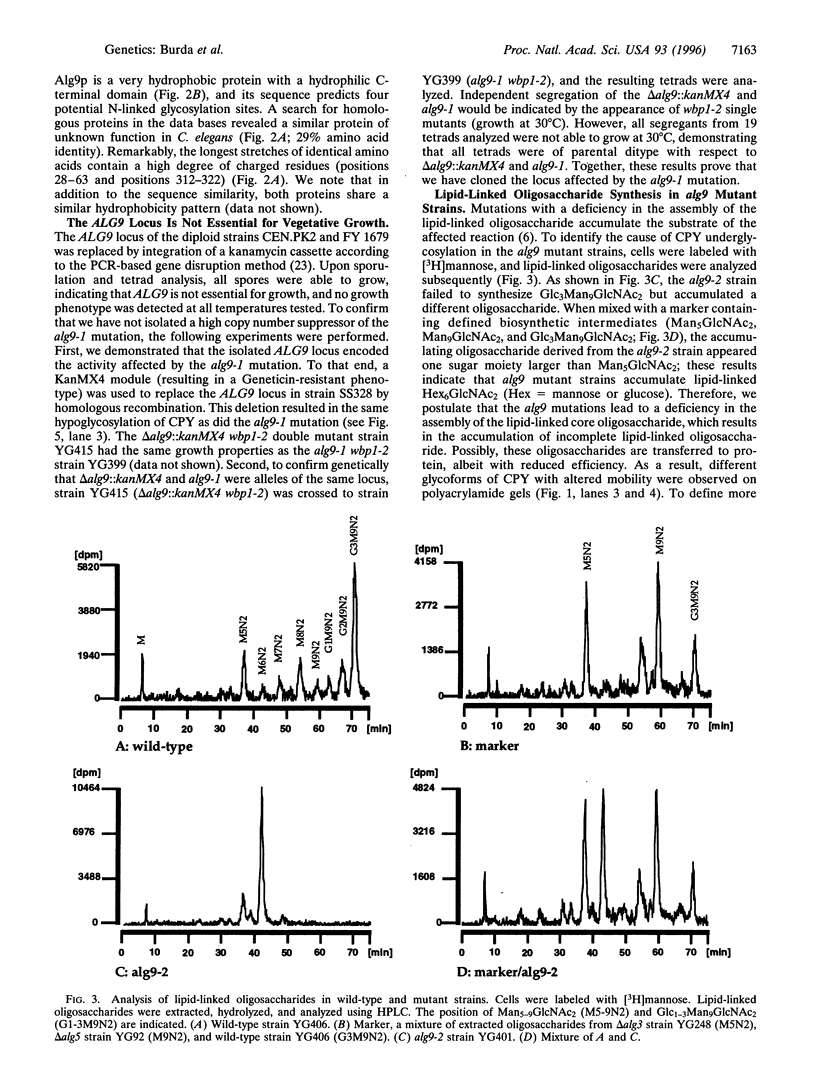
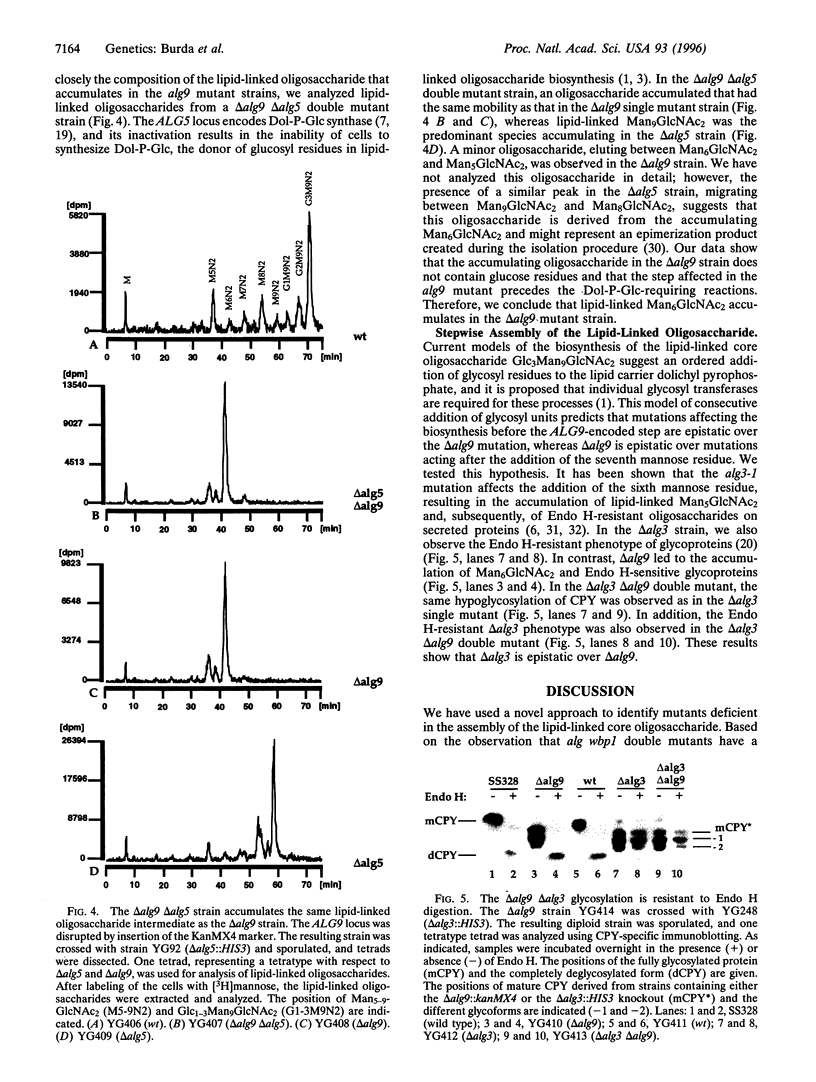
The core oligosaccharide Glc3Man9GlcNAc2 is assembled at the membrane of the endoplasmic reticulum on the lipid carrier dolichyl pyrophosphate and transferred to selected asparagine residues of nascent polypeptide chains. This transfer is catalyzed by the oligosaccharyl transferase complex. Based on the synthetic phenotype of the oligosaccharyl transferase mutation wbp1 in combination with a deficiency in the assembly pathway of the oligosaccharide in Saccharomyces cerevisiae, we have identified the novel ALG9 gene. We conclude that this locus encodes a putative mannosyl transferase because deletion of the gene led to accumulation of lipid-linked Man6GlcNAc2 in vivo and to hypoglycosylation of secreted proteins. Using an approach combining genetic and biochemical techniques, we show that the assembly of the lipid-linked core oligosaccharide in the lumen of the endoplasmic reticulum occurs in a stepwise fashion.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abeijon C., Hirschberg C. B. Topography of glycosylation reactions in the endoplasmic reticulum. Trends Biochem Sci. 1992 Jan;17(1):32–36. doi: 10.1016/0968-0004(92)90424-8. [DOI] [PubMed] [Google Scholar]
- Albright C. F., Robbins R. W. The sequence and transcript heterogeneity of the yeast gene ALG1, an essential mannosyltransferase involved in N-glycosylation. J Biol Chem. 1990 Apr 25;265(12):7042–7049. [PubMed] [Google Scholar]
- Ballou L., Gopal P., Krummel B., Tammi M., Ballou C. E. A mutation that prevents glucosylation of the lipid-linked oligosaccharide precursor leads to underglycosylation of secreted yeast invertase. Proc Natl Acad Sci U S A. 1986 May;83(10):3081–3085. doi: 10.1073/pnas.83.10.3081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein M., Kepes F., Schekman R. Sec59 encodes a membrane protein required for core glycosylation in Saccharomyces cerevisiae. Mol Cell Biol. 1989 Mar;9(3):1191–1199. doi: 10.1128/mcb.9.3.1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeze H. H., Koza-Taylor P., Saunders A., Cardelli J. A. The effects of altered N-linked oligosaccharide structures on maturation and targeting of lysosomal enzymes in Dictyostelium discoideum. J Biol Chem. 1989 Nov 15;264(32):19278–19286. [PubMed] [Google Scholar]
- Hanover J. A., Lennarz W. J. Transmembrane assembly of N-linked glycoproteins. Studies on the topology of saccharide synthesis. J Biol Chem. 1982 Mar 25;257(6):2787–2794. [PubMed] [Google Scholar]
- Heesen S., Lehle L., Weissmann A., Aebi M. Isolation of the ALG5 locus encoding the UDP-glucose:dolichyl-phosphate glucosyltransferase from Saccharomyces cerevisiae. Eur J Biochem. 1994 Aug 15;224(1):71–79. doi: 10.1111/j.1432-1033.1994.tb19996.x. [DOI] [PubMed] [Google Scholar]
- Herscovics A., Orlean P. Glycoprotein biosynthesis in yeast. FASEB J. 1993 Apr 1;7(6):540–550. doi: 10.1096/fasebj.7.6.8472892. [DOI] [PubMed] [Google Scholar]
- Huffaker T. C., Robbins P. W. Yeast mutants deficient in protein glycosylation. Proc Natl Acad Sci U S A. 1983 Dec;80(24):7466–7470. doi: 10.1073/pnas.80.24.7466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson B. J., Kukuruzinska M. A., Robbins P. Biosynthesis of asparagine-linked oligosaccharides in Saccharomyces cerevisiae: the alg2 mutation. Glycobiology. 1993 Aug;3(4):357–364. doi: 10.1093/glycob/3.4.357. [DOI] [PubMed] [Google Scholar]
- Kaplan H. A., Welply J. K., Lennarz W. J. Oligosaccharyl transferase: the central enzyme in the pathway of glycoprotein assembly. Biochim Biophys Acta. 1987 Jun 24;906(2):161–173. doi: 10.1016/0304-4157(87)90010-4. [DOI] [PubMed] [Google Scholar]
- Kepes F., Schekman R. The yeast SEC53 gene encodes phosphomannomutase. J Biol Chem. 1988 Jul 5;263(19):9155–9161. [PubMed] [Google Scholar]
- Kornfeld R., Kornfeld S. Assembly of asparagine-linked oligosaccharides. Annu Rev Biochem. 1985;54:631–664. doi: 10.1146/annurev.bi.54.070185.003215. [DOI] [PubMed] [Google Scholar]
- Kranz J. E., Holm C. Cloning by function: an alternative approach for identifying yeast homologs of genes from other organisms. Proc Natl Acad Sci U S A. 1990 Sep;87(17):6629–6633. doi: 10.1073/pnas.87.17.6629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kukuruzinska M. A., Bergh M. L., Jackson B. J. Protein glycosylation in yeast. Annu Rev Biochem. 1987;56:915–944. doi: 10.1146/annurev.bi.56.070187.004411. [DOI] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Mellis S. J., Baenziger J. U. Separation of neutral oligosaccharides by high-performance liquid chromatography. Anal Biochem. 1981 Jul 1;114(2):276–280. doi: 10.1016/0003-2697(81)90480-2. [DOI] [PubMed] [Google Scholar]
- Orlean P., Albright C., Robbins P. W. Cloning and sequencing of the yeast gene for dolichol phosphate mannose synthase, an essential protein. J Biol Chem. 1988 Nov 25;263(33):17499–17507. [PubMed] [Google Scholar]
- Rearick J. I., Fujimoto K., Kornfeld S. Identification of the mannosyl donors involved in the synthesis of lipid-linked oligosaccharides. J Biol Chem. 1981 Apr 25;256(8):3762–3769. [PubMed] [Google Scholar]
- Rost B., Casadio R., Fariselli P., Sander C. Transmembrane helices predicted at 95% accuracy. Protein Sci. 1995 Mar;4(3):521–533. doi: 10.1002/pro.5560040318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Runge K. W., Huffaker T. C., Robbins P. W. Two yeast mutations in glucosylation steps of the asparagine glycosylation pathway. J Biol Chem. 1984 Jan 10;259(1):412–417. [PubMed] [Google Scholar]
- Runge K. W., Robbins P. W. A new yeast mutation in the glucosylation steps of the asparagine-linked glycosylation pathway. Formation of a novel asparagine-linked oligosaccharide containing two glucose residues. J Biol Chem. 1986 Nov 25;261(33):15582–15590. [PubMed] [Google Scholar]
- Sharma C. B., Kaushal G. P., Pan Y. T., Elbein A. D. Purification and characterization of dolichyl-P-mannose:Man5(GlcNAc)2-PP-dolichol mannosyltransferase. Biochemistry. 1990 Sep 25;29(38):8901–8907. doi: 10.1021/bi00490a004. [DOI] [PubMed] [Google Scholar]
- Silberstein S., Collins P. G., Kelleher D. J., Gilmore R. The essential OST2 gene encodes the 16-kD subunit of the yeast oligosaccharyltransferase, a highly conserved protein expressed in diverse eukaryotic organisms. J Cell Biol. 1995 Oct;131(2):371–383. doi: 10.1083/jcb.131.2.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snider M. D., Sultzman L. A., Robbins P. W. Transmembrane location of oligosaccharide-lipid synthesis in microsomal vesicles. Cell. 1980 Sep;21(2):385–392. doi: 10.1016/0092-8674(80)90475-4. [DOI] [PubMed] [Google Scholar]
- Stagljar I., te Heesen S., Aebi M. New phenotype of mutations deficient in glucosylation of the lipid-linked oligosaccharide: cloning of the ALG8 locus. Proc Natl Acad Sci U S A. 1994 Jun 21;91(13):5977–5981. doi: 10.1073/pnas.91.13.5977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanner W., Lehle L. Protein glycosylation in yeast. Biochim Biophys Acta. 1987 Apr 27;906(1):81–99. doi: 10.1016/0304-4157(87)90006-2. [DOI] [PubMed] [Google Scholar]
- Verostek M. F., Atkinson P. H., Trimble R. B. Glycoprotein biosynthesis in the alg3 Saccharomyces cerevisiae mutant. I. Role of glucose in the initial glycosylation of invertase in the endoplasmic reticulum. J Biol Chem. 1993 Jun 5;268(16):12095–12103. [PubMed] [Google Scholar]
- Verostek M. F., Atkinson P. H., Trimble R. B. Glycoprotein biosynthesis in the alg3 Saccharomyces cerevisiae mutant. II. Structure of novel Man6-10GlcNAc2 processing intermediates on secreted invertase. J Biol Chem. 1993 Jun 5;268(16):12104–12115. [PubMed] [Google Scholar]
- Verostek M. F., Atkinson P. H., Trimble R. B. Structure of Saccharomyces cerevisiae alg3, sec18 mutant oligosaccharides. J Biol Chem. 1991 Mar 25;266(9):5547–5551. [PubMed] [Google Scholar]
- Wach A., Brachat A., Pöhlmann R., Philippsen P. New heterologous modules for classical or PCR-based gene disruptions in Saccharomyces cerevisiae. Yeast. 1994 Dec;10(13):1793–1808. doi: 10.1002/yea.320101310. [DOI] [PubMed] [Google Scholar]
- Winston F., Dollard C., Ricupero-Hovasse S. L. Construction of a set of convenient Saccharomyces cerevisiae strains that are isogenic to S288C. Yeast. 1995 Jan;11(1):53–55. doi: 10.1002/yea.320110107. [DOI] [PubMed] [Google Scholar]
- Zufferey R., Knauer R., Burda P., Stagljar I., te Heesen S., Lehle L., Aebi M. STT3, a highly conserved protein required for yeast oligosaccharyl transferase activity in vivo. EMBO J. 1995 Oct 16;14(20):4949–4960. doi: 10.1002/j.1460-2075.1995.tb00178.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- te Heesen S., Janetzky B., Lehle L., Aebi M. The yeast WBP1 is essential for oligosaccharyl transferase activity in vivo and in vitro. EMBO J. 1992 Jun;11(6):2071–2075. doi: 10.1002/j.1460-2075.1992.tb05265.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- te Heesen S., Knauer R., Lehle L., Aebi M. Yeast Wbp1p and Swp1p form a protein complex essential for oligosaccharyl transferase activity. EMBO J. 1993 Jan;12(1):279–284. doi: 10.1002/j.1460-2075.1993.tb05654.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Heijne G. A new method for predicting signal sequence cleavage sites. Nucleic Acids Res. 1986 Jun 11;14(11):4683–4690. doi: 10.1093/nar/14.11.4683. [DOI] [PMC free article] [PubMed] [Google Scholar]






