Abstract
Objectives/Hypothesis
To establish the relevance of the bone morphogenetic protein (BMP) signaling pathway in human oral squamous cell carcinoma (OSCCA) cell lines and determine if there is a biologic impact of stimulating this pathway with recombinant human (rh) BMP-2.
Study Design
In vitro laboratory investigations and in vivo analysis using an orthotopic animal model for oral cancer.
Methods
Gene expression profiles for BMP-2 and components of the BMP-signaling pathway were determined using reverse transcriptase-polymerase chain reaction. In vivo effects were evaluated using Kaplan-Meier survival analysis and studying histopathologic changes in established tumor xenografts with or without rhBMP-2 pretreatment. A phosphokinase array was used to detect levels of activation in signaling kinases.
Results
The BMP-2 gene was expressed in 90% of the 30 OSCCA cell lines tested. Gene expression of all components of the BMP-signaling pathway was highly conserved. Tumor xenografts established with rhBMP-2-treated cells showed more rapid local growth that resulted in worse animal survival as compared to the control group. These tumors had a more poorly differentiated morphology. Changes in protein kinases suggested interactions of BMP-2 signaling with the Wnt-β-catenin, and Janus kinase/signal transducers and activators of transcription (JAK/STAT) pathways.
Conclusions
Human OSCCA cell lines frequently express BMP-2 and all necessary components of the BMP-signaling pathway. Exogenous treatment of human OSCCA cell lines with rhBMP-2 prior to engraftment in an orthotopic animal model caused the subsequent tumors to be more locally aggressive with worse survival. Continued caution should be used for considering rhBMP-2 for reconstruction of bone defects in oral cancer patients.
Keywords: Bone morphogenetic protein, oral cancer, squamous cell carcinoma
INTRODUCTION
Current methods for craniofacial bone reconstruction involve vascularized or non-vascularized autologous bone grafts, which have inherent limitations and create donor site morbidity. There are great prospects for current reconstructive techniques to be replaced by approaches using tissue engineering. Preclinical animal models and clinical experience from orthopedics show that bone morphogenetic proteins (BMPs) are critically important osteoinductive molecules for these regenerative approaches.1 Two recombinant human BMPs (rhBMP-2 and rhBMP-7) are now commercially available and have already led to improvements in the treatment of patients undergoing surgery for spinal fusion and long bone nonunions.2,3 Human recombinant BMP-2 is also U.S. Food and Drug Administration-approved for some orodental applications, but the use for patients with craniomaxillofacial or mandibular defects after oral cancer resection remains contraindicated due to the paucity of data regarding the biologic effects of BMPs on human oral squamous cell carcinoma (OSCCA).4,5
The potential role of BMPs in malignant transformation and progression of cancer is poorly understood.6 Evidence of cancer development induced by rhBMP-2 at the local application site has not been apparent in extensive preclinical experience, and the incidence of subsequent cancer development in orthopedic patients treated with these proteins does not appear to be greater than that of the general population.7,8 With regard to oral carcinoma, a recent report showed that rhBMP-2 did not have any adverse effects on proliferation or angiogenesis in human OSCCA cell lines when tested in vitro or in vivo using an ectopic site animal model.9 However, Jin et al. studied 29 oral carcinomas by immunohistochemistry and found that BMP-2/4, BMP-5, and BMP type IA receptor (BMPR-IA) were expressed in 73%, 73%, and 83% of tumors, respectively. They concluded that the BMP pathway is involved in carcinogenesis of oral epithelium.10 These authors, however, did not comprehensively study the BMP signaling pathway components or investigate whether BMPs produced adverse biologic effects on any of the key processes of tumorigenesis as outlined by Hanahan and Weinberg.11 Subsequently, Zhou et al. performed gene microarray analysis with validation using quantitative polymerase chain reaction (PCR) on 25 oral tongue squamous cell carcinomas.12 These investigators found that increased BMP-2 gene expression was associated with regional lymph node metastasis and extracapsular spread within the metastatic lymph node, an established marker of particularly poor prognosis.
There are no unifying conclusions that can be made from the currently available data as to whether rhBMP-2 promotes, inhibits, or has no role in tumorigenesis of oral carcinoma. From a practical standpoint, our knowledge in this area needs to be increased before considering the translation of basic science advances in bone tissue engineering using BMPs for reconstruction of craniomaxillofacial and mandible defects in patients treated for OSCCA.
The objectives of this study were 1) to determine the gene expression profile for BMP-2 and the components of the BMP-signaling pathway in a large number of human OSCCA cell lines, 2) to detect whether stimulation of the BMP-2 signaling pathway has adverse biologic effects in vivo on xenografts established from human OSCCA cell lines in an orthotopic animal model, and 3) to establish if rhBMP-2 treatment changes expression profiles of protein kinases involved with tumorigenesis in an oral cancer cell line.
MATERIALS AND METHODS
Cell Culture and Media
Twenty-seven human oral squamous carcinoma cell lines were provided by Dr. Susanne M. Gollin (University of Pittsburgh, Pittsburgh, PA; UPCI:SCC). Cells were maintained in minimum essential medium (MEM) (Invitrogen, Carlsbad, CA) supplemented with Earle's salt, L-glutamine, 1X NEAA, 10% fetal bovine serum (FBS), and gentamicin. Three human oral squamous carcinoma cell lines were provided by Dr. Thomas E. Carey (University of Michigan, Ann Arbor, MI; UMSCC). These cells and MG-63, an osteosarcoma-derived osteoblastic cell line, were grown in Dulbecco's modified Eagle's medium (Mediatech Inc., Manasses, VA) supplemented with 4.5 g/L glucose, L-glutamine, 10% FBS, 1X NEAA, and penicillin-streptomycin. Cultures were maintained at 37° C in an atmosphere of 5% CO2. All 30 cell lines were derived from previously untreated primary disease sites, including the tongue, alveolar ridge, retromolar trigone, buccal, and floor-of-mouth subsites.
Gene Expression Profiling by Reverse Transcriptase-Polymerase Chain Reaction
Total RNA was isolated by TRIzol Reagent (Invitrogen) according to the manufacturer's protocol. Reverse transcription to produce first strand cDNA was performed with 2 μg of total RNA by High-Capacity cDNA Reverse Transcription kit with RNase inhibitor (Applied Biosystems, Foster City, CA) in accordance with the manufacturer's protocols. The BMP-2, BMP receptors, and Smads transcripts were amplified from the cDNA by conventional PCR using gene-specific primers13–18 (Table I). For BMP-2 gene expression, normal human oral mucosa was used as negative control, and MG-63 was used as positive control.10,19 Each sample was tested in duplicate in three independent experimental runs.
TABLE I.
Gene-Specific Primer Sequences Used for Reverse Transcriptase-Polymerase Chain Reaction.
| Name | Primer sequence | Product size (bp) | Reference |
|---|---|---|---|
| BMP-2 | For: 5′-CGAGGTCCTGAGCGAGTTCGAG-3′ | 837 | 13 |
| Rev: 5′-TGGCAGTAAAAGGCGTGATACC-3′ | |||
| BMPR-IA | For: 5′-GGGTGGGCACCAAACGCTAC-3′ | 474 | 14 |
| Rev: 5′-CCACTCTAATTCCACCCATGCC-3′ | |||
| BMPR-IB | For: 5′-ACTTGCTGTATTGCTGACCTGG-3′ | 512 | 14 |
| Rev: 5′-GGCTTTCTGCAGAGATGCTTAC-3′ | |||
| BMPR-II | For: 5′-AACATTTACAGAGTGCCTTTGATG-3′ | 468 | 14 |
| Rev: 5′-AGCTGATTCACAGTCCCTCAAG-3′ | |||
| ActR-I | For: 5′-TTGCATAGCAGATTTGGGCCTG-3′ | 556 | 14 |
| Rev: 5′-CAGTCAGGCCAGCATTAGGTCC-3′ | |||
| ActR-IIA | For: 5′-CGGGAAAATGGGAGCTGCTGC-3′ | 467 | 14 |
| Rev: 5′-CAATCCCCGCAATTAACATAAGTG-3′ | |||
| ActR-IIB | For: 5′-GACACGGGAGTGCATCTACTAC-3′ | 442 | 14 |
| Rev: 5′-GATGTCCACATGACCGTAGGG-3′ | |||
| Smad 1 | For: 5′-GTATGCCGAATGCCTTAGTG-3′ | 486 | 15 |
| Rev: 5′-CACAGAGGTCAAGTATTATC-3′ | |||
| Smad 5 | For: 5′-CGGTAGCCACTGACTTTGAGTTAC-3′ | 701 | 16 |
| Rev: 5′-AGCTGAAATGGACTTCCTGGTC-3′ | |||
| Smad 8 | For: 5′-CCATCAGCTCCCTCTTCTCC-3′ | 429 | 15 |
| Rev: 5′-CGCTGTGTCTTGGTACCAGC-3′ | |||
| Smad 4 | For: 5′-GTGGAATAGCTCCAGCTATC-3′ | 206 | 17 |
| Rev: 5′-CGGCATGGTATGAAGTACTCC-3′ | |||
| GAPDH | For: 5′-ACCACAGTCCATGCCATCAC-3′ | 451 | 18 |
| Rev: 5′-TCCACCACCCTGTTGCTGTA-3′ |
Establishment of Xenografts
All procedures involving animals were performed in accordance with protocols approved by the Animal Studies Committee at the Washington University School of Medicine. Six-week-old athymic NCr-nu/nu males were purchased from the National Cancer Institute (Frederick, MD) and housed under pathogen-free conditions. Two oral squamous carcinoma cell lines were used to establish xenografts. The cell line UMSCC-14A was originally derived from an OSCCA from the floor of the mouth, and the cell line UMSCC-74A was derived from an OSCCA from the tongue. Preliminary tumor growth experiments established the appropriate cell inoculums of 2 × 106 cells for UMSCC-14A and 1.5 × 106 cells for UMSCC-74A cells (data not shown). There were two treatment groups for each cell line. Group 1 consisted of cells that were initially kept in OPTIMEM reduced-serum medium (Invitrogen) for 48 hours prior to injection to establish control groups. Group 2 consisted of cells that were pretreated with rhBMP-2 (Sigma-Aldrich, St. Louis, MO) at 100 ng/mL for 48 hours in OPTIMEM reduced-serum medium prior the injection. For orthotopic injections, cells were suspended in 50 μl of phosphate buffered saline (Mediatech Inc.). After the mice were anesthetized, cells were injected into the tongue tip using an orthotopic nude mouse model for oral cancer that has been previously described.20 Xenograft formation and animal survival were monitored and recorded weekly and at the end of the experiment. The following criteria were used to terminate the experiment: animal weight loss >25% of preinjected weight, inability to access food/water, tumor ulceration/bleeding, respiratory symptoms, or moribund appearance. The tumors were surgically removed, separated from surrounding tissue under the microscope, and divided in half with one portion placed in 2% paraformaldehyde and the other fresh frozen in liquid nitrogen. Regional lymph nodes, brain, lung, and liver tissue were harvested from each animal and preserved in 2% paraformaldehyde.
Histopathologic Analysis
Formalin-fixed primary tumors were paraffin embedded and serially sectioned at 5 μm thickness. Staining was performed with standard technique using hematoxylin and eosin. All cases were reviewed by the study pathologist (J.S.L.), who was blinded to the treatment groups or other details regarding the tumors. Parameters evaluated with light microscopy included tumors graded as well, moderately, or poorly differentiated based on the degree of maturation, nuclear to cytoplasmic ratios, presence of intercellular bridges, and amount of extracellular keratin formation. Tumor grade also included evaluation for spindle cell (or sarcomatoid) features, wherein tumor cells develop fusiform, cigar-shaped nuclei and grew in fascicles rather than nests. Other parameters evaluated included host inflammatory response (as none, minimal, mild, or brisk), pattern of invasion (as pushing or infiltrative borders, and infiltrative consisting of small nests or individual cells growing away from the tumor mass at the invasive front), perineural invasion, and lymphovascular space invasion. Other features, such as mucosal ulceration, tumor necrosis, tumor degeneration and vacuolar change, and peritumoral edema were noted as they were observed. All tissue removed as regional lymph nodes, which frequently included major salivary gland tissue, was submitted for histologic examination. Only representative lung, liver, and brain tissue was submitted for histologic examination after gross prosection.
Protein Kinase Array
UMSCC-74A cells were treated with or without rhBMP-2 (100 ng/mL) for 48 hours in OPTIMEM medium. Total protein lysates were used to analyze the phosphorylation profiles of kinases using a Human Phospho-Kinase array kit (R&D Systems, Minneapolis, MN). Bio-Rad Chemiluminescent Imaging System and Quantity One software (Bio-Rad Laboratories, Hercules, CA) was used to analyze pixel density in each spot of the array. The average signal of the pair of duplicate spots was normalized on average signal from negative control spots as a background value. The relative changes in the treated cells were determined by comparing the corresponding signals to the control array. Two independent experiments were performed.
Statistical Analyses
Relations between treatments (BMP-2 and control) and histopathological characteristics (histological grade, host inflammatory response, pattern of invasion, perineural invasion, and lymphovascular space invasion) within each cell line were analyzed by applying the Fisher exact test to the corresponding contingency tables. The nonparametric Kaplan-Meier method with right censoring was used for survival analysis. Differences between the survival curves were tested using the Wilcoxon test. All analyses were done using SAS 9.1.3 (SAS Institute Inc., Cary, NC). P values <.05 were considered significant.
RESULTS
Gene Expression of BMP-2 and Components of the BMP-Signaling Pathway in OSCCA Cell Lines
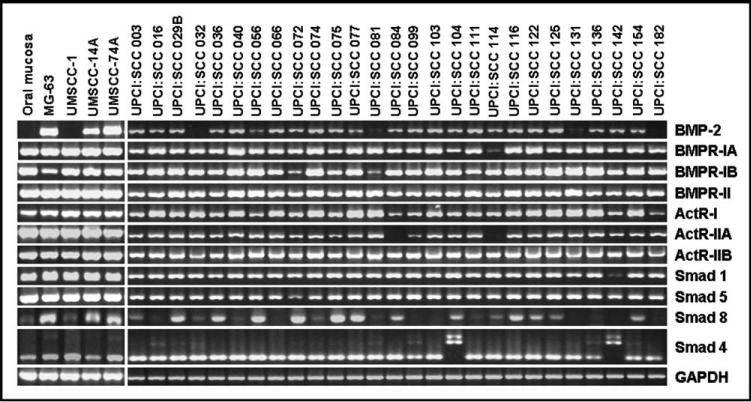
Semiquantitative reverse transcriptase-polymerase chain reaction analysis was performed to determine the in vitro baseline gene expression levels of BMP-2, BMPR-IA, BMPR-IB, BMPR-II, ActR-I, ActR-IIA, ActRIIB), BMPR-activating Smads (Smad 1, Smad 5, Smad 8, and the common partner Smad (Smad 4) in 30 OSCCA cell lines. The results shown in Figure 1 indicate that the gene expression levels of BMP-2, BMPRs, and the BMP-related Smads were highly conserved among the great majority of OSCCA cell lines. Notably, 27 cell lines (90%) expressed BMP-2 at baseline and usually at a similar level of expression. Normal mucosa did not show baseline gene expression of BMP-2.
Fig. 1.
Gene expression in cell lines for bone morphogenetic protein (BMP)-2, BMP receptors (BMPRs), and Smads. Reverse transcriptase-polymerase chain reaction of normal mucosa, MG-63, and 30 human oral squamous carcinoma cell lines derived from the tongue, alveolar ridge, retromolar trigone, buccal, and floor-of-mouth subsites.
In Vivo Biologic Effects of rhBMP-2 Treatment
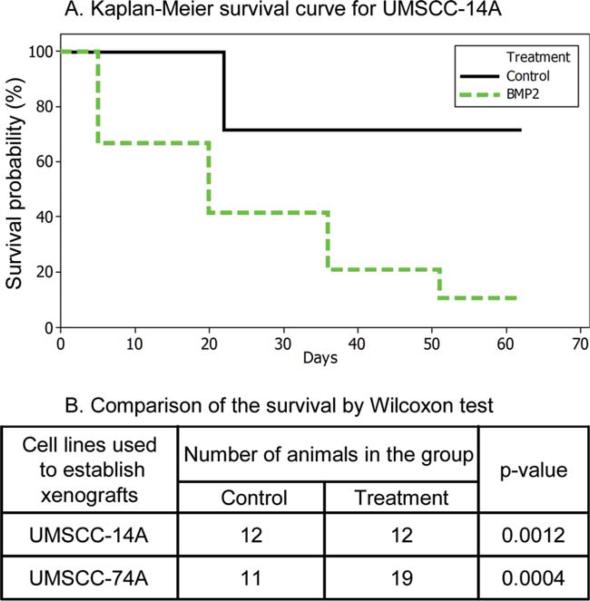
Two OSCCA cell lines (UMSCC-14A and UMSCC-74A) were selected for these experiments because their baseline gene expression was representative of the common pattern observed among the 30 tested cell lines. All recipient mice formed established tumors at the orthotopic site in both the treatment and control groups. Time of xenograft formation and animal survival were recorded. For the cells treated with rhBMP-2, the time of xenograft formation was rapid (<1 week as compared to 2 weeks for controls, data not shown). Animals in the rhBMP-2 treated groups had significantly worse survival as compared to the controls for both UMSCC-14A (P = .0012) and UMSCC-74A (P = .0004) (Fig. 2).
Fig. 2.
Recombinant human bone morphogenetic protein-2 (rhBMP-2) in vivo treatment effects on survival. (A) Kaplan-Meier survival curve for animals injected orthotopically with UMSCC-14A cells pretreated with rhBMP-2 or control. Animals injected with rhBMP-2–treated cells had worse survival secondary to rapid local tumor growth (P = .0012). (B) Data summarized for two representative human oral cancer cell lines showing statistically significant worse survival in the animals orthotopically injected with rhBMP-2–treated cells.
Histopathological Characteristics of Xenografts of BMP-2–Treated Oral Carcinoma Cell Lines
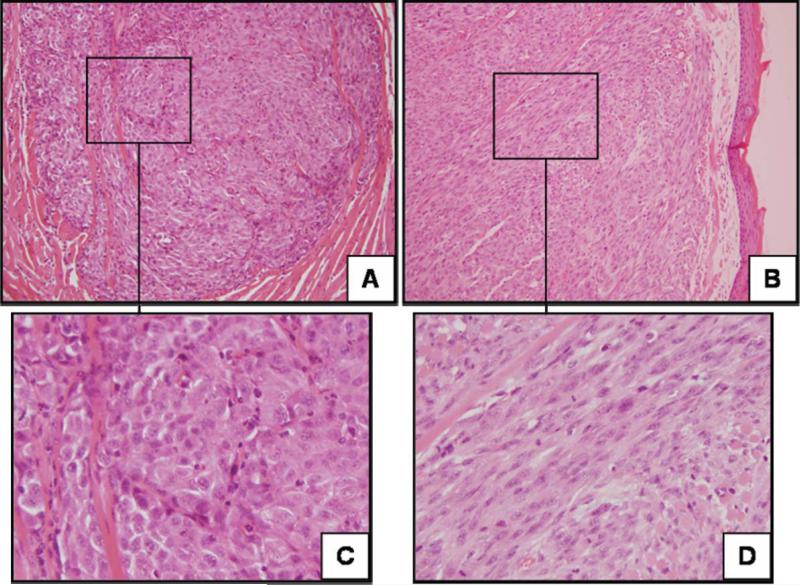
Groups where the cell lines were treated with rhBMP-2 prior to establishment of the xenografts consistently showed changes in morphology toward more poorly differentiated or undifferentiated tumors (Fig. 3). The tumors went from showing nests of epithelioid tumor cells with abundant cytoplasm and varying degrees of keratin formation to poorly differentiated carcinomas, many with spindle cell (or sarcomatoid) features. This finding was statistically significant for UMSCC-14A and trended toward being significant for UMSCC-74A (Table II). Host inflammatory response was more intense for the rhBMP-2 treatment group in UMSCC-14A, but this was not observed for UMSCC-74A. Lymphovascular space invasion was more prevalent in the rhBMP-2 treatment group for UMSCC-74A, but this did not reach statistical significance. There was no observed difference in pattern of invasion or perineural invasion for either cell line. Evaluation of the cervical lymph nodes and distant organs showed no evidence of metastatic carcinoma in the control or treatment groups for either cell line.
Fig. 3.
Xenograft histology from orthotopically established tumors. Representative histologic sections of xenografts established from UMSCC-74A treated with control (A and C) or recombinant human bone morphogenetic protein-2 (B and D). The xenografts showed changes in morphology toward more poorly differentiated tumors with spindle cell features. Hematoxylin and eosin; original magnification, 200 (A and B) and 400× (C and D).
TABLE II.
Recombinant Human Bone Morphogenetic Protein-2 Treatment Effects on Xenograft Histology.
| UMSCC-14A |
UMSCC-74A |
|||||
|---|---|---|---|---|---|---|
| Histopathologic Characteristics | Control (%) | BMP-2 (%) | Fisher's exact test (p-value) | Control(%) | BMP-2 (%) | Fisher's exact test (p-value) |
| Histological Grade: | ||||||
| Moderate | 100 | 50 | 0.0294 | 0 | 0 | 0.1222 |
| Poor | 0 | 50 | 40 | 7 | ||
| Poor with Spindle Cells | 0 | 0 | 60 | 86 | ||
| Spindle Cell Carcinoma | 0 | 0 | 0 | 7 | ||
| Host Inflammation: | ||||||
| None | 11 | 0 | 0.0216 | 20 | 43 | 0.3875 |
| Minimum | 78 | 25 | 80 | 57 | ||
| Mild | 11 | 25 | 0 | 0 | ||
| Brisk | 0 | 50 | 0 | 0 | ||
| Pattern of invasion: | ||||||
| Infiltrative | 44 | 25 | 0.6199 | 60 | 64 | 1.0000 |
| Pushing | 56 | 75 | 40 | 36 | ||
| Perineural invasion: | ||||||
| Yes | 0 | 0 | – | 10 | 8 | 1.0000 |
| No | 100 | 100 | 90 | 92 | ||
| Lymphovascular space invasion: | ||||||
| Yes | 33 | 38 | 1.0000 | 0 | 31 | 0.1045 |
| No | 67 | 62 | 100 | 69 | ||
The histopathologic characteristics between tumors established after treatment with recombinant human bone morphogenetic protein-2 and control were compared. P values for the treatment effects are shown. P values <.05 were considered significant. BMP-2 = bone morphogenetic protein-2.
Effect of rhBMP-2 Treatment on Expression Profiles of Signaling Kinases
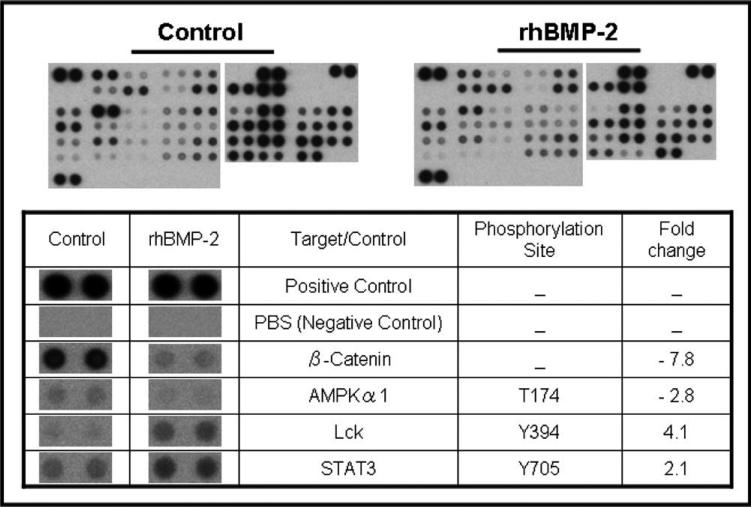
Stimulation of the BMP-2 signaling pathway resulted in alteration of phosphorylation levels of several protein kinases in UMSCC-74A (Fig. 4). STAT3 and Lckc were upregulated, whereas β-catenin and AMPKα1 were downregulated. It was difficult to determine statistically significant differences in phosphorylation of some other important kinases, because many had already been phosphorylated at high levels.
Fig. 4.
Human phospho-kinase array. Fold changes in protein expression levels of kinases after recombinant human bone morphogenetic protein-2 (rhBMP-2) treatment for UMSCC-74A. Only proteins showing statistically significant changes (P < .05) are shown.
DISCUSSION
The potential role of BMPs in malignant transformation and progression of cancer is poorly understood. Investigation in this area has proceeded to varying degrees in vitro and in vivo with different findings based on the tumor site, tumor type, and BMP studied.6 For OSCCA, previously reported data showed that BMP-2 and the receptor BMPR-IA were frequently expressed in human tumors as detected by immunohistochemistry.10 Our study shows that a large number of human OSCCA cell lines consistently expressed the BMP-2 gene and the components of the BMP-2 signaling pathway. Furthermore, the in vitro effects on expression profiles of protein kinases and the in vivo effects on animal survival after transient exposure of OSCCA cell lines to rhBMP-2 suggest that this pathway is functional and capable of being activated. Our data also confirmed the findings from other studies that the BMP-2 expression levels of normal oral mucosal cells are absent to weak.10,21
The nude mice engrafted orthotopically with OSCCA cell lines treated with rhBMP-2 had a significantly worse survival rate as compared to the control group. A previous study showed that rhBMP-2 did not appear to have an adverse biologic effect in OSCCA cells using a nude mouse model with an ectopic site of tumor engraftment.9 Physiologically, the ectopic model does not mimic the complexity of the microenvironment surrounding cancer cells in the oral cavity, which could significantly influence the tumor's growth and its response to treatment.22 Thus, an orthotopic nude mouse model for oral cancer was chosen because it reliably allows for tumor engraftment in the oral cavity and recapitulates the natural course of the disease.20 In this study, metastases were not found in the harvested cervical lymph nodes or distant organs, likely due to the early deaths of the animals from the local disease burden.
Several histopathological characteristics were compared in the xenografts, including known histologic prognosticators for oral cancer.23 For the cells treated with rhBMP-2, the xenografts consistently had a change in morphology toward a poorly or dedifferentiated appearance. This was statistically significant for UMSCC-14A and trended toward significance for UMSCC-74A. The moderately differentiated tumors became more poorly differentiated (UMSCC-14A), and the poorly differentiated tumors changed in morphology toward a spindle cell carcinoma (UMSCC-74A). This morphologic change to a more mesenchymal phenotype is suggestive of epithelial-mesenchymal transition (EMT) and will be a focus of future investigations. Bone morphogenetic proteins are already known to have a role in the normal physiologic induction of EMT during developmental processes and wound healing, and in the pathological induction of EMT leading to more invasive tumors for gastric and colon cancers.24–28
A human phospho-kinase array was used to address the potential mechanisms underlying the more aggressive tumor behavior observed in response to rhBMP-2 treatment. This technique enables 46 different phosphorylated cytoplasmic kinases to be screened in one sample of whole cell protein lysate, which is a powerful tool for gaining a global view of changes in signal transduction events within cells. In our study, a significant finding was close to an 8-fold decrease in expression of β-catenin. This was unexpected, because BMP-2 acts synergistically with β-catenin to promote osteoblast differentiation.29 Although activation of the Wnt-β-catenin pathway is frequently observed in several cancers, the role of this signaling pathway in OSCCA cells is poorly understood.30 Overexpression of β-catenin–promoted tumor growth in an OSSCA cell line using an ectopic nude mouse model, and a reduced cytoplasmic/nuclear pattern of β-catenin was associated with an invasive growth pattern and shorter survival in patients with oral cancers.31,32 However, a decreased level of β-catenin expression was associated with worse overall survival in patients with OSCCA and nasopharyngeal carcinoma.33,34 The roles of downstream target genes of BMP-2 signaling and subsequent interactions with other signaling pathways in OSCCA are not currently understood and warrant further investigation with regard to the Wnt-β-catenin pathway.
Another interesting finding was the 2-fold increased expression of STAT3 in the rhBMP-2 treated cells. The signal transducer and activator of transcription (STAT) proteins are important for relaying signals from activation of the epidermal growth factor receptor (EGFR). Inhibition of EGFR-STAT3 signaling is thought to be a possible therapeutic target for head and neck squamous cell carcinoma, because it is constitutively active in these cancers and causes adverse cellular effects through mediating invasion, proliferation, EMT, and prevention of apoptosis.35,36 There is currently a paucity of data describing the interactions of the BMP-2 signaling pathway with Janus kinase (JAK)-STAT signaling, which are limited to studies investigating osteoblast differentiation. These studies showed that BMP-2 activates this signaling pathway through STAT3 phosphorylation in human mesenchymal stem cells and accelerates early osteoblastic differentiation through a STAT3-dependent mechanism in mouse embryonic fibroblasts.37,38 These data are consistent with our finding of increased in vitro expression of phosphorylated STAT3 in response to rhBMP-2 treatment and also might explain the increased local tumor aggressiveness observed in vivo. Further investigations focusing on the interactions between the BMP2 and JAK/STAT signaling pathways in oral cancer appear warranted.
For the baseline expression analysis of the cell lines, only gene expression was determined rather than also analyzing protein expression with Western blots or doing fluorescence activated cell scanning analysis to show that the BMP-receptors were located on the cell surface. Independent of these additional investigations being performed, we were able to show that the BMP-2 signaling pathway is functional in vitro based on the observed changes in expression levels of kinases, and in vivo based on the effects on the xenografts that were established after rhBMP-2 treatment. Another limitation of this study is the single dose that was used for rhBMP-2 treatment, which was a standard dose used in other published experimental studies (100 ng/mL) rather than the dose used therapeutically in patients (1.5 mg/mL). Although increasing the concentration of rhBMP-2 to 500 ng/mL did not show an additional dose-dependent effect on proliferation or angiogenesis of OSCCA cell lines, the biologic effect of supramaximal stimulation similar to the dose used in patients could be unpredictable in this animal model and warrants future investigation.9 Last, the cell inoculums that were calculated from the preliminary tumor growth experiments were too high to compensate for the treatment effect, which resulted in rapid death of the animals in these groups. This did not allow for development of regional or distant metastases for further analysis. These data now provide important information that will assist us with calculating cell inoculums used for future experiments.
CONCLUSION
The gene expression levels of BMP-2 and the components of the BMP-signaling pathway are highly conserved among most OSCCA cell lines. Using an orthotopic nude mouse model for oral cancer, treatment of OSCCA cell lines with rhBMP-2 prior to engraftment increases the local aggressiveness of tumor growth and worsens animal survival. Histopathologic analysis of the xenografts and observed changes in the expression profile of protein kinases support further mechanistic studies focusing on EMT, the JAK/STAT pathway, and the Wnt-β-catenin pathway. Given the difference in expression levels of BMP-2 in OSCCA cell lines as compared to normal oral mucosa and the negative treatment effect, this signaling pathway might be a novel therapeutic target for treating OSCCA. Continued caution should be used for considering the therapeutic use of rhBMP-2 for reconstruction of bone defects in oral cancer patients.
Acknowledgment
The authors would like to thank Christine E. Stewart for technical assistance.
This work was supported by funding from an RO3 grant (DE017137-01A1) from the National Institute of Dental and Craniofacial Research/National Institutes of Health (NIDCR/NIH), and from a donation from the Bernard Hemann family. The authors have no other funding, financial relationships, or conflicts of interest to disclose.
BIBLIOGRAPHY
- 1.Schliephake H. Bone growth factors in maxillofacial skeletal reconstruction. Int J Oral Maxillofac Surg. 2002;31:469–484. doi: 10.1054/ijom.2002.0244. [DOI] [PubMed] [Google Scholar]
- 2.Burkus JK, Gornet MF, Dickman CA, et al. Anterior lumbar interbody fusion using rhBMP-2 with tapered interbody cages. J Spinal Disord Tech. 2002;15:337–349. doi: 10.1097/00024720-200210000-00001. [DOI] [PubMed] [Google Scholar]
- 3.Friedlaender GE, Perry CR, Cole JD, et al. Osteogenic protein-1 (bone morphogenetic protein-7) in the treatment of tibial nonunions. J Bone Joint Surg. 2001;83(suppl 1):151–158. [PMC free article] [PubMed] [Google Scholar]
- 4.INFUSE Bone Graft [package insert] Medtronic Sofamor Danek USA, Inc.; Memphis, TN: 2008. Available at: https://www.infusebonegraft.com/omf_package_insert.pdf. [Google Scholar]
- 5.Nussenbaum B, Krebsbach PH. Practical matters in the application of tissue engineered products for skeletal regeneration in the head and neck region. In: Sandell LJ, Grodzinsky AJ, editors. Tissue Engineering in Musculoskeletal Clinical Practice. American Academy of Orthopedic Surgeons; Rosemont, IL: 2004. pp. 151–159. [Google Scholar]
- 6.Thawani JP, Wang AC, Than KD, et al. Bone morphogenetic proteins and cancer: review of the literature. Neurosurgery. 2010;66:233–246. doi: 10.1227/01.NEU.0000363722.42097.C2. [DOI] [PubMed] [Google Scholar]
- 7.Poynton AR, Lane JM. Safety profile for the clinical use of bone morphogenetic proteins in the spine. Spine. 2002;27(16 suppl 1):S40–S48. doi: 10.1097/00007632-200208151-00010. [DOI] [PubMed] [Google Scholar]
- 8.Golden JD, Jones AL, Bucholz RW, et al. Recombinant human BMP-2 and allograft compared with autogenous bone graft for reconstruction of diaphyseal tibial fractures with cortical defects [letter]. J Bone Joint Surg. 2008;90:1168–1169. [PubMed] [Google Scholar]
- 9.Gao Q, Tong W, Luria JS, Wang Z, Nussenbaum B, Krebsbach PH. Effects of bone morphogenetic protein-2 on proliferation and angiogenesis in oral squamous cell carcinoma. Int J Oral Maxillofac Surg. 2010;39:266–271. doi: 10.1016/j.ijom.2009.11.015. [DOI] [PubMed] [Google Scholar]
- 10.Jin Y, Tipoe GL, Liong EC, Lau TY, Fung PC, Leung KM. Overexpression of BMP-2/4, -5 and BMPR-1A is associated with malignancy of oral epithelium. Oral Oncology. 2001;37:225–233. doi: 10.1016/s1368-8375(00)00087-7. [DOI] [PubMed] [Google Scholar]
- 11.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 12.Zhou X, Temam S, Oh M, et al. Global expression-based classification of lymph node metastasis and extracapsular spread of oral tongue squamous cell carcinoma. Neoplasia. 2006;8:925–932. doi: 10.1593/neo.06430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Willette RN, Gu JL, Lysko PG, et al. BMP-2 gene expression and effects on human vascular smooth muscle cells. J Vasc Res. 1999;36:120–125. doi: 10.1159/000025634. [DOI] [PubMed] [Google Scholar]
- 14.Gobbi G, Sangiorgi L, Lenzi L, et al. Seven BMPs and all their receptors are simultaneously expressed in osteosarcoma cells. Int J Oncol. 2002;20:143–147. [PubMed] [Google Scholar]
- 15.Valcourt U, Gouttenoire J, Moustakas A, et al. Functions of transforming growth factor-beta family type I receptors and Smad proteins in the hypertrophic maturation and osteoblastic differentiation of chondrocytes. J Biol Chem. 2002;277:33545–33558. doi: 10.1074/jbc.M202086200. [DOI] [PubMed] [Google Scholar]
- 16.Bau B, Haag J, Schmid E, et al. Bone morphogenetic protein-mediating receptor-associated Smads as well as common Smad are expressed in human articular chondrocytes but not up-regulated or down-regulated in osteoarthritic cartilage. J Bone Miner Res. 2002;17:2141–2150. doi: 10.1359/jbmr.2002.17.12.2141. [DOI] [PubMed] [Google Scholar]
- 17.Sebestyen A, Barna G, Nagy K, et al. Smad signal and TGFbeta induced apoptosis in human lymphoma cells. Cytokine. 2005;30:228–235. doi: 10.1016/j.cyto.2005.01.013. [DOI] [PubMed] [Google Scholar]
- 18.Simic P, Culej JB, Orlic I, et al. Systemically administered bone morphogenetic protein-6 restores bone in aged ovariectomized rats by increasing bone formation and suppressing bone resorption. J Biol Chem. 2006;281:25509–22521. doi: 10.1074/jbc.M513276200. [DOI] [PubMed] [Google Scholar]
- 19.Sun J, Wei L, Liu X, et al. Influences of ionic dissolution products of dicalcium silicate coating on osteoblastic proliferation, differentiation and gene expression. Acta Biomater. 2009;5:1284–1293. doi: 10.1016/j.actbio.2008.10.011. [DOI] [PubMed] [Google Scholar]
- 20.Myers JN, Holsinger FC, Jasser SA, et al. An orthotopic nude mouse model of oral tongue squamous cell carcinoma. Clin Cancer Res. 2002;8:293–298. [PubMed] [Google Scholar]
- 21.Soares AF, Xavier RL, daCosta Miguel MC, et al. Bone morphogenetic protein-2/4 and bone morphogenetic protein receptor type IA expression in metastatic and nonmetastatic oral squamous cell carcinoma. Am J Otolaryngol. 2010;31:266–271. doi: 10.1016/j.amjoto.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 22.Kupferman ME, Myers JN. Molecular biology of oral cavity squamous cell carcinoma. Otolaryngol Clin North Am. 2006;39:229–247. doi: 10.1016/j.otc.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 23.Brandwein-Gensler M, Teixeira MS, Lewis CM, et al. Oral squamous cell carcinoma: histologic risk assessment, but not margin status, is strongly predictive of local disease-free and overall survival. Am J Surg Pathol. 2005;29:167–178. doi: 10.1097/01.pas.0000149687.90710.21. [DOI] [PubMed] [Google Scholar]
- 24.Park KS, Gumbiner BM. Cadherin 6B induces BMP signaling and de-epithelialization during the epithelial mesenchymal transition of the neural crest. Development. 2010;137:2691–2701. doi: 10.1242/dev.050096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Townsend TA, Robinson JY, Deig CR, et al. BMP-2 and TGFb2 shared path-ways regulate endocardial cell transformation. Cells Tissues Organs. 2011;194:1–12. doi: 10.1159/000322035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yan C, Grimm WA, Garner WL, et al. Epithelial to mesenchymal transition in human skin wound healing is induced by tumor necrosis factor-α through bone morphogenetic protein-2. Am J Surg Pathol. 2010;176:2247–2258. doi: 10.2353/ajpath.2010.090048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kang MH, Kim JS, Seo JS, et al. BMP2 accelerates the motility and invasiveness of gastric cancer cells via activation of the phosphatidylinositol 3-kinase (PI3K)/Akt pathway. Exp Cell Res. 2010;316:24–37. doi: 10.1016/j.yexcr.2009.10.010. [DOI] [PubMed] [Google Scholar]
- 28.Kang MH, Kang HN, Kim JL, et al. Inhibition of PI3 kinase/Akt pathway is required for BMP2-induced EMT and invasion. Oncol Rep. 2009;22:525–534. doi: 10.3892/or_00000467. [DOI] [PubMed] [Google Scholar]
- 29.Zhang M, Yan Y, Lim YB, et al. BMP-2 modulates β-catenin signaling through stimulation of Lrp5 expression and inhibition of β-TrCP expression in osteoblasts. J Cell Biochem. 2009;108:896–905. doi: 10.1002/jcb.22319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Molinolo AA, Amornphimoltham P, Squarize CH, et al. Dysregulated molecular networks in head and neck carcinogenesis. Oral Oncol. 2009;45:324–334. doi: 10.1016/j.oraloncology.2008.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang F, Zeng Q, Yu G, et al. Wnt/β-catenin signaling inhibits death receptor-mediated apoptosis and promotes invasive growth of HNSCC. Cell Signal. 2006;18:679–687. doi: 10.1016/j.cellsig.2005.06.015. [DOI] [PubMed] [Google Scholar]
- 32.Odajima T, Sasaki Y, Tanaka N, et al. Abnormal β-catenin expression in oral cancer with no gene mutation: correlation with expression of cyclin D1 and epidermal growth factor receptor, Ki-67labeling index, and clinicopathological features. Hum Pathol. 2005;36:234–241. doi: 10.1016/j.humpath.2004.12.009. [DOI] [PubMed] [Google Scholar]
- 33.Zheng Z, Pan J, Chu B, et al. Downregulation and abnormal expression of E-cadherin and β-catenin in nasopharyngeal carcinoma: close association with advanced disease stage and lymph node metastasis. Hum Pathol. 1999;30:458–466. doi: 10.1016/s0046-8177(99)90123-5. [DOI] [PubMed] [Google Scholar]
- 34.Liu LK, Jiang XY, Zhou XX, et al. Upregulation of vimentin and aberrant expression of E-cadherin/β-catenin complex in oral squamous cell carcinomas: correlation with the clinicopathological features and patient out-come. Mod Pathol. 2010;23:213–224. doi: 10.1038/modpathol.2009.160. [DOI] [PubMed] [Google Scholar]
- 35.Leeman RJ, Lui VW, Grandis JR. STAT3 as a therapeutic target in head and neck cancer. Expert Opin Biol Ther. 2006;6:231–241. doi: 10.1517/14712598.6.3.231. [DOI] [PubMed] [Google Scholar]
- 36.Wheeler SE, Suzuki S, Thomas SM, et al. Epidermal growth factor receptor variant III mediates head and neck cancer cell invasion via STAT3 activation. Oncogene. 2010;29:5135–5145. doi: 10.1038/onc.2009.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Levy O, Ruvinov E, Reem T, et al. Highly efficient osteogenic differentiation of human mesenchymal stem cells by eradication of STAT3 signaling. Int J Biochem Cell Biol. 2010;42:1823–1830. doi: 10.1016/j.biocel.2010.07.017. [DOI] [PubMed] [Google Scholar]
- 38.Mikami Y, Asano M, Honda MJ, Takagi M. Bone morphogenetic protein 2 and dexamethasone synergistically increase alkaline phosphatase levels through JAK/STAT signaling in C3H10T1/2 cells. J Cell Physiol. 2010;223:123–133. doi: 10.1002/jcp.22017. [DOI] [PubMed] [Google Scholar]