Abstract
Mitochondrial RNA polymerase activity from rat liver has previously been demonstrated in intact organelles. This activity has now been solubilized, partially purified, and shown to be a true polymerase, free of nuclease. The enzyme is derived from mitochondria and is not from contaminating bacteria or nuclear components. The enzyme is distinguished from its nuclear counterparts by its behavior on ammonium sulfate fractionation and lack of inhibition by α-amanitin. Rifamycin inhibits the crude enzyme, but only inconsistently inhibits the more purified preparation.
Keywords: rifampicin, α-amanitin, (NH4)2SO4, DEAE-Sephadex, divalent cations
Full text
PDF

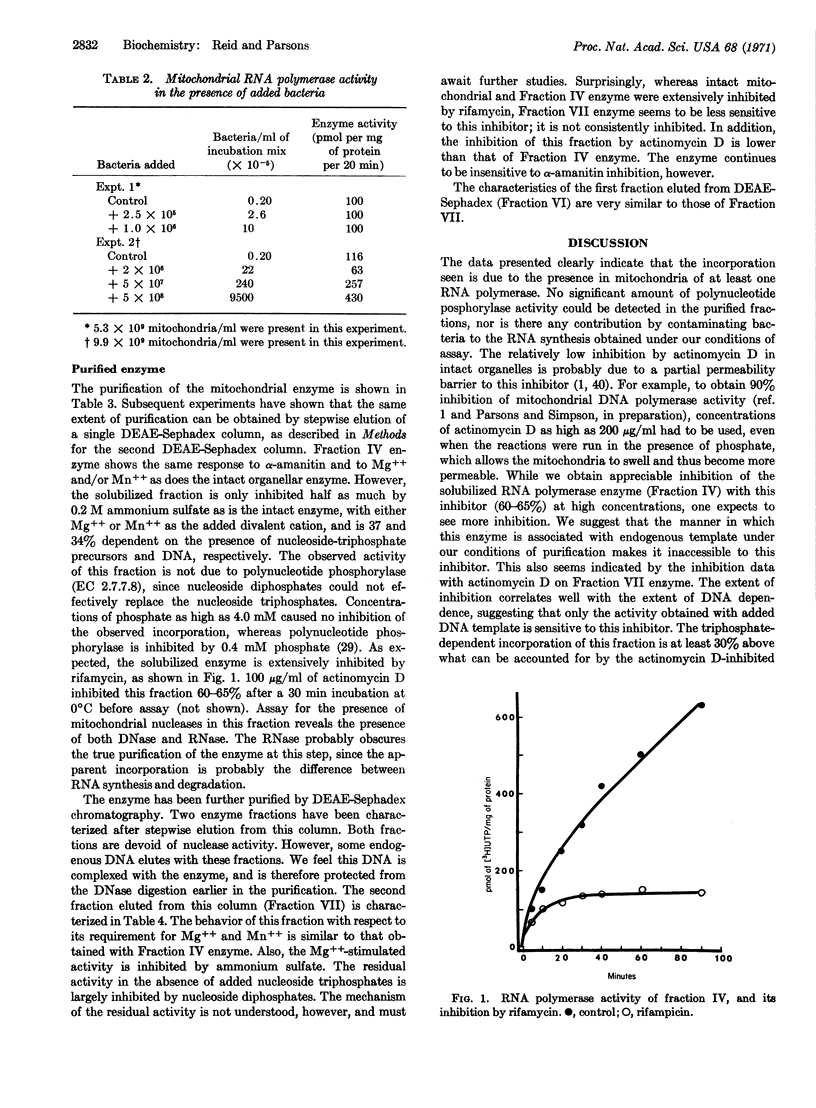
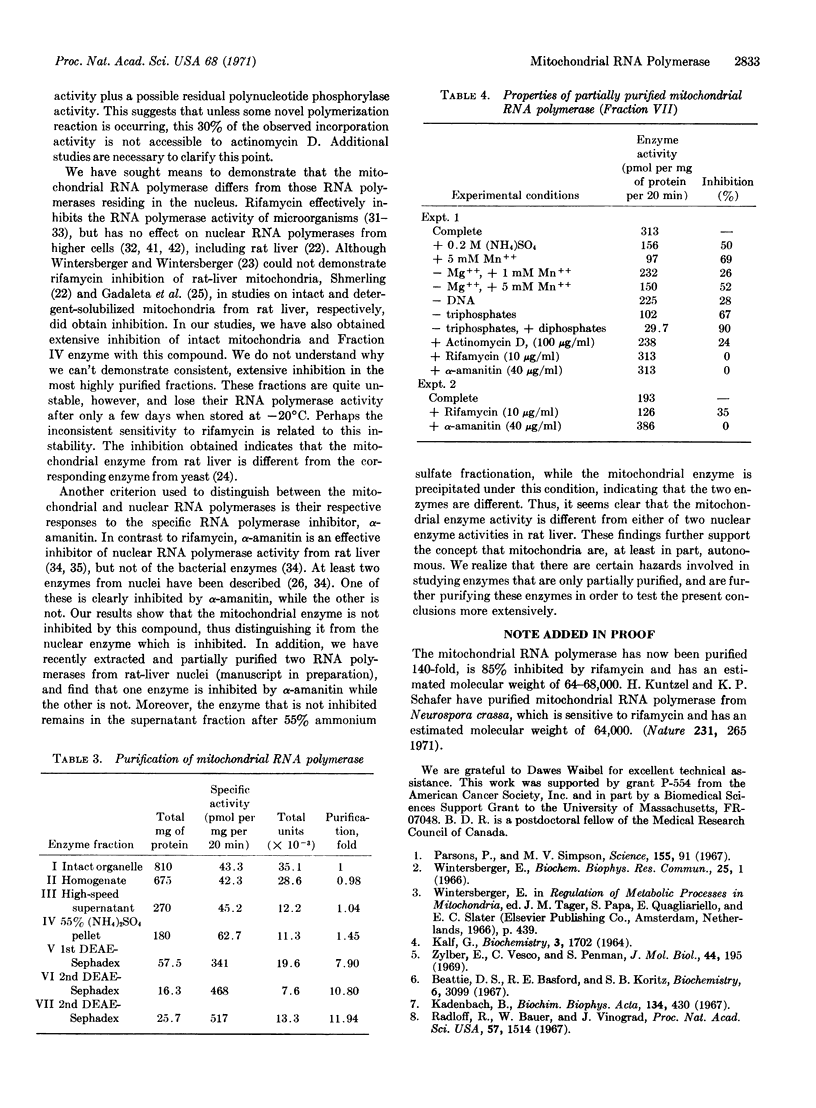

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Attardi G., Attardi B. Mitochondrial origin of membrane-associated heterogeneous RNA in HeLa cells. Proc Natl Acad Sci U S A. 1968 Sep;61(1):261–268. doi: 10.1073/pnas.61.1.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnett W. E., Brown D. H. Mitochondrial transfer ribonucleic acids. Proc Natl Acad Sci U S A. 1967 Feb;57(2):452–458. doi: 10.1073/pnas.57.2.452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beattie D. S., Basford R. E., Koritz S. B. The inner membrane as the site of the in vitro incorporation of L-[14C]leucine into mitochondrial protein. Biochemistry. 1967 Oct;6(10):3099–3106. doi: 10.1021/bi00862a017. [DOI] [PubMed] [Google Scholar]
- Buck C. A., Nass M. M. Studies on mitochondrial tRNA from animal cells. I. A comparison of mitochondrial and cytoplasmic trna and aminoacyl-tRNA synthetases. J Mol Biol. 1969 Apr 14;41(1):67–82. doi: 10.1016/0022-2836(69)90126-0. [DOI] [PubMed] [Google Scholar]
- Burgess R. R. A new method for the large scale purification of Escherichia coli deoxyribonucleic acid-dependent ribonucleic acid polymerase. J Biol Chem. 1969 Nov 25;244(22):6160–6167. [PubMed] [Google Scholar]
- Comorosan S., Gaspar A., Sandru D. Isolation and characterization of a rapidly labelled RNA from mouse liver mitochondria. Biochim Biophys Acta. 1968 Sep 24;166(2):394–402. doi: 10.1016/0005-2787(68)90227-x. [DOI] [PubMed] [Google Scholar]
- Dubin D. T. The effect of actinomycin on the synthesis of mitochondrial RNA in hamster cells. Biochem Biophys Res Commun. 1967 Dec 15;29(5):655–660. doi: 10.1016/0006-291x(67)90266-5. [DOI] [PubMed] [Google Scholar]
- Fukamachi S., Bartoov B., Mitra R. S., Freeman K. B. The synthesis of ribosomal-type RNA by isolated rat liver mitochondria. Biochem Biophys Res Commun. 1970 Aug 24;40(4):852–857. doi: 10.1016/0006-291x(70)90981-2. [DOI] [PubMed] [Google Scholar]
- Gadaleta M. N., Greco M., Saccone C. The effect of rifampicin on mitochondrial RNA polymerase from rat liver. FEBS Lett. 1970 Sep 18;10(1):54–56. doi: 10.1016/0014-5793(70)80414-8. [DOI] [PubMed] [Google Scholar]
- Hartmann G., Honikel K. O., Knüsel F., Nüesch J. The specific inhibition of the DNA-directed RNA synthesis by rifamycin. Biochim Biophys Acta. 1967;145(3):843–844. doi: 10.1016/0005-2787(67)90147-5. [DOI] [PubMed] [Google Scholar]
- Jacob S. T., Sajdel E. M., Munro H. N. Different responses of soluble whole nuclear RNA polymerase and soluble nucleolar RNA polymerase to divalent cations and to inhibition by alpha-amanitin. Biochem Biophys Res Commun. 1970 Feb 20;38(4):765–770. doi: 10.1016/0006-291x(70)90647-9. [DOI] [PubMed] [Google Scholar]
- KALF G. F. DEOXYRIBONUCLEIC ACID IN MITOCHONDRIA AND ITS ROLE IN PROTEIN SYNTHESIS. Biochemistry. 1964 Nov;3:1702–1706. doi: 10.1021/bi00899a018. [DOI] [PubMed] [Google Scholar]
- Kedinger C., Gniazdowski M., Mandel J. L., Jr, Gissinger F., Chambon P. Alpha-amanitin: a specific inhibitor of one of two DNA-pendent RNA polymerase activities from calf thymus. Biochem Biophys Res Commun. 1970 Jan 6;38(1):165–171. doi: 10.1016/0006-291x(70)91099-5. [DOI] [PubMed] [Google Scholar]
- Kroon A. M., de Vries H. The effect of chloramphenicol on the biogenesis of mitochondria of rat liver in vivo. FEBS Lett. 1969 May;3(3):208–210. doi: 10.1016/0014-5793(69)80137-7. [DOI] [PubMed] [Google Scholar]
- Küntzel H., Noll H. Mitochondrial and cytoplasmic polysomes from Neurospora crassa. Nature. 1967 Sep 23;215(5108):1340–1345. doi: 10.1038/2151340a0. [DOI] [PubMed] [Google Scholar]
- Küntzel H., Schäfer K. P. Mitochondrial RNA polymerase from Neurospora crassa. Nat New Biol. 1971 Jun 30;231(26):265–269. doi: 10.1038/newbio231265a0. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Meyer R. R., Simpson M. V. DNA biosynthesis in mitochondria: partial purification of a distinct DNA polymerase from isolated rat liver mitochondria. Proc Natl Acad Sci U S A. 1968 Sep;61(1):130–137. doi: 10.1073/pnas.61.1.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novello F., Stirpe F. Simultaneous assay of RNA polymerase I and II in nuclei isolated from resting and growing rat liver with the use of alpha-amanitin. FEBS Lett. 1970 May 11;8(1):57–60. doi: 10.1016/0014-5793(70)80225-3. [DOI] [PubMed] [Google Scholar]
- Parsons P., Simpson M. V. Biosynthesis of DNA by isolated mitochondria: incorporation of thymidine triphosphate-2-C-14. Science. 1967 Jan 6;155(3758):91–93. doi: 10.1126/science.155.3758.91. [DOI] [PubMed] [Google Scholar]
- ROODYN D. B., FREEMAN K. B., TATA J. R. THE STIMULATION BY TREATMENT IN VIVO WITH TRI-IODOTHYRONINE OF AMINO ACID INCORPORATION INTO PROTEIN BY ISOLATED RAT-LIVER MITOCHONDRIA. Biochem J. 1965 Mar;94:628–641. doi: 10.1042/bj0940628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROODYN D. B., REIS P. J., WORK T. S. Protein synthesis in mitochondria. Requirements for the incorporation of radioactive amino acids into mitochondrial protein. Biochem J. 1961 Jul;80:9–21. doi: 10.1042/bj0800009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radloff R., Bauer W., Vinograd J. A dye-buoyant-density method for the detection and isolation of closed circular duplex DNA: the closed circular DNA in HeLa cells. Proc Natl Acad Sci U S A. 1967 May;57(5):1514–1521. doi: 10.1073/pnas.57.5.1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shmerling Z. G. The effect of rifamycin on RNA synthesis in the rat liver mitochondria. Biochem Biophys Res Commun. 1969 Dec 4;37(6):965–969. doi: 10.1016/0006-291x(69)90225-3. [DOI] [PubMed] [Google Scholar]
- Sippel A., Hartmann G. Mode of action of rafamycin on the RNA polymerase reaction. Biochim Biophys Acta. 1968 Mar 18;157(1):218–219. doi: 10.1016/0005-2787(68)90286-4. [DOI] [PubMed] [Google Scholar]
- Stirpe F., Fiume L. Studies on the pathogenesis of liver necrosis by alpha-amanitin. Effect of alpha-amanitin on ribonucleic acid synthesis and on ribonucleic acid polymerase in mouse liver nuclei. Biochem J. 1967 Nov;105(2):779–782. doi: 10.1042/bj1050779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suyama Y., Eyer J. Ribonucleic acid synthesis in isolated mitochondria from Tetrahymena. J Biol Chem. 1968 Jan 25;243(2):320–328. [PubMed] [Google Scholar]
- Tsai M. J., Michaelis G., Criddle R. S. DNA-dependent RNA polymerase from yeast mitochondria. Proc Natl Acad Sci U S A. 1971 Feb;68(2):473–477. doi: 10.1073/pnas.68.2.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umezawa H., Mizuno S., Yamazaki H., Nitta K. Inhibition of DNA-dependent RNA synthesis by rifamycins. J Antibiot (Tokyo) 1968 Mar;21(3):234–236. doi: 10.7164/antibiotics.21.234. [DOI] [PubMed] [Google Scholar]
- Walker J. E., Abraham E. P. Isolation of bacilysin and a new amino acid from culture filtrates of Bacillus subtilis. Biochem J. 1970 Jul;118(4):557–561. doi: 10.1042/bj1180557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehrli W., Knüsel F., Staehelin M. Action of rifamycin on RNA-polymerase from sensitive and resistant bacteria. Biochem Biophys Res Commun. 1968 Jul 26;32(2):284–288. doi: 10.1016/0006-291x(68)90382-3. [DOI] [PubMed] [Google Scholar]
- Wehrli W., Nüesch J., Knüsel F., Staehelin M. Action of rifamycins on RNA polymerase. Biochim Biophys Acta. 1968 Mar 18;157(1):215–217. doi: 10.1016/0005-2787(68)90285-2. [DOI] [PubMed] [Google Scholar]
- Wintersberger E. Occurrence of a DNA-polymerase in isolated yeast mitochondria. Biochem Biophys Res Commun. 1966 Oct 5;25(1):1–7. doi: 10.1016/0006-291x(66)90630-9. [DOI] [PubMed] [Google Scholar]
- Wintersberger E., Wintersberger U. Rifamycin insensitivity of RNA synthesis in yeast. FEBS Lett. 1970 Jan 15;6(1):58–60. doi: 10.1016/0014-5793(70)80043-6. [DOI] [PubMed] [Google Scholar]
- Zylber E., Vesco C., Penman S. Selective inhibition of the synthesis of mitochondria-associated RNA by ethidium bromide. J Mol Biol. 1969 Aug 28;44(1):195–204. doi: 10.1016/0022-2836(69)90414-8. [DOI] [PubMed] [Google Scholar]
- de Vries H., Kroon A. M. On the effect of chloramphenicol and oxytetracycline on the biogenesis of mammalian mitochondria. Biochim Biophys Acta. 1970 Apr 15;204(2):531–541. doi: 10.1016/0005-2787(70)90173-5. [DOI] [PubMed] [Google Scholar]
- van Bruggen E. F., Runner C. M., Borst P., Ruttenberg G. J., Kroon A. M., Stekhoven F. M. Mitochondrial DNA. 3. Electron microscopy of DNA released from mitochondria by osmotic shock. Biochim Biophys Acta. 1968 Jul 23;161(2):402–414. [PubMed] [Google Scholar]


