Abstract
Introduction
Substantial heterogeneity remains across studies investigating changes in gray matter in schizophrenia. Differences in methodology, heterogeneous symptom patterns and symptom trajectories may contribute to inconsistent findings. To address this problem, we recently proposed to group patients by symptom dimensions, which map on the language, the limbic and the motor systems. The aim of the present study was to investigate whether patients with prevalent symptoms of emotional dysregulation would show structural neuronal abnormalities in the limbic system.
Method
43 right-handed medicated patients with schizophrenia were assessed with the Bern Psychopathology Scale (BPS). The patients and a control group of 34 healthy individuals underwent structural imaging at a 3T MRI scanner. Whole brain voxel-based morphometry (VBM) was compared between patient subgroups with different severity of emotional dysregulation. Group comparisons (comparison between patients with severe emotional dysregulation, patients with mild emotional dysregulation, patients with no emotional dysregulation and healthy controls) were performed using a one way ANOVA and ANCOVA respectively.
Results
Patients with severe emotional dysregulation had significantly decreased gray matter density in a large cluster including the right ventral striatum and the head of the caudate compared to patients without emotional dysregulation. Comparing patients with severe emotional dysregulation and healthy controls, several clusters of significant decreased GM density were detected in patients, including the right ventral striatum, head of the caudate, left hippocampus, bilateral thalamus, dorsolateral prefrontal and orbitofrontal cortex. The significant effect in the ventral striatum was lost when patients with and without emotional dysregulation were pooled and compared with controls.
Discussion
Decreased gray matter density in a large cluster including the right ventral striatum was associated with severe symptoms of emotional dysregulation in patients with schizophrenia. The ventral striatum is an important part of the limbic system, and was indicated to be involved in the generation of incentive salience and psychotic symptoms. Only patients with severe emotional dysregulation had decreased gray matter in several brain structures associated with emotion and reward processing compared to healthy controls. The results support the hypothesis that grouping patients according to specific clinical symptoms matched to the limbic system allows identifying patient subgroups with structural abnormalities in the limbic network.
Keywords: Dimensions, Emotional dysregulation, Limbic system, Brain circuits, Psychopathology
Highlights
-
•
We examined whole brain VBM in schizophrenia patients and healthy controls.
-
•
We compared patients with different severity of emotional dysregulation (ED).
-
•
Symptoms of ED were associated with GM density in the ventral striatum.
-
•
Grouping patients according to symptoms identified specific GM abnormalities.
1. Introduction
Structural alterations in cerebral gray matter have been repeatedly reported in schizophrenia, particularly in the prefrontal cortex, the superior temporal gyrus, the limbic system (medial temporal lobe, hippocampus, entorhinal cortex, amygdala) as well as in the insula, the thalamus and the cerebellum (Bora et al., 2011; Ellison-Wright et al., 2008; Glahn et al., 2008; Horn et al., 2009). Considerable heterogeneity of results across studies on structural anatomy has been noted, however (Haijma et al., 2012; Honea et al., 2008). In addition to differences in methodology and population demographics, heterogeneous symptom patterns and symptom trajectories may critically contribute to the inconsistent findings. Furthermore, the distribution of anatomical alterations does not yield unique hints to understand the pathophysiology of the different symptoms associated with schizophrenia. Linking dimensional assessment of behavioral domains and mental states to brain circuitry may be necessary to progress in schizophrenia research (Heckers, 2011).
Investigations of more homogeneous patient groups aimed to overcome this issue. In addition to the classical consideration of positive and negative symptoms, there is growing evidence that investigating core symptoms may lead to meaningful results, as demonstrated for formal thought disorder (Horn et al., 2010; McCarley et al., 1993; Shenton et al., 1992), hallucinations (Barta et al., 1990; Gaser et al., 2004; Nestor et al., 2007) and delusions (Pankow et al., 2012; Spencer et al., 2007). In addition, factor analysis to psychopathology as rated with the scales for assessment of positive and negative symptoms has been applied to reduce symptom variance in a large patient group; this resulted in distinct associations with structural alterations, e.g. between the paranoid/hallucinatory subsyndrome and the superior temporal cortex (Nenadic et al., 2010).
If psychopathology in schizophrenia spectrum disorders is the result of a functional imbalance of higher order brain systems, it should be possible to match specific psychotic symptoms on the respective brain functions and systems. Based on this assumption, we have developed the Bern Psychopathology Scale (BPS) as a research instrument to group psychotic symptoms into three biologically relevant dimensions referring to the language, limbic and motor systems (Strik et al., 2010).
Dysfunction in the limbic system in schizophrenia is a growing area of interest. Particularly, abnormalities in emotion processing and regulation are cardinal features in psychiatric disorders (Taylor and Liberzon, 2007). They may be pivotal to produce abnormal salience (Kapur, 2003) and threat beliefs, possibly underlying specific psychotic symptoms such as persecutory delusions (Freeman and Garety, 2003). Biased emotion processing may cause aggression, suspiciousness and poor social performance of patients with schizophrenia (Kee et al., 2003; Phillips et al., 2003), and was associated with positive psychotic symptoms (Abi-Dargham et al., 2000; Pankow et al., 2012). In addition, patients with schizophrenia show aberrant functional brain activation in neuronal regions implicated in emotion and reward processing e.g. the amygdala, insula, anterior cingulate, orbitofrontal cortex and ventral striatum (Aleman and Kahn, 2005; Brunet-Gouet and Decety, 2006; Kirsch et al., 2007; Pankow et al., 2013; Schlagenhauf et al., 2008; Walter et al., 2009).
In the light of these findings from functional brain imaging, we expected changes in limbic structures to be associated with emotional dysregulation and delusions of threat, covered in the BPS by the affectivity dimension. Particularly, we hypothesized that gray matter volumes in the limbic system would differ between patients presenting with severe emotional dysregulation and patients without emotional dysregulation. In addition, the structural alterations in patients presenting with severe emotional dysregulation would also differ from controls.
2. Methods
2.1. Subjects and clinical assessment
In total, 43 patients (16 women and 27 men) of the University Hospital, Bern, Switzerland, meeting DSM IV (American Psychiatric Association 1994) criteria for schizophrenia were included. Diagnoses were given following thorough clinical interviews and review of all records available, however the structured clinical interview for DSM IV (SCID) was not applied. 40 patients were treated with atypical or typical antipsychotics. Three patients were drug free at the time of the study. Five patients received typical antipsychotics (four typical and atypical and one only typical). A control group of 34 healthy individuals was included (18 women and 16 men). All subjects were right handed. Groups did not differ in age or gender distribution (see Table 1). However, controls had longer duration of education (t = − 3.4, df = 75, p = 0.001).
Table 1.
Sample demographic variables.
| Schizophrenia | Controls | t/χ2 | df | p-Values | |
|---|---|---|---|---|---|
| No. of subjects | 43a | 34 | |||
| Gender (% female) | 62.8 | 37.2 | 1.3 | 1 | 0.354 |
| Age (years) | 34.1 ± 10.9 | 37.1 ± 12.3 | − 1.2 | 75 | 0.231 |
| Education (years) | 12.9 ± 4.0 | 16.2 ± 4.1 | − 3.4 | 75 | 0.001 |
| PANSS total score | 57.4 ± 17.3 | – | – | – | – |
| PANSS positive syndrome score | 15.4 ± 5.7 | – | – | – | – |
| PANSS negative syndrome score | 14.6 ± 6.4 | – | – | – | – |
| Chlorpromazine equivalents (mg) | 457.4 ± 333.7 | – | – | – | – |
| Mean duration of illness (years) | 8.7 ± 9.3 | – | – | – | – |
| Number of psychotic episodes | 4.1 ± 5.1 | – | – | – | – |
| Gray matter volume (ml) | 705.9 ± 113.1 | 756.9 ± 80.2 | − 2.2 | 75 | 0.029 |
Patient subgroups: sED: n = 14; mED: n = 22; nED: n = 7; − sED: n = 6; + sED: n = 8. Severe emotional dysregulation (sED), mild emotional dysregulation (mED), and no emotional dysregulation (nED).
Participants provided written informed consent. The protocol was approved by the local ethics committee.
Psychopathology was assessed using the Positive and Negative Syndrome Scale (PANSS; Kay et al., 1987) as well as the Bern Psychopathology Scale. The BPS was explicitly developed as a research instrument to attribute psychotic symptoms to behavioral dimensions and the suspected underlying brain systems, namely the language, the motor and the limbic systems. It is neither intended as a diagnostic tool in terms of the ICD-10 or DSM-IV categories, nor attempted to cover every phenomenon associated with psychoses.
The affectivity dimension includes symptoms of emotional dysregulation, attributed to the limbic system (http://www.puk.unibe.ch/BPS) e.g. delusions of threat or supernatural power, tension, psychotic anxiety, suspiciousness, aggressiveness or social avoidance and unpleasant body sensations. Global severity of symptoms is rated on 7 point Likert scales ranging from − 3 (e.g. most severe psychotic anxiety) to + 3 (e.g. most severe psychotic grandiosity), whereas 0 refers to normal behavior; (Strik et al., 2010). The global rating does not represent a sum score of the single items but instead refers to a global clinical impression after assessing the presence and severity of all items. One or two very severe impairing signs therefore may drive the global impression, whereas multiple mild signs may not sum up to high global severity ratings. The major differences of the BPS compared to other psychopathological scales (e.g. the PANSS) are first the possibility to attribute psychotic symptoms to behavioral domains and second the dual rating system that focuses on both single signs and the global impression. The latter allows weighting the impact of single signs with prominent severity.
We chose a prototypical approach to data analysis, assuming that severe emotional dysregulation was reliably related to psychosis, while the mildest forms (+ 1 and − 1 on the global BPS scale) may overlap with normal or reactive emotional dysregulation. Based on this consideration and lacking precedents to provide a rationale for the cut-offs, we defined three patient subgroups according to the severity of emotional dysregulation, regardless of the direction (+ or −) on the global rating. The first group consisted of patients with evident and severe emotional dysregulation (sED; n = 14; 7 women and 7 men) defined by high ratings in the global affectivity scale (≤ − 2 or ≥ + 2); the second group of patients with less evident and mild emotional dysregulation (mED; n = 22; 5 women and 17 men; BPS global affectivity − 1 or + 1); the third group of patients with normal affectivity (no symptom of emotional dysregulation, nED; n = 7; 4 women and 3 men; BPS global affectivity = 0). Patient subgroups did not differ in duration of illness, number of episodes, chlorpromazine equivalent dosage, PANSS scores (negative, positive and total scores), duration of education, age or gender distribution and total gray matter volume (see Table A, Supplementary data).
2.2. Structural MRI acquisition and data processing
Imaging was performed on a 3T MRI scanner (Siemens Magnetom Trio; Siemens Medical Solutions, Erlangen, Germany) with a standard head coil. 3D-T1-weighted (Modified Driven Equilibrium Fourier Transform Pulse Sequence; MDEFT) images for each subject have been obtained (Deichmann et al., 2004), providing 176 sagittal slices with 256 × 224 matrix points with a non-cubic field of view (FOV) of 256 × 224, yielding a nominal isotopic resolution of 1 mm3 (i.e. 1 mm × 1 mm × 1 mm). Further scan parameters were 7.92 ms repetition time (TR), 2.48 ms echo time (TE) and a flip angle of 16° (FA).
Structural images were processed using SPM8 (Wellcome Trust Center for Neuroimaging, London; http://www.fil.ion.ucl.ac.uk/spm). All preprocessing steps were conducted using standard procedures as implemented in SPM8 (Matlab version 7, release 2008a; The MathWorks, Inc., Natick, MA, USA), in particular the voxel-based morphometry (VBM) toolbox. The images have been normalized, modulated and smoothed with 8 mm full-width at half maximum (FWHM) kernel.
2.3. Statistical analyses
Statistical tests were performed using SPM routines and SPSS 17.0 (SPSS Inc., Chicago, IL, USA). Independent two-sample t tests, one way ANOVAs and chi-square tests (χ2) were used to test the continuous and categorical variables between patients and healthy controls. Effects of categorical and continuous variables on GM density have been investigated using one way ANOVAs and multiple regression analysis respectively.
The primary focus of the analyses was the effect of emotional dysregulation on gray matter density as derived from voxel-based morphometry (VBM). Gray matter (GM) density differences were therefore explored among patient subgroups and healthy controls (sED vs. nED, mED vs. nED, mED vs. sED) using whole brain ANOVAs to VBM data. As patients differed from controls in total GM volume and duration of education, we calculated contrasts between patients and controls with whole brain ANCOVAs, including total GM volume and duration of education as covariates (sED vs. healthy controls and all patients vs. healthy controls).
In addition, for an exploratory analysis we split the group of patients presenting with severe emotional dysregulation into two samples according to the pole of Global Affectivity scores. We then explored gray matter density differences between both samples and those patients without emotional dysregulation. First, the sample of patients with severe emotional dysregulation on the positive pole + sED (+ 3, + 2 BPS Global scale scores; n = 8) was contrasted with patients without emotional dysregulation (nED; n = 7) using whole brain ANOVA to VBM data. Second, the sample of patients with severe emotional dysregulation on the negative pole − sED (− 3, − 2 BPS Global scale scores; n = 6) was contrasted with patients without emotional dysregulation (nED) using whole brain ANOVA to VBM data.
The resulting sets of voxels from each contrast represent a statistical parametric map of the t-statistic (SPM-t). We excluded all voxels with gray matter values of less than 0.01 (absolute threshold masking). For all statistical analyses of VBM data, we used a uniform threshold of p < 0.001 (uncorrected) and a minimum cluster size threshold of 17 voxels has been introduced for each contrast. This threshold is equivalent to a map-wise false positive rate of alpha < 0.0001 using a Monte Carlo procedure as implemented in the AlphaSim program in the Analysis of Functional Neuroimages software package (Stepens et al., 2010).
Finally we defined a post hoc region of interest (ROI) using the cluster from the whole brain VBM group comparison (sED vs. nED) including the right ventral striatum extending to the head of the caudate. The ROI data was then extracted with the SPM toolbox MarsBaR (Brett et al., 2002). Gray matter density values of the ROI were extracted from the modulated and normalized GM images for each subject and were compared between the patients with severe emotional dysregulation and no emotional dysregulation.
3. Results
3.1. GM density in patients: severe vs. no emotional dysregulation
The whole brain analysis revealed decreased GM density within a region including the right ventral striatum extending to the head of the caudate in the patients with severe limbic symptoms compared to patients with normal affectivity (sED vs. nED). Local statistical maximum reached a peak p-value of 0.046, FDR-corrected (MNI-coordinates: x = 18, y = − 2, z = 16; peak T-value of 7.01, and MNI-coordinates: x = 12, y = 8, z = 4; peak T-value of 5.63; cluster size of 149 voxels, volume: 1192 mm; x: max/min = 8/20; y: max/min = − 4/12; z: max/min = − 2/18 and at: x = 14, y = 16 and z = 0). Thus, one part of the cluster is located in the ventral striatum and the other posteriorly in the caudate head (see Fig. 1).
Fig. 1.
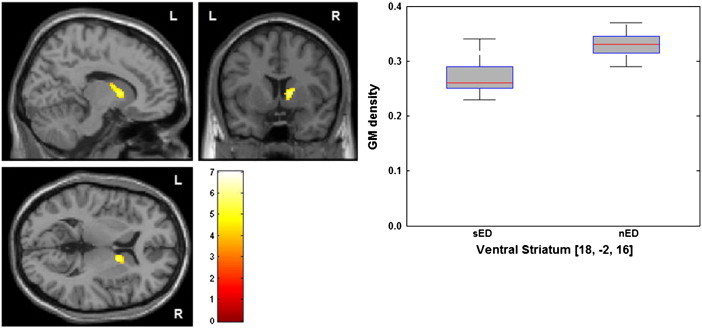
Decreased GM density of the ventral striatum of patients with severe emotional dysregulation. Group map of volumes with lower concentration of GM was statistically thresholded at p < 0.001, uncorrected and displayed on the section of the standard MNI-template; minimum cluster size threshold of 17 voxels. Local difference maxima in the depicted brain region reached a peak p-value of 0.046, FDR-corrected. GM density values in the cluster including the right ventral striatum of patients with severe and no emotional dysregulation. sED: severe emotional dysregulation and nED: no emotional dysregulation.
For an exploratory analysis gray matter density of patients with severe emotional dysregulation of both poles of the BPS vs. no emotional dysregulation was analyzed. To investigate the effect of paranoid threat, we compared − sED patients with nED patients (p < 0.001 uncorrected, minimum cluster size: 17 voxels) and detected gray matter density differences in a cluster including the ventral striatum extending to the head of the caudate (cluster size: 124; cluster peak at MNI-coordinates: x = 18, y = − 2, z = 16, peak T-value = 6.29, and x = 12, y = 8, z = 4, peak T-value = 5.15), as well as in the right orbitofrontal cortex (cluster size: 31; cluster peak at MNI-coordinates: x = 16, y = 50, z = − 8, BA 11). However, the test of the effects of the opposite direction of emotional dysregulation, i.e. grandiosity (+ sED vs. nED), failed to detect significant differences at a threshold of p < 0.001 (uncorrected, minimum cluster size of 17). When contrasting patients with schizophrenia of the two poles of the axis + sED and –sED we found no significant gray matter differences.
Likewise, no differences were detected when comparing patients with mild emotional dysregulation and the other patient groups.
3.2. GM density: severe emotional dysregulation vs. healthy controls
Several clusters of decreased GM density were detected in patients with sED compared to healthy controls (see Table 2 and Fig. 2), including the right ventral striatum and head of the caudate nucleus, bilateral thalamus, dorsolateral prefrontal cortex, orbitofrontal cortex, insula and left hippocampus.
Table 2.
Regions with significant group differences in brain density (sED < healthy controls). The values given are the stereotactic (MNI) coordinates and the T values of each anatomical region. SPM maps were thresholded at p < 0.001 (uncorrected, voxel-level); minimum cluster size threshold of 17 voxels.
| Lobe | Region |
Statistical effects |
|||||
|---|---|---|---|---|---|---|---|
| Brodmann area | Anatomical region | MNI (mm) |
Cluster size (no. of voxels) | Peak T value | |||
| x | y | z | |||||
| Midbrain, thalamus | L thalamus | − 8 | − 14 | 10 | 74 | 3.89 | |
| R thalamus | 12 | − 18 | 12 | 169 | 4.58 | ||
| Frontal lobe, superolateral, prefrontal | BA 10 | L middle frontal gyrus | − 32 | 54 | 0 | 77 | 4.57 |
| BA 10 | L superior frontal gyrus | − 20 | 56 | 20 | 29 | 4.28 | |
| BA 46 | L inferior frontal gyrus | − 50 | 36 | 2 | 74 | 5.16 | |
| BA 46 | R inferior frontal gyrus | 44 | 48 | − 2 | 41 | 3.89 | |
| BA 10 | R middle frontal gyrus | 40 | 44 | 12 | 19 | 3.87 | |
| BA 11 | L middle frontal gyrus | − 24 | 34 | − 20 | 85 | 4.84 | |
| BA 11 | R Middle frontal gyrus | 26 | 36 | − 16 | 72 | 4.19 | |
| BA 11 | L middle frontal gyrus | − 36 | 46 | 18 | 19 | 3.78 | |
| BA 11 | R middle frontal gyrus | 40 | 44 | − 16 | 24 | 4.28 | |
| BA 11 | L Medial orbital gyrus/ | − 6 | 36 | − 26 | 732 | 4.95 | |
| R medial orbital gyrus | 4 | 46 | − 16 | 4.71 | |||
| Frontal lobe, medial/inferior, prefrontal | BA 10 | R medial frontal gyrus | 12 | 62 | − 2 | 17 | 4.07 |
| Limbic lobe | R ventral striatum | 12 | 12 | − 4 | 114 | 3.83 | |
| Caudate nucleus, head | 14 | 14 | 6 | 3.63 | |||
| Hippocampal formation | L hippocampus | − 32 | − 28 | − 8 | 37 | 4.36 | |
| Insular lobe | BA 13 | L insula | − 32 | 22 | 6 | 74 | 3.77 |
| BA 13 | R insula | 36 | 24 | − 2 | 99 | 5.21 | |
BA: Brodmann area; R: right; L: left; MNI: Montreal Neurological Institute; sED: severe emotional dysregulation. Patients with sED, n = 14; healthy controls, n = 34.
Fig. 2.
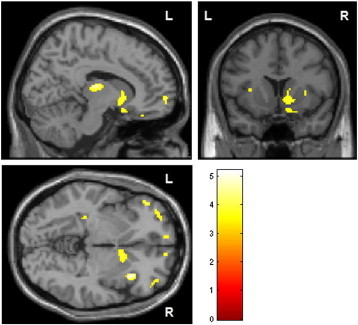
Decreased GM density in patients with severe emotional dysregulation compared to healthy controls. Group map of volumes with lower concentration of GM was statistically thresholded at p < 0.001, uncorrected and displayed on the section of the standard MNI brain; minimum cluster size threshold of 17 voxels.
3.3. GM density: all patients vs. controls
GM density of all patients, irrespective of the psychopathology, was decreased in widespread and well-known brain areas, including the right thalamus, bilateral ventral prefrontal cortex, and left superior temporal gyrus compared to controls (see Table 3 and Fig. 3).
Table 3.
Regions with significant group differences in brain density (all patients < healthy controls). The values given are the stereotactic (MNI) coordinates and the T values of each anatomical region. SPM maps were thresholded at p < 0.001 (uncorrected, voxel-level); minimum cluster size threshold of 17 voxels.
| Lobe | Region |
Statistical effects |
|||||
|---|---|---|---|---|---|---|---|
| Brodmann area | Anatomical region | MNI (mm) |
Cluster size (No. of voxels) | Peak T value | |||
| x | y | z | |||||
| Midbrain, thalamus | R thalamus | 10 | − 16 | 14 | 42 | 3.95 | |
| Frontal lobe | BA 11 | L middle frontal gyrus | − 24 | 34 | − 20 | 49 | 4.28 |
| BA 11 | L orbital gyrus, | − 4 | 40 | − 22 | 118 | 4.21 | |
| R orbital gyrus | 4 | 48 | − 20 | 3.34 | |||
| BA 10 | R medial frontal gyrus | 10 | 60 | 0 | 19 | 3.88 | |
| Temporal lobe | BA 38 | L superior temporal gyrus | − 26 | 14 | − 38 | 327 | 4.19 |
BA: Brodmann area; R: right; L: left; MNI: Montreal Neurological Institute; all patients, n = 43; healthy controls, n = 34. sED: severe emotional dysregulation; nED: no emotional dysregulation. Patients with sED, n = 14; patients with nED: n = 7.
Fig. 3.
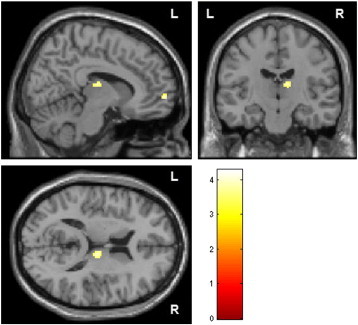
Decreased GM density of all patients compared to healthy controls. Group map of volumes with lower concentration of GM was statistically thresholded at p < 0.001, uncorrected and displayed on the section of the standard MNI brain; minimum cluster size threshold of 17 voxels.
No differences in GM have been found when comparing patients with mild emotional dysregulation and healthy controls. Results of the contrast of patients without emotional dysregulation and healthy controls are given in Supplementary Table B. Using an ANCOVA with total GM volume as covariate to determine the differences across all patient subgroups and healthy controls we found GM density differences in the right thalamus, the bilateral orbitofrontal cortex and a larger cluster including the ventral striatum and the head of the caudate (see Supplementary analysis A, Fig. A, Table C).
In order to rule out the putative effects of medication and total GM volume on our findings, we have provided additional ANCOVAs of the contrasts with chlorpromazine equivalents and total GM volume as covariates, which corroborated the main results (see Supplementary material, analysis B, Table D).
In addition post hoc ANCOVA analyses were performed within the region of interest (ROI) using the cluster from the whole brain VBM comparison (sED vs. nED) including the right ventral striatum. Results supported the main findings (see Supplementary material analysis C and Fig. B).
3.4. Correlations of PANSS scores and BPS Global scores
Using the five-factor model (positive symptoms, negative symptoms, disorganization, excitement, emotional distress) of the PANSS according to van der Gaag et al. (2006) a significant negative correlation of the factor emotional distress and the BPS-Affectivity Global scores (ranging from − 3 to + 3) has been found (r = − 0.38; p = 0.012). No significant correlation between the other factors according to van der Gaag and the BPS-Global scores emerged. Results of an exploratory group analysis of the single PANSS item delusions indicated higher scores for delusion in patients with severe emotional dysregulation (see Supplementary material, analysis D).
4. Discussion
The current study aimed to test whether patients with schizophrenia presenting with severe emotional disturbances would differ in terms of GM density from patients without such emotional disturbances. In line with our hypothesis, patients with schizophrenia with symptoms of severe emotional dysregulation demonstrated structural alterations in the limbic system. Particularly, gray matter density in a larger cluster including the right ventral striatum was decreased compared to patients without emotional dysregulation. Compared to healthy controls patients with emotional dysregulation were found to have decreased GM density in several brain structures associated with emotion processing such as a large cluster including the right ventral striatum and head of the caudate, left hippocampus, bilateral thalamus, dorsolateral prefrontal and orbitofrontal cortex. When we compared the GM density of all schizophrenia patients with that of all healthy controls, the focus on the limbic system was lost, and a pattern of GM deficits consistent with previous studies in schizophrenia was found, including the right thalamus, bilateral ventral prefrontal cortex, and left superior temporal gyrus but not the ventral striatum (Bora et al., 2011; Ellison-Wright and Bullmore, 2010; Ellison-Wright et al., 2008; Glahn et al., 2008; Honea et al., 2008). The main finding suggests severe emotional disturbance in schizophrenia to be particularly associated with reduced GM in a large cluster including the ventral striatum. In fact, this decrease of GM in the limbic system was specific only to severe emotional disturbances in schizophrenia but unrelated to general cerebral changes associated with schizophrenia.
4.1. GM alterations in regions of emotion processing
Compared to healthy controls, in emotionally disturbed patients decreased GM density was found in several brain structures that are associated with emotion processing and reward processing via a limbic cortico–striato–pallido–thalamic circuit that is closely associated with the mesolimbic dopamine pathway (for review see Dichter et al., 2012). Specifically, the dorsolateral prefrontal cortex has consistently been associated with cognitive control processes such as top-down modulation of task-relevant information processing and is considered a key area for the integration of sensory information with behavioral intentions, rules and rewards (Cieslik et al., 2012). The most ventral part of the caudate has traditionally been suggested as part of the limbic system (Alexander et al., 1990) and recently Arsalidou et al. (2012) provided a topographical model suggesting that reward is processed in the anterior caudate head while superior structures in the caudate body and putamen are active during emotion processing (elicitation and perception). Regarding the orbitofrontal cortex there is growing evidence for its critical role in reward processing (Levy and Glimcher, 2012). Thus, the regions with reduced GM density in sED compared to controls have been associated with reward and emotion processing. However, GM reductions in the thalamus and orbitofrontal cortex are less specific as GM was also reduced when comparing all patients vs. controls. Gray matter density reductions of the dorsolateral prefrontal cortex and the hippocampus have been repeatedly reported in patients with schizophrenia independent of specific symptoms (Bora et al., 2011; Ellison-Wright and Bullmore, 2010; Ellison-Wright et al., 2008; Glahn et al., 2008; Honea et al., 2008). Our results therefore corroborate the numerous findings of unspecific GM deficits in schizophrenia, while the larger cluster including the ventral striatum seems to be specifically related to the affectivity dimension. In other words: our focus on emotional dysregulation when assessing state psychopathology allowed for the identification of specific gray matter alterations in a biologically plausible brain system, i.e. the limbic system.
4.2. The ventral striatum and psychotic symptoms
Several studies assessed ventral striatum dopamine dysfunction in schizophrenia (Hietala et al., 1995; Kumakura et al., 2007; Laruelle et al., 1996; McGowan et al., 2004; Meyer-Lindenberg et al., 2002; Reith et al., 1994; Wong et al., 1986). Dopamine dysfunction is one of the core theories that aim to explain the neurobiological correlates of schizophrenia and specific symptoms (Pankow et al., 2012). Specifically increased, chaotic firing of dopaminergic neurons in the ventral striatum may lead to incentive salience to irrelevant stimuli, which is supposed to be involved in the generation of delusional mood and delusions of reference and may thereby lead to suspiciousness, aggressiveness and delusions of persecution (Heinz, 1999; Heinz and Schlagenhauf, 2010; Heinz et al., 1998; Kapur, 2003; Laruelle and Abi-Dargham, 1999; Morrison and Murray, 2009). The applied Bern Psychopathology Scale groups these symptoms into the domain of affectivity, referring to emotional dysregulation and the limbic system (Strik et al., 2010). Therefore, our results of altered GM in the ventral striatum in the patients with severe emotional dysregulation support the notion of an association between ventral striatal dysfunction and delusions in schizophrenia.
4.3. Possible explanations for variance of emotional symptoms
In the light of these previous findings, our results can be interpreted as an indication of a structural pathology in a key region involved in emotion processing, which may have a crucial role in triggering psychotic symptoms with high emotional valence in both positive (e.g. grandiosity) and negative (e.g. persecutory delusions) directions.
Further studies need to demonstrate whether the detected structural differences for both poles of the axis may reflect the “trait” of the neuronal system while the direction (paranoid threat vs. grandiosity) may possibly reflect the systems' “state”, if we assume that the affective content may change over time. Marneros et al. (1992) found that most of the schizophrenia patients (76%) presented a bimorphous course i.e. showing positive and negative symptomatology according to the Andreasen's criteria of positive and negative and mixed symptomatology during the course of the illness. Likewise, different emotional contents of both poles (paranoid threat and grandiosity) may be present at the same time. Garety et al. (2013) reported considerable overlap of grandiosity and persecutory delusions in the same patients. When we explored the impact of the direction of severe emotional dysregulation on brain structure, we found reduced gray matter density in a region including the ventral striatum and the orbitofrontal cortex exclusively for the negative pole (− sED), i.e. threat. However, this exploratory finding has to be interpreted with caution given the limitations of such analyses.
We may hypothesize that alterations in striatal GM may represent structural changes following specific severe symptoms. In fact, several studies described activity-dependent selective changes in gray matter (Draganski et al., 2004; Maguire et al., 2000). These structural changes may occur even after short time periods (May and Gaser, 2006; May et al., 2007). For instance symptoms such as delusions are likely to cause structural changes as they seem to persist in most patients over at least ten weeks (Appelbaum et al., 2004). Thus, the major structural finding of decreased ventral striatal gray matter density in our study could reflect structural adaption to the present symptoms of emotional dysregulation. On the other hand, structural alterations may cause the symptoms that we now associated with structural changes in this cross-sectional study. However, longitudinal studies are necessary to clarify whether the structural alterations in the ventral striatum vary over time and whether different changes are found during the course of the disease (Howes and Kapur, 2009).
4.4. Laterality of GM alterations of the ventral striatum
Emotional processing is considered to be lateralized (attributed) to the non-dominant hemisphere. In addition, right/left asymmetry of dopamine deficits may impact emotion processing in patients with schizophrenia (Hietala et al., 1999; Morris et al., 2012; Murray et al., 2008). Subcortical structures of the limbic system such as the hippocampus, the amygdala and the habenula display some degree of structural asymmetry (Pedraza et al., 2004; Shi et al., 2009). These asymmetries of the human brain were interpreted as hemispheric specialization. The description of deviation from such a functional asymmetry may help to understand the pathophysiology of patients with schizophrenia. For instance abnormal asymmetry has been proposed as a risk factor, genetic marker and possible endophenotype (Woolard and Heckers, 2012). The structural changes in patients with severe emotional dysregulation in our study were present unilaterally in the right ventral striatum. Likewise, Hietala et al. (1999) specifically observed more pronounced presynaptic dopamine dysfunction and paranoid symptoms correlated more markedly with right hemispheric changes. These are interesting results since they correlate abnormal lateralization and specific psychotic symptoms. The direction of association, however, remains unclear. Lateralization may be a cause or consequence of some psychotic symptoms that may provoke specific psychotic symptoms. On the other hand it can be hypothesized that psychotic symptoms may be triggered by abnormal functional or structural lateralization.
4.5. Strengths and limitations
The strength of the study is that patients were specifically grouped according to clear-cut symptoms of emotional dysregulation (patients with severe emotional dysregulation and patients without emotional dysregulation). The patient subgroups did not differ in duration of illness, chlorpromazine-equivalents, number of episodes, age of onset or PANSS ratings (positive, negative or total). Therefore, the structural findings in the limbic system are likely to be related specifically to the presence of severe emotional dysregulation rather than to general clinical differences. A possible limitation of the study is an effect of current and past antipsychotic treatment, which may affect brain structure. There is an ongoing discussion on the influence of medication on gray matter volume. In particular, typical versus atypical antipsychotics, dosage and treatment duration have been hypothesized as partly responsible for brain volume changes (Navari and Dazzan, 2009). With respect to subcortical structures, volumes of the thalamus and putamen were detected as positively correlated with the degree of positive symptoms in unmedicated patients with schizophrenia (Gur et al., 1998; Rao et al., 2010). These findings are particularly important since they point to subcortical volume changes independent of treatment and possibly dependent of psychopathology. However, in our study, all patients were on a stable medication with first- or second-generation antipsychotics, except for three patients who were drug free. Further, antipsychotic dose neither differed between the compared patient subgroups, nor affected the main finding when entered as a covariate. Diagnoses were given after thorough clinical psychiatric examination and chart review; however, SCID was not applied.
4.6. Conclusion
Using the BPS affectivity domain, we identified a subgroup of patients with schizophrenia with severe emotional disturbances. On a structural level, this group had particular deficits in the ventral striatum, which was absent in the other subgroup without emotional dysregulation. The results intriguingly provide further evidence that the biological and clinical heterogeneity of schizophrenia can be disentangled by specific symptom dimensions. As previously shown in the case of hallucinations, formal thought (Horn et al., 2010; Hubl et al., 2004; Strik et al., 2008) and movement disorders (Bracht et al., 2012; Walther and Strik, 2012; Walther et al., 2011), this strategy helps to find meaningful links of psychopathological features with specific, well known brain systems. The present study suggests that structural deficits in the ventral striatum contribute to severe emotional dysregulation in schizophrenia.
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike License, which permits non-commercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
Appendix A. Supplementary data
Supplementary material.
References
- Abi-Dargham A. Increased baseline occupancy of D2 receptors by dopamine in schizophrenia. Proc. Natl. Acad. Sci. U. S. A. 2000;97(14):8104–8109. doi: 10.1073/pnas.97.14.8104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aleman A., Kahn R.S. Strange feelings: do amygdala abnormalities dysregulate the emotional brain in schizophrenia? Prog. Neurobiol. 2005;77(5):283–298. doi: 10.1016/j.pneurobio.2005.11.005. [DOI] [PubMed] [Google Scholar]
- Alexander G.E., Crutcher M.D., DeLong M.R. Basal ganglia–thalamocortical circuits: parallel substrates for motor, oculomotor, “prefrontal” and “limbic” functions. Prog. Brain Res. 1990;85:119–146. [PubMed] [Google Scholar]
- Appelbaum P.S., Robbins P.C., Vesselinov R. Persistence and stability of delusions over time. Compr. Psychiatry. 2004;45(5):317–324. doi: 10.1016/j.comppsych.2004.06.001. [DOI] [PubMed] [Google Scholar]
- Arsalidou M., Duerden E.G., Taylor M.J. The centre of the brain: topographical model of motor, cognitive, affective, and somatosensory functions of the basal ganglia. Hum. Brain Mapp. 2013;34:3031–3054. doi: 10.1002/hbm.22124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barta P.E., Pearlson G.D., Powers R.E., Richards S.S., Tune L.E. Auditory hallucinations and smaller superior temporal gyral volume in schizophrenia. Am. J. Psychiatry. 1990;147(11):1457–1462. doi: 10.1176/ajp.147.11.1457. [DOI] [PubMed] [Google Scholar]
- Bora E. Neuroanatomical abnormalities in schizophrenia: a multimodal voxelwise meta-analysis and meta-regression analysis. Schizophr. Res. 2011;127(1–3):46–57. doi: 10.1016/j.schres.2010.12.020. [DOI] [PubMed] [Google Scholar]
- Bracht T. Comparison of objectively measured motor behavior with ratings of the motor behavior domain of the Bern Psychopathology Scale (BPS) in schizophrenia. Psychiatry Res. 2012;198(2):224–229. doi: 10.1016/j.psychres.2011.12.038. [DOI] [PubMed] [Google Scholar]
- Brett Matthew, Anton Jean-Luc, Valabregue Romain, Poline Jean-Baptiste. Region of interest analysis using an SPM toolbox [abstract] Presented at the 8th International Conference on Functional Mapping of the Human Brain; Sendai: June 2-6, 2002. Vol 16, No 2. [Google Scholar]
- Brunet-Gouet E., Decety J. Social brain dysfunctions in schizophrenia: a review of neuroimaging studies. Psychiatry Res. 2006;148(2–3):75–92. doi: 10.1016/j.pscychresns.2006.05.001. [DOI] [PubMed] [Google Scholar]
- Cieslik E.C. Is there “one” DLPFC in cognitive action control? Evidence for heterogeneity from co-activation-based parcellation. Cereb. Cortex. 2013;23(11):2677–2689. doi: 10.1093/cercor/bhs256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deichmann R., Schwarzbauer C., Turner R. Optimisation of the 3D MDEFT sequence for anatomical brain imaging: technical implications at 1.5 and 3T. Neuroimage. 2004;21(2):757–767. doi: 10.1016/j.neuroimage.2003.09.062. [DOI] [PubMed] [Google Scholar]
- Dichter G.S., Damiano C.A., Allen J.A. Reward circuitry dysfunction in psychiatric and neurodevelopmental disorders and genetic syndromes: animal models and clinical findings. J. Neurodev. Disord. 2012;4(1):19. doi: 10.1186/1866-1955-4-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draganski B. Neuroplasticity: changes in grey matter induced by training. Nature. 2004;427(6972):311–312. doi: 10.1038/427311a. [DOI] [PubMed] [Google Scholar]
- Ellison-Wright I., Bullmore E. Anatomy of bipolar disorder and schizophrenia: a meta-analysis. Schizophr. Res. 2010;117(1):1–12. doi: 10.1016/j.schres.2009.12.022. [DOI] [PubMed] [Google Scholar]
- Ellison-Wright I., Glahn D.C., Laird A.R., Thelen S.M., Bullmore E. The anatomy of first-episode and chronic schizophrenia: an anatomical likelihood estimation meta-analysis. Am. J. Psychiatry. 2008;165(8):1015–1023. doi: 10.1176/appi.ajp.2008.07101562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman D., Garety P.A. Connecting neurosis and psychosis: the direct influence of emotion on delusions and hallucinations. Behav. Res. Ther. 2003;41(8):923–947. doi: 10.1016/s0005-7967(02)00104-3. [DOI] [PubMed] [Google Scholar]
- Garety P.A. Differences in cognitive and emotional processes between persecutory and grandiose delusions. Schizophr. Bull. 2013;39(3):629–639. doi: 10.1093/schbul/sbs059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaser C., Nenadic I., Buchsbaum B.R., Hazlett E.A., Buchsbaum M.S. Ventricular enlargement in schizophrenia related to volume reduction of the thalamus, striatum, and superior temporal cortex. Am. J. Psychiatry. 2004;161(1):154–156. doi: 10.1176/appi.ajp.161.1.154. [DOI] [PubMed] [Google Scholar]
- Glahn D.C. Meta-analysis of gray matter anomalies in schizophrenia: application of anatomic likelihood estimation and network analysis. Biol. Psychiatry. 2008;64(9):774–781. doi: 10.1016/j.biopsych.2008.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gur R.E. Subcortical MRI volumes in neuroleptic-naive and treated patients with schizophrenia. Am. J. Psychiatry. 1998;155(12):1711–1717. doi: 10.1176/ajp.155.12.1711. [DOI] [PubMed] [Google Scholar]
- Haijma S.V. Brain volumes in schizophrenia: a meta-analysis in over 18 000 subjects. Schizophr. Bull. 2013;39(5):1129–1138. doi: 10.1093/schbul/sbs118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckers S. Bleuler and the neurobiology of schizophrenia. Schizophr. Bull. 2011;37(6):1131–1135. doi: 10.1093/schbul/sbr108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinz A. Psychopathological correlates of dopaminergic dysfunction in alcoholic and schizophrenic patients. Nervenarzt. 1999;70(5):399–407. doi: 10.1007/s001150050455. [DOI] [PubMed] [Google Scholar]
- Heinz A., Schlagenhauf F. Dopaminergic dysfunction in schizophrenia: salience attribution revisited. Schizophr. Bull. 2010;36(3):472–485. doi: 10.1093/schbul/sbq031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinz A. Psychomotor slowing, negative symptoms and dopamine receptor availability—an IBZM SPECT study in neuroleptic-treated and drug-free schizophrenic patients. Schizophr. Res. 1998;31(1):19–26. doi: 10.1016/s0920-9964(98)00003-6. [DOI] [PubMed] [Google Scholar]
- Hietala J. Presynaptic dopamine function in striatum of neuroleptic-naive schizophrenic patients. Lancet. 1995;346(8983):1130–1131. doi: 10.1016/s0140-6736(95)91801-9. [DOI] [PubMed] [Google Scholar]
- Hietala J. Depressive symptoms and presynaptic dopamine function in neuroleptic-naive schizophrenia. Schizophr. Res. 1999;35(1):41–50. doi: 10.1016/s0920-9964(98)00113-3. [DOI] [PubMed] [Google Scholar]
- Honea R.A. Is gray matter volume an intermediate phenotype for schizophrenia? A voxel-based morphometry study of patients with schizophrenia and their healthy siblings. Biol. Psychiatry. 2008;63(5):465–474. doi: 10.1016/j.biopsych.2007.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn H. Structural and metabolic changes in language areas linked to formal thought disorder. Br. J. Psychiatry. 2009;194(2):130–138. doi: 10.1192/bjp.bp.107.045633. [DOI] [PubMed] [Google Scholar]
- Horn H. Gray matter volume differences specific to formal thought disorder in schizophrenia. Psychiatry Res. 2010;182(2):183–186. doi: 10.1016/j.pscychresns.2010.01.016. [DOI] [PubMed] [Google Scholar]
- Howes O.D., Kapur S. The dopamine hypothesis of schizophrenia: version III—the final common pathway. Schizophr. Bull. 2009;35(3):549–562. doi: 10.1093/schbul/sbp006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubl D. Pathways that make voices: white matter changes in auditory hallucinations. Arch. Gen. Psychiatry. 2004;61(7):658–668. doi: 10.1001/archpsyc.61.7.658. [DOI] [PubMed] [Google Scholar]
- Kapur S. Psychosis as a state of aberrant salience: a framework linking biology, phenomenology, and pharmacology in schizophrenia. Am. J. Psychiatry. 2003;160(1):13–23. doi: 10.1176/appi.ajp.160.1.13. [DOI] [PubMed] [Google Scholar]
- Kay S.R., Fiszbein A., Opler L.A. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr. Bull. 1987;13(2):261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- Kee K.S., Green M.F., Mintz J., Brekke J.S. Is emotion processing a predictor of functional outcome in schizophrenia? Schizophr. Bull. 2003;29(3):487–497. doi: 10.1093/oxfordjournals.schbul.a007021. [DOI] [PubMed] [Google Scholar]
- Kirsch P., Ronshausen S., Mier D., Gallhofer B. The influence of antipsychotic treatment on brain reward system reactivity in schizophrenia patients. Pharmacopsychiatry. 2007;40(5):196–198. doi: 10.1055/s-2007-984463. [DOI] [PubMed] [Google Scholar]
- Kumakura Y. Elevated [18F]fluorodopamine turnover in brain of patients with schizophrenia: an [18F]fluorodopa/positron emission tomography study. J. Neurosci. 2007;27(30):8080–8087. doi: 10.1523/JNEUROSCI.0805-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laruelle M., Abi-Dargham A. Dopamine as the wind of the psychotic fire: new evidence from brain imaging studies. J. Psychopharmacol. 1999;13(4):358–371. doi: 10.1177/026988119901300405. [DOI] [PubMed] [Google Scholar]
- Laruelle M. Single photon emission computerized tomography imaging of amphetamine-induced dopamine release in drug-free schizophrenic subjects. Proc. Natl. Acad. Sci. U. S. A. 1996;93(17):9235–9240. doi: 10.1073/pnas.93.17.9235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy D.J., Glimcher P.W. The root of all value: a neural common currency for choice. Curr. Opin. Neurobiol. 2012;22(6):1027–1038. doi: 10.1016/j.conb.2012.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire E.A. Navigation-related structural change in the hippocampi of taxi drivers. Proc. Natl. Acad. Sci. U. S. A. 2000;97(8):4398–4403. doi: 10.1073/pnas.070039597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marneros A., Deister A., Rohde A. Validity of the negative/positive dichotomy for schizophrenic disorders under long-term conditions. Schizophr. Res. 1992;7(2):117–123. doi: 10.1016/0920-9964(92)90041-3. [DOI] [PubMed] [Google Scholar]
- May A., Gaser C. Magnetic resonance-based morphometry: a window into structural plasticity of the brain. Curr. Opin. Neurol. 2006;19(4):407–411. doi: 10.1097/01.wco.0000236622.91495.21. [DOI] [PubMed] [Google Scholar]
- May A. Structural brain alterations following 5 days of intervention: dynamic aspects of neuroplasticity. Cereb. Cortex. 2007;17(1):205–210. doi: 10.1093/cercor/bhj138. [DOI] [PubMed] [Google Scholar]
- McCarley R.W. Auditory P300 abnormalities and left posterior superior temporal gyrus volume reduction in schizophrenia. Arch. Gen. Psychiatry. 1993;50(3):190–197. doi: 10.1001/archpsyc.1993.01820150036003. [DOI] [PubMed] [Google Scholar]
- McGowan S., Lawrence A.D., Sales T., Quested D., Grasby P. Presynaptic dopaminergic dysfunction in schizophrenia: a positron emission tomographic [18F]fluorodopa study. Arch. Gen. Psychiatry. 2004;61(2):134–142. doi: 10.1001/archpsyc.61.2.134. [DOI] [PubMed] [Google Scholar]
- Meyer-Lindenberg A. Reduced prefrontal activity predicts exaggerated striatal dopaminergic function in schizophrenia. Nat. Neurosci. 2002;5(3):267–271. doi: 10.1038/nn804. [DOI] [PubMed] [Google Scholar]
- Morris R.W. Disambiguating ventral striatum fMRI-related BOLD signal during reward prediction in schizophrenia. Mol. Psychiatry. 2012;17(3):280–289. doi: 10.1038/mp.2011.75. (235) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison P.D., Murray R.M. From real-world events to psychosis: the emerging neuropharmacology of delusions. Schizophr. Bull. 2009;35(4):668–674. doi: 10.1093/schbul/sbp049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray G.K. Substantia nigra/ventral tegmental reward prediction error disruption in psychosis. Mol. Psychiatry. 2008;13(3):267–276. doi: 10.1038/sj.mp.4002058. (239) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navari S., Dazzan P. Do antipsychotic drugs affect brain structure? A systematic and critical review of MRI findings. Psychol. Med. 2009;39(11):1763–1777. doi: 10.1017/S0033291709005315. [DOI] [PubMed] [Google Scholar]
- Nenadic I., Sauer H., Gaser C. Distinct pattern of brain structural deficits in subsyndromes of schizophrenia delineated by psychopathology. Neuroimage. 2010;49(2):1153–1160. doi: 10.1016/j.neuroimage.2009.10.014. [DOI] [PubMed] [Google Scholar]
- Nestor P.G. Dissociable contributions of MRI volume reductions of superior temporal and fusiform gyri to symptoms and neuropsychology in schizophrenia. Schizophr. Res. 2007;91(1–3):103–106. doi: 10.1016/j.schres.2006.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pankow A., Knobel A., Voss M., Heinz A. Neurobiological correlates of delusion: beyond the salience attribution hypothesis. Neuropsychobiology. 2012;66(1):33–43. doi: 10.1159/000337132. [DOI] [PubMed] [Google Scholar]
- Pankow A. Altered amygdala activation in schizophrenia patients during emotion processing. Schizophr. Res. 2013;150(1):101–106. doi: 10.1016/j.schres.2013.07.015. [DOI] [PubMed] [Google Scholar]
- Pedraza O., Bowers D., Gilmore R. Asymmetry of the hippocampus and amygdala in MRI volumetric measurements of normal adults. J. Int. Neuropsychol. Soc. 2004;10(5):664–678. doi: 10.1017/S1355617704105080. [DOI] [PubMed] [Google Scholar]
- Phillips M.L., Drevets W.C., Rauch S.L., Lane R. Neurobiology of emotion perception II: implications for major psychiatric disorders. Biol. Psychiatry. 2003;54(5):515–528. doi: 10.1016/s0006-3223(03)00171-9. [DOI] [PubMed] [Google Scholar]
- Rao N.P., Kalmady S., Arasappa R., Venkatasubramanian G. Clinical correlates of thalamus volume deficits in anti-psychotic-naive schizophrenia patients: a 3-Tesla MRI study. Indian J. Psychiatry. 2010;52(3):229–235. doi: 10.4103/0019-5545.70975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reith J. Elevated dopa decarboxylase activity in living brain of patients with psychosis. Proc. Natl. Acad. Sci. U. S. A. 1994;91(24):11651–11654. doi: 10.1073/pnas.91.24.11651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlagenhauf F. Reward system activation in schizophrenic patients switched from typical neuroleptics to olanzapine. Psychopharmacology (Berl) 2008;196(4):673–684. doi: 10.1007/s00213-007-1016-4. [DOI] [PubMed] [Google Scholar]
- Shenton M.E. Abnormalities of the left temporal lobe and thought disorder in schizophrenia. A quantitative magnetic resonance imaging study. N. Engl. J. Med. 1992;327(9):604–612. doi: 10.1056/NEJM199208273270905. [DOI] [PubMed] [Google Scholar]
- Shi F., Liu B., Zhou Y., Yu C., Jiang T. Hippocampal volume and asymmetry in mild cognitive impairment and Alzheimer's disease: meta-analyses of MRI studies. Hippocampus. 2009;19(11):1055–1064. doi: 10.1002/hipo.20573. [DOI] [PubMed] [Google Scholar]
- Spencer M.D. Grey matter correlates of early psychotic symptoms in adolescents at enhanced risk of psychosis: a voxel-based study. Neuroimage. 2007;35(3):1181–1191. doi: 10.1016/j.neuroimage.2007.01.008. [DOI] [PubMed] [Google Scholar]
- Stepens A. White matter abnormalities in methcathinone abusers with an extrapyramidal syndrome. Brain. 2010;133:3676–3684. doi: 10.1093/brain/awq281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strik W., Dierks T., Hubl D., Horn H. Hallucinations, thought disorders, and the language domain in schizophrenia. Clin. EEG Neurosci. 2008;39(2):91–94. doi: 10.1177/155005940803900214. [DOI] [PubMed] [Google Scholar]
- Strik W. The Bern psychopathology scale for the assessment of system-specific psychotic symptoms. Neuropsychobiology. 2010;61(4):197–209. doi: 10.1159/000297737. [DOI] [PubMed] [Google Scholar]
- Taylor S.F., Liberzon I. Neural correlates of emotion regulation in psychopathology. Trends Cogn. Sci. 2007;11(10):413–418. doi: 10.1016/j.tics.2007.08.006. [DOI] [PubMed] [Google Scholar]
- van der Gaag M. The five-factor model of the Positive and Negative Syndrome Scale II: a ten-fold cross-validation of a revised model. Schizophr. Res. 2006;85(1–3):280–287. doi: 10.1016/j.schres.2006.03.021. [DOI] [PubMed] [Google Scholar]
- Walter H., Kammerer H., Frasch K., Spitzer M., Abler B. Altered reward functions in patients on atypical antipsychotic medication in line with the revised dopamine hypothesis of schizophrenia. Psychopharmacology (Berl) 2009;206(1):121–132. doi: 10.1007/s00213-009-1586-4. [DOI] [PubMed] [Google Scholar]
- Walther S., Strik W. Motor symptoms and schizophrenia. Neuropsychobiology. 2012;66(2):77–92. doi: 10.1159/000339456. [DOI] [PubMed] [Google Scholar]
- Walther S. Alterations of white matter integrity related to motor activity in schizophrenia. Neurobiol. Dis. 2011;42(3):276–283. doi: 10.1016/j.nbd.2011.01.017. [DOI] [PubMed] [Google Scholar]
- Wong D.F. Positron emission tomography reveals elevated D2 dopamine receptors in drug-naive schizophrenics. Science. 1986;234(4783):1558–1563. doi: 10.1126/science.2878495. [DOI] [PubMed] [Google Scholar]
- Woolard A.A., Heckers S. Anatomical and functional correlates of human hippocampal volume asymmetry. Psychiatry Res. 2012;201(1):48–53. doi: 10.1016/j.pscychresns.2011.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material.


