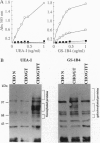
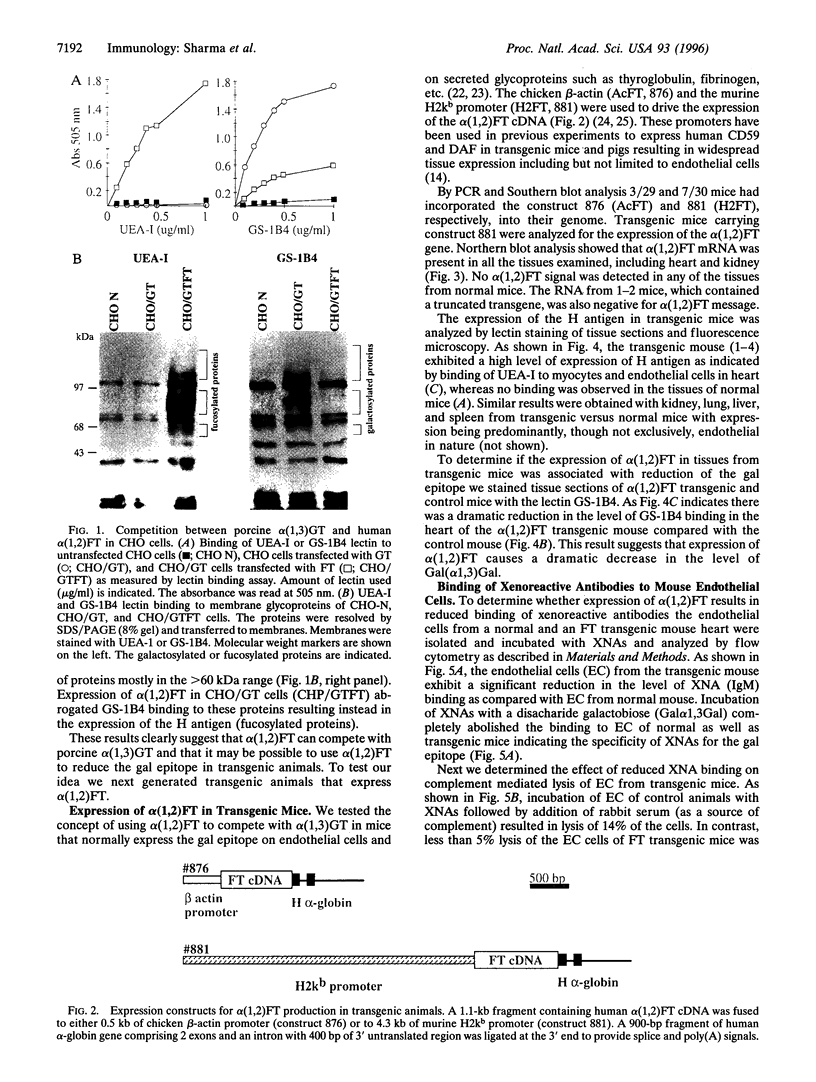
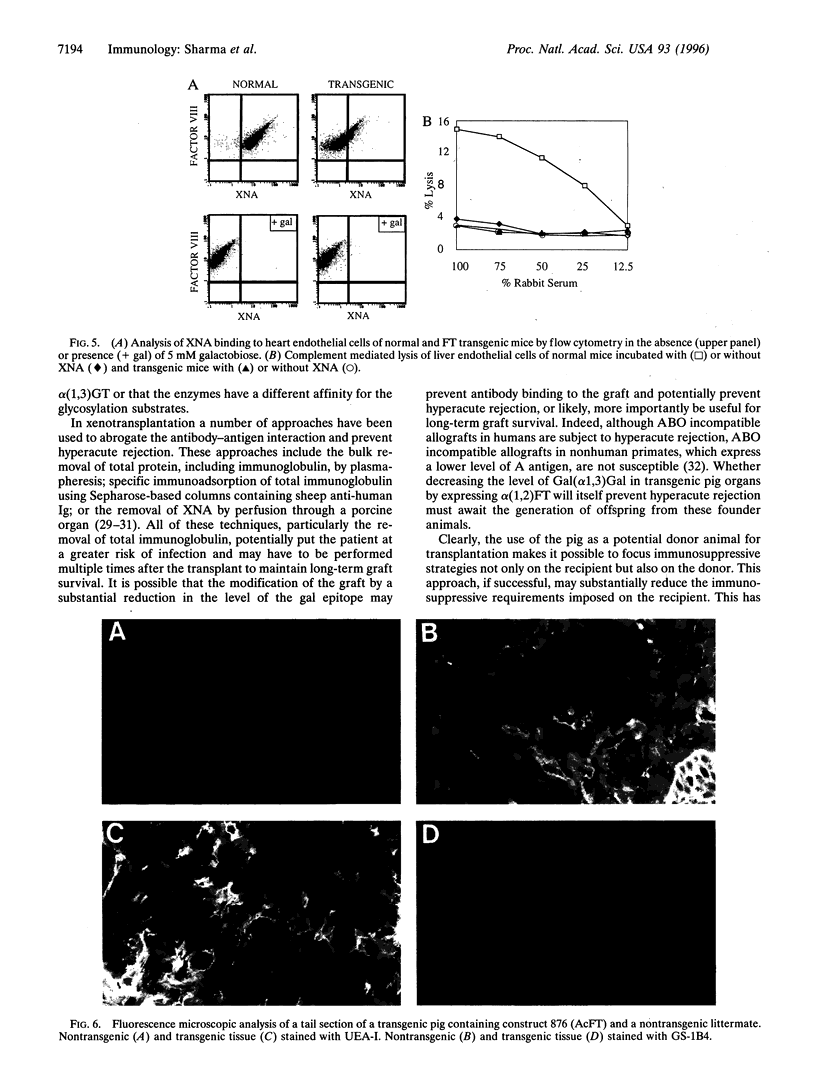
Abstract
Hyperacute rejection of a porcine organ by higher primates is initiated by the binding of xenoreactive natural antibodies of the recipient to blood vessels in the graft leading to complement activation. The majority of these antibodies recognize the carbohydrate structure Gal(alphal,3)Gal (gal epitope) present on cells of pigs. It is possible that the removal or lowering of the number of gal epitopes on the graft endothelium could prevent hyperacute rejection. The Gal(alpha1,3) Gal structure is formed by the enzyme Galbeta1,4GlcNAc3-alpha-D-galactosyltransferase [alpha(1,3)GT; EC 2.4.1.51], which transfers a galactose molecule to terminal N-acetyllactosamine (N-lac) present on various glycoproteins and glycolipids. The N-lac structure might be utilized as an acceptor by other glycosyltransferases such as Galbeta1,4GlcNAc 6-alpha-D-sialyltransferase [alpha(2,6)ST], Galbeta1,4GlcNAc 3-alpha-D-Sialyltransferase [alpha(2,3)ST], or Galbeta 2-alpha-L-fucosyltransferase [alpha(1,2)FT; EC 2.4.1.691, etc. In this report we describe the competition between alpha(1,2)FT and alpha(1,3)GT in cells in culture and the generation of transgenic mice and transgenic pigs that express alpha(1,2)Fr leading to synthesis of Fucalpha,2Galbeta- (H antigen) and a concomitant decrease in the level of Gal(alpha1,3)Gal. As predicted, this resulted in reduced binding of xenoreactive natural antibodies to endothelial cells of transgenic mice and protection from complement mediated lysis.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blanken W. M., Van den Eijnden D. H. Biosynthesis of terminal Gal alpha 1----3Gal beta 1----4GlcNAc-R oligosaccharide sequences on glycoconjugates. Purification and acceptor specificity of a UDP-Gal:N-acetyllactosaminide alpha 1----3-galactosyltransferase from calf thymus. J Biol Chem. 1985 Oct 25;260(24):12927–12934. [PubMed] [Google Scholar]
- Byrne G. W., McCurry K. R., Kagan D., Quinn C., Martin M. J., Platt J. L., Logan J. S. Protection of xenogeneic cardiac endothelium from human complement by expression of CD59 or DAF in transgenic mice. Transplantation. 1995 Nov 27;60(10):1149–1156. doi: 10.1097/00007890-199511270-00016. [DOI] [PubMed] [Google Scholar]
- Chang K. S., Zimmer W. E., Jr, Bergsma D. J., Dodgson J. B., Schwartz R. J. Isolation and characterization of six different chicken actin genes. Mol Cell Biol. 1984 Nov;4(11):2498–2508. doi: 10.1128/mcb.4.11.2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper D. K., Koren E., Oriol R. Oligosaccharides and discordant xenotransplantation. Immunol Rev. 1994 Oct;141:31–58. doi: 10.1111/j.1600-065x.1994.tb00871.x. [DOI] [PubMed] [Google Scholar]
- Dalmasso A. P., Vercellotti G. M., Fischel R. J., Bolman R. M., Bach F. H., Platt J. L. Mechanism of complement activation in the hyperacute rejection of porcine organs transplanted into primate recipients. Am J Pathol. 1992 May;140(5):1157–1166. [PMC free article] [PubMed] [Google Scholar]
- Fodor W. L., Williams B. L., Matis L. A., Madri J. A., Rollins S. A., Knight J. W., Velander W., Squinto S. P. Expression of a functional human complement inhibitor in a transgenic pig as a model for the prevention of xenogeneic hyperacute organ rejection. Proc Natl Acad Sci U S A. 1994 Nov 8;91(23):11153–11157. doi: 10.1073/pnas.91.23.11153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galili U., Clark M. R., Shohet S. B., Buehler J., Macher B. A. Evolutionary relationship between the natural anti-Gal antibody and the Gal alpha 1----3Gal epitope in primates. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1369–1373. doi: 10.1073/pnas.84.5.1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galili U., Shohet S. B., Kobrin E., Stults C. L., Macher B. A. Man, apes, and Old World monkeys differ from other mammals in the expression of alpha-galactosyl epitopes on nucleated cells. J Biol Chem. 1988 Nov 25;263(33):17755–17762. [PubMed] [Google Scholar]
- Good A. H., Cooper D. K., Malcolm A. J., Ippolito R. M., Koren E., Neethling F. A., Ye Y., Zuhdi N., Lamontagne L. R. Identification of carbohydrate structures that bind human antiporcine antibodies: implications for discordant xenografting in humans. Transplant Proc. 1992 Apr;24(2):559–562. [PubMed] [Google Scholar]
- Hanasaki K., Varki A., Stamenkovic I., Bevilacqua M. P. Cytokine-induced beta-galactoside alpha-2,6-sialyltransferase in human endothelial cells mediates alpha 2,6-sialylation of adhesion molecules and CD22 ligands. J Biol Chem. 1994 Apr 8;269(14):10637–10643. [PubMed] [Google Scholar]
- Ikematsu S., Kaname T., Ozawa M., Yonezawa S., Sato E., Uehara F., Obama H., Yamamura K., Muramatsu T. Transgenic mouse lines with ectopic expression of alpha-1,3-galactosyltransferase: production and characteristics. Glycobiology. 1993 Dec;3(6):575–580. doi: 10.1093/glycob/3.6.575. [DOI] [PubMed] [Google Scholar]
- Joziasse D. H., Shaper J. H., Van den Eijnden D. H., Van Tunen A. J., Shaper N. L. Bovine alpha 1----3-galactosyltransferase: isolation and characterization of a cDNA clone. Identification of homologous sequences in human genomic DNA. J Biol Chem. 1989 Aug 25;264(24):14290–14297. [PubMed] [Google Scholar]
- Kimura A., Israël A., Le Bail O., Kourilsky P. Detailed analysis of the mouse H-2Kb promoter: enhancer-like sequences and their role in the regulation of class I gene expression. Cell. 1986 Jan 31;44(2):261–272. doi: 10.1016/0092-8674(86)90760-9. [DOI] [PubMed] [Google Scholar]
- Kroshus T. J., Dalmasso A. P., Leventhal J. R., John R., Matas A. J., Bolman R. M., 3rd Antibody removal by column immunoabsorption prevents tissue injury in an ex vivo model of pig-to-human xenograft hyperacute rejection. J Surg Res. 1995 Jul;59(1):43–50. doi: 10.1006/jsre.1995.1130. [DOI] [PubMed] [Google Scholar]
- Larsen R. D., Ernst L. K., Nair R. P., Lowe J. B. Molecular cloning, sequence, and expression of a human GDP-L-fucose:beta-D-galactoside 2-alpha-L-fucosyltransferase cDNA that can form the H blood group antigen. Proc Natl Acad Sci U S A. 1990 Sep;87(17):6674–6678. doi: 10.1073/pnas.87.17.6674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen R. D., Rivera-Marrero C. A., Ernst L. K., Cummings R. D., Lowe J. B. Frameshift and nonsense mutations in a human genomic sequence homologous to a murine UDP-Gal:beta-D-Gal(1,4)-D-GlcNAc alpha(1,3)-galactosyltransferase cDNA. J Biol Chem. 1990 Apr 25;265(12):7055–7061. [PubMed] [Google Scholar]
- Looker D., Mathews A. J., Neway J. O., Stetler G. L. Expression of recombinant human hemoglobin in Escherichia coli. Methods Enzymol. 1994;231:364–374. doi: 10.1016/0076-6879(94)31025-4. [DOI] [PubMed] [Google Scholar]
- Lowe J. B. Molecular cloning, expression, and uses of mammalian glycosyltransferases. Semin Cell Biol. 1991 Oct;2(5):289–307. [PubMed] [Google Scholar]
- McCurry K. R., Kooyman D. L., Alvarado C. G., Cotterell A. H., Martin M. J., Logan J. S., Platt J. L. Human complement regulatory proteins protect swine-to-primate cardiac xenografts from humoral injury. Nat Med. 1995 May;1(5):423–427. doi: 10.1038/nm0595-423. [DOI] [PubMed] [Google Scholar]
- Oriol R., Mollicone R., Coullin P., Dalix A. M., Candelier J. J. Genetic regulation of the expression of ABH and Lewis antigens in tissues. APMIS Suppl. 1992;27:28–38. [PubMed] [Google Scholar]
- Oriol R., Ye Y., Koren E., Cooper D. K. Carbohydrate antigens of pig tissues reacting with human natural antibodies as potential targets for hyperacute vascular rejection in pig-to-man organ xenotransplantation. Transplantation. 1993 Dec;56(6):1433–1442. doi: 10.1097/00007890-199312000-00031. [DOI] [PubMed] [Google Scholar]
- Paulson J. C., Colley K. J. Glycosyltransferases. Structure, localization, and control of cell type-specific glycosylation. J Biol Chem. 1989 Oct 25;264(30):17615–17618. [PubMed] [Google Scholar]
- Platt J. L., Fischel R. J., Matas A. J., Reif S. A., Bolman R. M., Bach F. H. Immunopathology of hyperacute xenograft rejection in a swine-to-primate model. Transplantation. 1991 Aug;52(2):214–220. doi: 10.1097/00007890-199108000-00006. [DOI] [PubMed] [Google Scholar]
- Platt J. L., Holzknecht Z. E. Porcine platelet antigens recognized by human xenoreactive natural antibodies. Transplantation. 1994 Feb;57(3):327–335. doi: 10.1097/00007890-199402150-00003. [DOI] [PubMed] [Google Scholar]
- Platt J. L., LeBien T. W., Michael A. F. Interstitial mononuclear cell populations in renal graft rejection. Identification by monoclonal antibodies in tissue sections. J Exp Med. 1982 Jan 1;155(1):17–30. doi: 10.1084/jem.155.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platt J. L., Lindman B. J., Chen H., Spitalnik S. L., Bach F. H. Endothelial cell antigens recognized by xenoreactive human natural antibodies. Transplantation. 1990 Nov;50(5):817–822. doi: 10.1097/00007890-199011000-00015. [DOI] [PubMed] [Google Scholar]
- Sandrin M. S., Fodor W. L., Mouhtouris E., Osman N., Cohney S., Rollins S. A., Guilmette E. R., Setter E., Squinto S. P., McKenzie I. F. Enzymatic remodelling of the carbohydrate surface of a xenogenic cell substantially reduces human antibody binding and complement-mediated cytolysis. Nat Med. 1995 Dec;1(12):1261–1267. doi: 10.1038/nm1295-1261. [DOI] [PubMed] [Google Scholar]
- Sandrin M. S., Vaughan H. A., Dabkowski P. L., McKenzie I. F. Anti-pig IgM antibodies in human serum react predominantly with Gal(alpha 1-3)Gal epitopes. Proc Natl Acad Sci U S A. 1993 Dec 1;90(23):11391–11395. doi: 10.1073/pnas.90.23.11391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D. F., Larsen R. D., Mattox S., Lowe J. B., Cummings R. D. Transfer and expression of a murine UDP-Gal:beta-D-Gal-alpha 1,3-galactosyltransferase gene in transfected Chinese hamster ovary cells. Competition reactions between the alpha 1,3-galactosyltransferase and the endogenous alpha 2,3-sialyltransferase. J Biol Chem. 1990 Apr 15;265(11):6225–6234. [PubMed] [Google Scholar]
- Thall A., Galili U. Distribution of Gal alpha 1----3Gal beta 1----4GlcNAc residues on secreted mammalian glycoproteins (thyroglobulin, fibrinogen, and immunoglobulin G) as measured by a sensitive solid-phase radioimmunoassay. Biochemistry. 1990 Apr 24;29(16):3959–3965. doi: 10.1021/bi00468a024. [DOI] [PubMed] [Google Scholar]
- Wrenshall L. E., Platt J. L., Chopek M. W., Fischel R., Matas A. J. ABO-incompatible transplantation in pigtail macaque monkeys. Transplant Proc. 1991 Feb;23(1 Pt 1):703–704. [PubMed] [Google Scholar]