Abstract
Aims
Distal symmetric polyneuropathy increases fall risk due to inability to cope with perturbations. We aimed to 1) identify the frontal plane lower limb sensorimotor functions which are necessary for robustness to a discrete, underfoot perturbation during gait; and 2) determine whether changes in the post-perturbed step parameters could distinguish between fallers and non fallers.
Methods
Forty-two subjects (16 healthy old and 26 with diabetic PN) participated. Frontal plane lower limb sensorimotor functions were determined using established laboratory-based techniques. The subjects' most extreme alterations in step width or step length in response to a perturbation were measured. In addition, falls and fall-related injuries were prospectively recorded.
Results
Ankle proprioceptive threshold (APrT; p=.025) and hip abduction rate of torque generation (RTG; p=.041) independently predicted extreme step length after medial perturbation, with precise APrT and greater hip RTG allowing maintenance of step length. Fallers demonstrated greater extreme step length changes after medial perturbation than non fallers (percent change = 16.41±8.42 vs 11.0±4.95; p=.06)
Conclusions
The ability to rapidly generate frontal plane hip strength and/or precisely perceive motion at the ankle is needed to maintain a normal step length after perturbation, a parameter, which distinguishes between fallers and non fallers.
Keywords: Peripheral neuropathy, sensorimotor functions, gait, falls, proprioception, muscle strength
Introduction
The World Health Organization noted a prevalence of Diabetes Mellitus (DM) of 171 million people in the year 2000 and predicted an increase to 366 million by 2030. The disease is generally more common in developed countries. For example, the lifetime risk of developing diabetes in the United States for those born in year 2000 is about 40% for women and 30% for men[1].
It is understood that type 2 DM leads to early mortality, as well as retinopathy, nephropathy, neuropathy and accelerated macro vascular diseases[2]. Therapies that normalize glycemia are thought to prevent generation and/or delay progression of such complications[3]. The most common non-pharmacologic approaches to improve glycemic control are weight loss and exercise[4]. Exercise provides an additive effect when combined with caloric restriction[5] and it has been recommended that patients should engage in at least 150 minutes of moderate-intensity aerobic exercise per week[5]. Most exercise regimens feature walking, and it has been shown that a walking program improves the metabolic profile of patients with type 2 DM[6].
Although prevalence varies, peripheral neuropathy (PN) is common in patients with type 2 DM. A 2007 French study found an 11% prevalence of PN in adults with DM[7], while a 1999-2000 United States study noted a 28.5% prevalence in those with DM aged 40 years and older [8]. Importantly, diabetic PN reduces sensory and motor neuron excitability which in turn leads to coarsened proprioceptive thresholds and distal muscle atrophy[9], resulting in prolonged muscle response latencies. These patho-physiological changes adversely affect motor control and alter balance[10] and gait[10, 11], markedly increasing risk for falls and fall-related injuries[12, 13]. Therefore older patients with PN are at increased risk for a fall-related injury, while endeavoring to improve their health by pursuing a walking program.
Falls occur most frequently among older subjects with, and without, neuropathy while walking on uneven surfaces[13, 14]. Given the importance of walking to the health of patients with Type 2 DM, there is a need to identify the lower limb sensorimotor functions essential to cope with perturbations and avoiding falls. To date few studies have investigated the relationship between lower limb neuromuscular function and surface perturbations and no study has evaluated the relationships between lower limb sensorimotor function and a discrete, unanticipated perturbation. To address this we performed laboratory-based evaluations of lower limb frontal plane sensorimotor function using established techniques, and then observed older subjects with a spectrum of peripheral neurologic function due to diabetes mellitus respond to an unexpected, discrete perturbation during stance phase of gait by means of a specifically designed shoe[15]. The shoe challenged lateral (i.e., frontal plane) control, which is relevant given the greater injury potential of lateral falls in older adults [16, 17]. The subjects were then followed prospectively to record falls and fall-related injuries.
The ideal response to a discrete perturbation was defined as per Reeves et al. (ref) who argue that a robust biologic system changes its behavior minimally in response to a perturbation. This means that the error between the disturbed and undisturbed motions should be minimal and converge to the undisturbed pattern in a short time interval following a perturbation. Therefore, the objectives of the present study were to: 1) Identify the specific frontal plane lower limb sensorimotor functions necessary for robustness (minimal change in response) to a discrete, underfoot perturbation during gait; and 2) Determine whether post-perturbation step parameter changes could distinguish between those who sustained falls and fall-related injuries, and those who did not, during one year of follow-up. Given prior work [10, 18] we hypothesized that Hip motor function (specifically, rate of torque Generation), ankle proprioceptive threshold (APrT) and the ratio of the former to the latter would be the predominant influences on post-perturbation step characteristics (H1). We further hypothesize that most extreme post-perturbation step length and/or step width (of the four analyzed) would identify subjects who were not robust to perturbation and distinguish between subjects who fell and/or sustained a fall-related injury and those who did not (H2).
Materials and Methods
Subjects
Forty-two subjects (16 healthy old and 26 with diabetic PN) aged between 50-85 years and with a weight lower than 136 kg, were recruited from the University of Michigan Orthotics and Prosthetics Clinic, Endocrinology Clinic, and the Older Americans Independence Center Human Subjects Core. The PN subjects had disease severity that ranged from minimal to moderate severity so that when they were included with the older subjects without PN, a spectrum of lower limb sensorimotor function within the study population was assured. To participate in the study, subjects had to be able to walk household distances without any assistance and without any assistive device. They further had to present strength of ankle dorsiflexors, invertors, and evertors at least anti-gravity (grade 3 by manual muscle testing). Full details of inclusion and exclusion are available [10]. In general, these criteria eliminated subjects with upper motor neuron dysfunction, vestibular and visual conditions that would interfere with stability, limiting pain, falls within the prior 6 months, or joint replacement within the prior year.
The entrance evaluation involved a physical examination during which in- and exclusion criteria were verified. For the subjects with diabetes, the presence of PN was confirmed by symptoms (subject report of altered distal lower limb sensation), signs (Michigan Diabetes Neuropathy Score > 10)[19, 20] and fibular motor nerve conduction studies. Bilaterally abnormal fibular nerve conduction studies (absent or amplitude <2 mV and/or latency >6.2 ms and/or conduction velocity <41.0 m/s, stimulating 9 cm from the recording site over the extensor digitorum brevis distally, and distal to the fibular head proximally) were essential for inclusion as PN [10]. Subjects without diabetes met the same inclusion and exclusion criteria except that they had no symptoms or signs of neuropathy, and had normal fibular motor nerve conduction studies. The study protocol was approved by the institutional review board. Written informed consent was obtained from all participants.
Evaluation of lower limb sensorimotor function
Frontal Ankle proprioceptive threshold (APrT)
The subjects stood with the foot and ankle being tested in a 40 × 25-cm cradle that rotated in the frontal plane (inversion and eversion). The cradle was rotated by a servomotor equipped with an 8000 line rotary encoder (Aerotech 1000 servomotor; Aerotech, Inc, Pittsburgh, PA). The subject responded to the direction of the rotation with a hand-held joystick. Four blocks of 25 trials (randomly, 10 eversion, 10 inversion, and 5 dummy trials) were presented. Each block was interspersed with 2- to 5-minute rest intervals. The outcome measure was the APrT, defined as the smallest rotational displacement of the ankle that a subject could reliably detect with 100% accuracy. The sum of the inversion proprioception threshold and eversion proprioception threshold was used for further analysis [21].
Ankle strength
Ankle muscle strength (maximum voluntary contraction; MVC and rate of torque Generation; RTG) was tested while subjects stood on the test foot on a force plate (OR-6; Advanced Mechanical Technology, Inc.) while touching hand rails. To assess the MVC, subjects shifted their center of gravity as far laterally under their foot as possible and lifted their hands from the rails for 3 seconds. The test was repeated 3 times for the lateral margin of the foot (maximum voluntary inversion) and repeated for the medial margin of the foot (maximum voluntary eversion).
To measure ankle RTG, the subjects stood on the test foot on the force plate and moved the center of ground support reaction from the lateral margin of the foot to the medial margin as quickly as possible and then back again to the lateral margin, as previously described. Three trials, each trial with 5 medial-lateral movements, were performed [10].
Hip Strength
Hip abduction and adduction (MVC) and (RTG) in the frontal plane at the hip were measured with a custom whole-body dynamometer (BioLogic Engineering, Inc.). The subject lay supine on a horizontal bench with the pelvis and upper body immobilized with adjustable harness straps, and the limb being tested was secured with straps against a lever, which allowed all measurements to be made in a gravity-free plane. During maximum voluntary strength tests, the subjects progressively increased their isometric effort to their maximum over a count of 3 seconds, held it for 2 seconds, and relaxed. To quantify the rate of isometric strength development, the subjects increased their effort as rapidly as possible for 3 seconds. Three trials were performed with 1-minute rests between trials. The subjects had a real-time visual display of the force generated to allow them to evaluate their efforts [10].
Perturbed gait analysis
Subjects were equipped with a specific pair of shoes with electronically controlled linear actuators (ModelPQ-12, Firgelli Technologies, Inc., Victoria, BC, Canada). Each shoe deployed a small rectangular (25.4*30.5*9.5 mm) aluminum flap hinged in the parasagittal plane. When neither flap was deployed, the shoe felt normal to walk in. When one of the concealed flaps was deployed, it rotated down about a parasagittal plane axis such as described by Kim et al. (Figure 1)[15].
Figure 1. Picture of the perturbing shoe.

Anterior view of the sandal, unloaded by body weight and with the 18.4mm-high lateral flap (circled) deployed in the parasagittal plane so as to invert the foot during a single mid stance phase
Subjects performed a total of 60 walking trials (three blocks of 20 trials with a break between every block) along a 6 m level walkway at a speed that is faster than individuals' normal speed, i.e. as though they were trying to cross a busy street before the signal changes. Sixteen trials were conducted with either a medial or a lateral perturbation (MP or LP, respectively), presented randomly under the left or right foot, and randomized among the 44 additional unperturbed (UnP) dummy trials. Subjects were told that, when it occurred, the stance phase perturbation would happen only once per gait trial; but they could not know if, when or where, a left or a right shoe MP or LP was to be deployed in that trial. Before the trials began, subjects practiced several times without the perturbation, to familiarize themselves with the apparatus and experimental environment.
Traditional step kinematic measures (including step width (SW) and step length (SL) were collected at 100 Hz using an Optotrak optoelectric motion analysis system Northern Digital Inc., Waterloo, ON, Canada)[22].
Fall assessments
A subgroup of 32 subjects (19 with DM and some degree of neuropathy and 13 without DM or significant neuropathy) was followed prospectively for one year. Subjects completed a falls calendar bi-weekly, and follow-up telephone calls were made when fall-related events occurred[14]. A fall was defined as an unintentional change in body posture that results in the subject coming to rest on the ground or other lower level that was not a consequence of a physical blow or loss of consciousness.
Statistics
Statistics were conducted using SPSS for Windows (release 11.0.1.2001; SPSS, Inc., Chicago, Illinois). A descriptive analysis of demographic data was performed.
H1:
Definition of unperturbed step measures
The mean step length and step width of the steps during the 44 trials during which no perturbation was offered.
Definition of most extreme post-perturbation step measures
The greatest difference between the four post-perturbed step lengths and widths for a given perturbation (medial or lateral of left or right foot) and the mean of that parameter for the unperturbed steps (as defined above).
In this way the most aberrant step length and width of the 4 post-perturbation steps[23] were identified for each of the 4 conditions (i.e., medial and lateral perturbations for the left and right foot). The relationships between the most extreme post-perturbation steps and subject lower limb sensorimotor function were assessed with bivariate correlations. This analysis included a compound variable that was the ratio of hip motor function to APrT. This ratio was identified prior to data analyses given previous work finding that the ratio predicted the majority of the variability in one legged balance times[10]. Multivariate analyses of lower limb sensorimotor functions that significantly correlated with relevant post-perturbation gait measures were used to determine relative strengths and independence of effects.
H2:
Significant fall group and fall-injury group differences in post-perturbation extreme step measures were identified using one way ANOVA.
Detection of Outliers
Given the quantity of data processed and the technical nature of the measurements, outlier data points were sought. Two extreme outliers (defined as the 3rd quartile + 3 times the inter quartile range) were detected for post perturbation step length and were excluded. One other subject was excluded from all analyses due to the potential for bias after it was learned that the subject had multiple prior exposures to the perturbing shoe device, and was aware of its hypothesized influence on gait.
Results
Demographic characteristics of the 42 subjects who entered the study, and the 32 subjects who agreed to one year prospective fall and fall-related injury follow-up, are shown in Table 1a and 1b.
Table 1a. Demographic characteristics of the study population.
| Variable | N | Mean (SD) |
|---|---|---|
| Age (years) | 42 | 69.07 (8.36) |
| BMI (kg/cm2) | 42 | 30.98 (6.94) |
| MDNS (points out of 46) | 42 | 9.12 (7.85) |
| Gender (N Males / N Females) | 42 | 21 / 21 |
| Falls (N of Persons) | 32 | 20 |
| Fall related Injury (N of Persons) | 32 | 18 |
N = Number
Table 1b. Demographic characteristics of the 32 subjects who agreed to one year prospective fall and fall-related injury follow-up.
| Variable | N | Mean (SD) |
|---|---|---|
| Age (years) | 32 | 68.53 (8.17) |
| BMI (kg/cm2) | 32 | 30.41 (6.57) |
| MDNS (points out of 46) | 32 | 8.19 (7.61) |
| Gender (N Males / N Females) | 32 | 17 / 15 |
N = Number
H1: Three neuromuscular functions (Hip Abduction RTG, Hip Adduction RTG and APrT) demonstrated significant correlations with extreme step length after a medial perturbation (Table 2). The relationships were such that the greater the hip RTG and smaller (more precise) the APrT the less aberrant the most extreme post perturbed step length. The Hip RTG to APrT ratio also demonstrated a significant correlation with the extreme step length after medial (p = .009) and lateral (p = .004) perturbations. Neuromuscular functions did not correlate with extreme post-perturbation step width, with the exception of APrT which weakly correlated with extreme step width after a lateral perturbation.
Table 2. Correlation coefficients between extreme post-perturbed steps and neuromuscular functions.
| Neuromuscular functions | Post perturbed step length after a lateral perturbation | Post perturbed step length after a medial perturbation | Post perturbed step width after a lateral perturbation | Post perturbed step width after a medial perturbation | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|
||||||||
| R | p-value | N | R | p-value | N | R | p-value | N | R | p-value | N | |
|
|
|
|
|
|
||||||||
| Hip Abd MVC | -0.182 | 0.295 | 35 | -0.137 | 0.433 | 35 | 0.021 | 0.900 | 37 | 0.046 | 0.790 | 36 |
| Hip Abd RTG | -0.479 | 0.004 | 35 | -0.432 | 0.010 | 35 | -0.037 | 0.830 | 37 | 0.108 | 0.530 | 36 |
| Hip Add MVC | -0.282 | 0.100 | 35 | -0.133 | 0.447 | 35 | 0.047 | 0.780 | 37 | 0.095 | 0.581 | 36 |
| Hip Add RTG | -0.363 | 0.032 | 35 | -0.371 | 0.028 | 35 | -0.201 | 0.233 | 37 | -0.112 | 0.515 | 36 |
| Ankle Inv MVC | 0.083 | 0.656 | 31 | -0.202 | 0.275 | 31 | -0.124 | 0.491 | 33 | -0.103 | 0.574 | 32 |
| Ankle Inv RTG | -0.144 | 0.423 | 33 | -0.228 | 0.202 | 33 | 0.186 | 0.284 | 35 | 0.169 | 0.339 | 34 |
| Ankle Ev MVC | -0.184 | 0.306 | 33 | -0.199 | 0.266 | 33 | -0.086 | 0.625 | 35 | -0.092 | 0.605 | 34 |
| Ankle Ev RTG | -0.107 | 0.553 | 33 | -0.171 | 0.340 | 33 | 0.069 | 0.693 | 35 | 0.040 | 0.822 | 34 |
| Ankle Proprioception | 0.194 | 0.263 | 35 | 0.457 | 0.006 | 35 | 0.375 | 0.022 | 37 | 0.259 | 0.128 | 36 |
| Hip RTG / AP | -0.478 | 0.004 | 34 | -0.439 | 0.009 | 34 | -0.134 | 0.435 | 36 | -0.017 | 0.923 | 35 |
MVC = Maximum voluntary contraction, RTG = Rate of Torque Generation, AP = Ankle Proprioception, Abd = Abduction, Add = Adduction, Inv = Inversion; Ev = Eversion
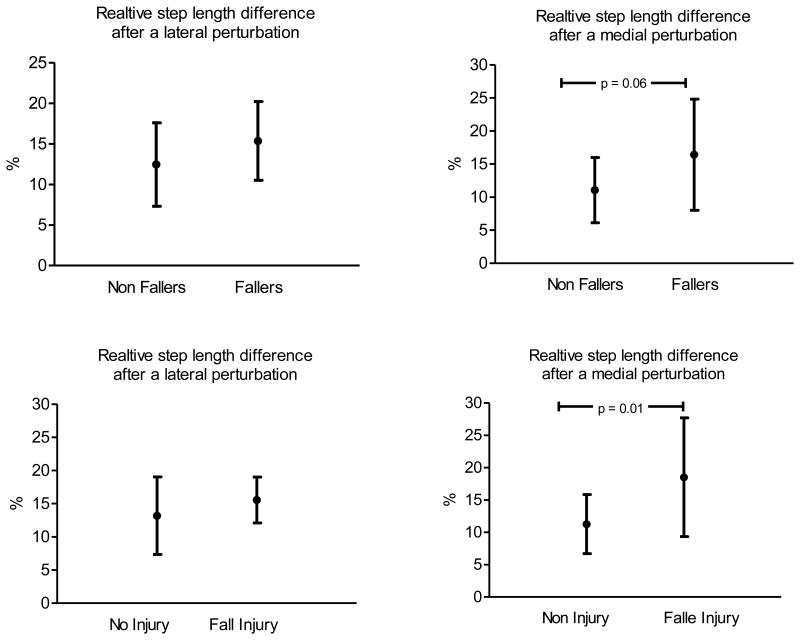
H2: During the 12 months of follow-up 20 of the 32 subjects (62.5%) reported a fall, and 14 of 32 (43.8%) reported a fall-related injury. When considered by group, 17 of the 19 subjects (89.5%) with DM and some degree of neuropathy reported a fall while 3 of the 13 (23.1%) subjects without DM reported a fall. Near significant fall group differences (16.41±8.42 vs 11.0 ±4.95; p = .06) and significant fall-related injury group differences (18.5 ±9.19 vs. 11.3±; p .01) in extreme step length after a medial perturbation were identified (Figure 2).
Figure 2. Differences in the most aberrant post perturbed step length between Fallers and Non Fallers and between Fall Injured and No Fall injured individuals.
The figures illustrate relative differences (in percentage on the y axis) in the most aberrant post perturbed step length between Fallers and Non Fallers and between Fall Injured and No Fall injured individuals (x-axis).
Mutivariate analyses
The group differences in post-perturbation extreme step measures suggest that subjects who sustained a fall and/or fall-related injury were less able to maintain their unperturbed mean step length after a medial perturbation as compared to subjects who did not fall or sustain a fall-related injury. Given this, multivariate analyses were used to determine the relative importance of the three neuromuscular functions that showed significance using bivariate correlations. The results demonstrated that APrT (p = .025) and Hip Abduction RTG (p = .041) significantly and independently predicted extreme step length after a medial perturbation, with more precise (smaller) APrT and greater Hip Abduction RTG leading to smaller extreme differences in post-perturbation step length. (Table 3)
Table 3. Multivariate analysis with the extreme post-perturbed step length after a medial perturbation as a dependent variable and neuromuscular functions as independent variables.
| Unstandardized Coefficients |
Standardized Coefficients |
95.0% Confidence Interval for B |
|||||
|---|---|---|---|---|---|---|---|
| B | Std. Error | Beta | t | Sig. | Lower Bound | Upper Bound | |
| (Constant) | 10.090 | 1.815 | 5.559 | .000 | 6.397 | 13.782 | |
| Ankle Proprioception | 2.314 | .785 | .457 | 2.950 | .006 | .718 | 3.911 |
|
| |||||||
| (Constant) | 13.949 | 2.505 | 5.569 | .000 | 8.847 | 19.052 | |
| Ankle Proprioception | 1.835 | .779 | .362 | 2.355 | .025 | .248 | 3.423 |
| Hip Abduction RTG | -14.987 | 7.051 | -.327 | -2.125 | .041 | -29.350 | -.624 |
RTG = Rate of Torque Generation, Abd = Abduction
Discussion
The major findings in this study of older persons with varying degrees of diabetic and age-related neuropathy was that a combination of rapidly available frontal plane strength at the hip and precise frontal plane APrT were necessary to consistently maintain step length following a discrete medial perturbation (H1). Moreover, the inability to consistently maintain step length after a perturbation identified those who fell and/or sustained a fall-related injury (H2). Further, the ratio of hip rate of torque generation to APrT was a strong predictor of step length consistency suggesting that the two may compensate for one another, as has been noted for unipedal stance time[10]. The optimal situation is a powerful frontal plane hip RTG and precise APrT. Nevertheless, the correlations between the hip RTG and APrT ratio and step length suggest that powerful hip musculature may compensate for coarse APrT and a precise threshold may compensate for a weak hip.
Most studies of perturbed gait have focused on challenges to postural stability in the sagittal plane, such as trips. To our knowledge only one study[24] included subjects at increased fall risk as is the case for those with diabetic neuropathy, and none of the studies quantified lower limb sensorimotor function or followed subjects prospectively after analysis. In the study including subjects at increased fall risk, Weerdesteyn et al[24] analyzed how young and older adults avoid obstacles when walking on a treadmill at 3 km/h. Success rates of avoidance was worse in elderly participants who sustained recurrent falls in the six-month period prior to the assessment compared to those who sustained no or only one fall. Pijnappels et al.[25] evaluated recovery from a standardized trip (via a quickly emerging obstacle that obstructed the swing limb) in healthy young and older subjects. Then they tried to divide the older adults into fallers and non-fallers based on their ability to successfully recover from the experimental trip. Consistent with our findings regarding importance of RTG of the hip musculature to reject a lateral perturbation, fallers in Pijnappels' work were unable to arrest the body's momentum due to a decreased rate of moment generation in the support limb[25]. Forner Codero et al.[23] analyzed the recovery from an induced stumble by braking the forward swing of the leg and disturbing its trajectory during treadmill walking. Subjects showed two different types of reactions to the experimental perturbation. In addition, the authors concluded that research protocols of recovery following gait perturbations should include at least three steps.
One other paper analyzed healthy young subjects laterally perturbed by a horizontally translating platform during gait, simulating a lateral slip[26]. A minority of these young subjects responded to the perturbation by abruptly terminating swing phase and placing the foot down, thereby shortening the first post-perturbation step and limiting a destabilizing extreme lateral step. A similar strategy in our subjects may be responsible for the absence of a relationship between extreme post-perturbation step width and falls or fall-related injury. It seems that subjects with insufficient hip strength have reduced step length in one or more of the post-perturbation steps so as to prevent a destabilizing extreme lateral step, thus giving rise to an extreme short step. This possibility links decreased hip abductor/adductor RTG and the inability to maintain step length following a perturbation. A similar reduction in step length so as to prevent extreme lateral steps in older subjects with diabetic neuropathy, in this case walking on an irregular surface, has been noted previously[27]. Finally, Otten et al.[28] analyzed younger subjects balancing on one foot on a thin beam, thereby effectively removing or minimizing ankle control, and found that hip adductor/abductor-generated moments varied the horizontal ground-reaction force so as to allow the maintenance of equilibrium. Frontal plane hip strength is critical to maintenance of unipedal balance given the sensorimotor impairments at the ankle in older people with diabetic neuropathy. Prior work found this to be the case during static unipedal balance. In that paper we showed that Hip abductor/adductor maximum strength was the dominant predictor of unipedal stance time[10]. The data reported here substitute hip abductor/adductor RTG for maximum strength, as might be expected given the more dynamic and time-contingent nature of the rejection of a perturbation while walking.
The findings have implications for investigators and clinicians. The former may, in future research, incorporate analyses of extreme steps after subjects are perturbed, physically or cognitively. Such steps are often statistically shunned as “outliers”, and yet their evaluation here appears to be meaningful. Many and perhaps most falls in the community occur as a result of the patient responding in a particularly unusual and disadvantageous way, that is indeed an outlier given that most steps taken throughout a patient's day do not result in falls. It is unlikely that the perturbing shoe apparatus would be useful clinically given the sophisticated equipment and analysis required. However, the detected critical role played by rapidly generated frontal plane strength at the hip after perturbation may fine tune rehabilitation efforts in older patients at increased fall risk due to declining diabetes-related peripheral nerve function. Further it has to be mentioned that falls are 3-dimensional events and examining them in one of the 3 orthogonal planes remains an over-simplification. However, frontal plane is an important and until now unilluminated part of the picture.
The strength of this study is that subjects were evaluated for neuropathy by history, physical examination and electrodiagnostic techniques as has been recommended[29]. The use of less specific techniques which often employ a single sensory modality was avoided. Also, the laboratory-based techniques for quantifying lower limb sensorimotor function are well-established and the specifically designed shoe is a novel way to provide a uniform, yet unexpected perturbing stimulus[15]. The prospective recording of falls was performed as per consensus recommendations[30]. The rates of falls and fall-related injuries were high, but not unexpected given prior experience with fall rates retrospectively determined[31, 32]. The relationship between the most extreme post-perturbation step length and fall-related injury is particularly compelling, and the ability of this variable to predict fall-related injury is noteworthy given the number of subjects. That number, although relative large for a highly quantified study, is still small and the conclusions must be tempered by sampling concerns.
Conclusion
The results suggest further research. Given the rate of falls and fall-related injury in a group of subjects who need to walk to maintain their health, the efficacy of an exercise regimen targeting rapid strength generation of hip abductors/adductors is necessary. As diabetic neuropathy is predominantly a distal process, such a training program seems feasible, and prior work suggests that older patients with diabetic neuropathy respond favorably to resistance training [33]. The concept that the most extreme step following a physical or cognitive perturbation is predictive of falls and/or fall-related injury is intuitively attractive but further evaluation is necessary before it is accepted.
Acknowledgments
National Institutes of Health RO1 AG026569 (HK, JAAM, TD, JKR).
Swiss National Foundation Section International collaboration (IZKOZ3_133925).
Abbreviations
- MVC
Maximum Voluntary Contraction
- MP
Medial Perturbed
- LP
Lateral Perturbed
- PN
Peripheral Neuropathy
- RTG
Rate of Torque Generation
- SW
Step width
- SL
Step length
- UnP
Unperturbed
Footnotes
Conflict of Interest Statement: The authors do not have any conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Narayan KM, et al. Lifetime risk for diabetes mellitus in the United States. JAMA. 2003;290(14):1884–90. doi: 10.1001/jama.290.14.1884. [DOI] [PubMed] [Google Scholar]
- 2.Nathan DM. Long-term complications of diabetes mellitus. N Engl J Med. 1993;328(23):1676–85. doi: 10.1056/NEJM199306103282306. [DOI] [PubMed] [Google Scholar]
- 3.Nathan DM. Prevention of long-term complications of non-insulin-dependent diabetes mellitus. Clin Invest Med. 1995;18(4):332–9. [PubMed] [Google Scholar]
- 4.Ismail-Beigi F. Clinical practice. Glycemic management of type 2 diabetes mellitus. N Engl J Med. 2012;366(14):1319–27. doi: 10.1056/NEJMcp1013127. [DOI] [PubMed] [Google Scholar]
- 5.Association AD Association, A.D. Standards of medical care in diabetes. Diabetes Care. 2012;35(Suppl 1):11–63. [Google Scholar]
- 6.Sung K, Bae S. Effects of a regular walking exercise program on behavioral and biochemical aspects in elderly people with type II diabetes. Nurs Health Sci. 2012 doi: 10.1111/j.1442-2018.2012.00690.x. [DOI] [PubMed] [Google Scholar]
- 7.Wu EQ, et al. Estimated prevalence of peripheral neuropathy and associated pain in adults with diabetes in France. Curr Med Res Opin. 2007;23(9):2035–42. doi: 10.1185/030079907X210516. [DOI] [PubMed] [Google Scholar]
- 8.Gregg EW, et al. Prevalence of lower-extremity disease in the US adult population >=40 years of age with and without diabetes: 1999-2000 national health and nutrition examination survey. Diabetes Care. 2004;27(7):1591–7. doi: 10.2337/diacare.27.7.1591. [DOI] [PubMed] [Google Scholar]
- 9.Narici MV, Maganaris C, Reeves N. Myotendinous alterations and effects of resistive loading in old age. Scand J Med Sci Sports. 2005;15(6):392–401. doi: 10.1111/j.1600-0838.2005.00458.x. [DOI] [PubMed] [Google Scholar]
- 10.Allet L, et al. Frontal plane hip and ankle sensorimotor function, not age, predicts unipedal stance time. Muscle Nerve. 2012;45(4):578–85. doi: 10.1002/mus.22325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Allet L, et al. Gait characteristics of diabetic patients: a systematic review. Diabetes Metab Res Rev. 2008;24(3):173–91. doi: 10.1002/dmrr.809. [DOI] [PubMed] [Google Scholar]
- 12.Wallace C, et al. Incidence of falls, risk factors for falls, and fall-related fractures in individuals with diabetes and a prior foot ulcer. Diabetes Care. 2002;25(11):1983–6. doi: 10.2337/diacare.25.11.1983. [DOI] [PubMed] [Google Scholar]
- 13.Allet L, et al. Gait alterations of diabetic patients while walking on different surfaces. Gait Posture. 2009;29(3):488–93. doi: 10.1016/j.gaitpost.2008.11.012. [DOI] [PubMed] [Google Scholar]
- 14.DeMott TK, et al. Falls and gait characteristics among older persons with peripheral neuropathy. Am J Phys Med Rehabil. 2007;86(2):125–32. doi: 10.1097/PHM.0b013e31802ee1d1. [DOI] [PubMed] [Google Scholar]
- 15.Kim H, Ashton-Miller JA. A shoe sole-based apparatus and method for randomly perturbing the stance phase of gait: Test-retest reliability in young adults. J Biomech. 2012;45(10):1850–3. doi: 10.1016/j.jbiomech.2012.05.003. [DOI] [PubMed] [Google Scholar]
- 16.Maki BE, Holliday PJ, Topper AK. A prospective study of postural balance and risk of falling in an ambulatory and independent elderly population. J Gerontol. 1994;49(2):M72–84. doi: 10.1093/geronj/49.2.m72. [DOI] [PubMed] [Google Scholar]
- 17.Cummings SR, Nevitt MC. Non-skeletal determinants of fractures: the potential importance of the mechanics of falls. Study of Osteoporotic Fractures Research Group. Osteoporos Int. 1994;4(Suppl 1):67–70. doi: 10.1007/BF01623439. [DOI] [PubMed] [Google Scholar]
- 18.Allet L, et al. Which Lower Limb Frontal Plane Sensory and Motor Functions Predict Gait Speed and Efficiency on Uneven Surfaces in Older Persons With Diabetic Neuropathy? PM R. 2012 doi: 10.1016/j.pmrj.2012.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Richardson JK. The clinical identification of peripheral neuropathy among older persons. Arch Phys Med Rehabil. 2002;83(11):1553–8. doi: 10.1053/apmr.2002.35656. [DOI] [PubMed] [Google Scholar]
- 20.Strotmeyer ES, et al. Long-term retention of older adults in the Cardiovascular Health Study: implications for studies of the oldest old. J Am Geriatr Soc. 2010;58(4):696–701. doi: 10.1111/j.1532-5415.2010.02770.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Son J, Ashton-Miller JA, Richardson JK. Frontal plane ankle proprioceptive thresholds and unipedal balance. Muscle Nerve. 2009;39(2):150–7. doi: 10.1002/mus.21194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thies SB, Ashton-Miller JA, Richardson JK. What causes a crossover step when walking on uneven ground? A study in healthy young women. Gait Posture. 2007;26(1):156–60. doi: 10.1016/j.gaitpost.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 23.Forner Cordero A, Koopman HF, van der Helm FC. Multiple-step strategies to recover from stumbling perturbations. Gait Posture. 2003;18(1):47–59. doi: 10.1016/s0966-6362(02)00160-1. [DOI] [PubMed] [Google Scholar]
- 24.Weerdesteyn V, Nienhuis B, Duysens J. Advancing age progressively affects obstacle avoidance skills in the elderly. Hum Mov Sci. 2005;24(5-6):865–80. doi: 10.1016/j.humov.2005.10.013. [DOI] [PubMed] [Google Scholar]
- 25.Pijnappels M, Bobbert MF, van Dieen JH. Push-off reactions in recovery after tripping discriminate young subjects, older non-fallers and older fallers. Gait Posture. 2005;21(4):388–94. doi: 10.1016/j.gaitpost.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 26.Oddsson LI, et al. Recovery from perturbations during paced walking. Gait Posture. 2004;19(1):24–34. doi: 10.1016/s0966-6362(03)00008-0. [DOI] [PubMed] [Google Scholar]
- 27.Richardson JK, Thies S, Ashton-Miller JA. An exploration of step time variability on smooth and irregular surfaces in older persons with neuropathy. Clin Biomech (Bristol, Avon) 2008;23(3):349–56. doi: 10.1016/j.clinbiomech.2007.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Otten E. Balancing on a narrow ridge: biomechanics and control. Philos Trans R Soc Lond B Biol Sci. 1999;354(1385):869–75. doi: 10.1098/rstb.1999.0439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.England JD, et al. Distal symmetrical polyneuropathy: a definition for clinical research. A report of the American Academy of Neurology, the American Association of Electrodiagnostic Medicine, and the American Academy of Physical Medicine and Rehabilitation. Arch Phys Med Rehabil. 2005;86(1):167–74. doi: 10.1016/j.apmr.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 30.Lamb SE, et al. Development of a common outcome data set for fall injury prevention trials: the Prevention of Falls Network Europe consensus. J Am Geriatr Soc. 2005;53(9):1618–22. doi: 10.1111/j.1532-5415.2005.53455.x. [DOI] [PubMed] [Google Scholar]
- 31.Richardson JK, Hurvitz EA. Peripheral neuropathy: a true risk factor for falls. J Gerontol A Biol Sci Med Sci. 1995;50(4):M211–5. doi: 10.1093/gerona/50a.4.m211. [DOI] [PubMed] [Google Scholar]
- 32.Cavanagh PR, et al. Problems with gait and posture in neuropathic patients with insulin-dependent diabetes mellitus. Diabet Med. 1992;9(5):469–74. doi: 10.1111/j.1464-5491.1992.tb01819.x. [DOI] [PubMed] [Google Scholar]
- 33.Praet SF, et al. Long-standing, insulin-treated type 2 diabetes patients with complications respond well to short-term resistance and interval exercise training. Eur J Endocrinol. 2008;158(2):163–72. doi: 10.1530/EJE-07-0169. [DOI] [PubMed] [Google Scholar]