Significance
In peatlands, the external sources of nitrogen are mainly atmospheric, but the atmospheric nitrogen deposition alone cannot explain the long-term annual nitrogen accumulation rates to these ecosystems. Because of methodological problems, methane-induced fixation of atmospheric dinitrogen gas has been previously overlooked as an additional nitrogen input mechanism. We found that the activity of methane-oxidizing bacteria provides not only carbon but also nitrogen to peat mosses and, thus, contributes to carbon and nitrogen accumulation in peatlands, which store approximately one-third of the global soil carbon pool. Our results imply that nitrogen fixation in wetlands may be strongly underestimated when methods inhibiting methane oxidizers are used.
Keywords: CH4, diazotrophy, mire, peat, phosphorus
Abstract
Nitrogen (N) accumulation rates in peatland ecosystems indicate significant biological atmospheric N2 fixation associated with Sphagnum mosses. Here, we show that the linkage between methanotrophic carbon cycling and N2 fixation may constitute an important mechanism in the rapid accumulation of N during the primary succession of peatlands. In our experimental stable isotope enrichment study, previously overlooked methane-induced N2 fixation explained more than one-third of the new N input in the younger peatland stages, where the highest N2 fixation rates and highest methane oxidation activities co-occurred in the water-submerged moss vegetation.
Peat-accumulating wetlands, i.e., peatlands, store approximately 30% of the global soil carbon (C) (1), and this value is even higher if the permafrost regions in the Northern Hemisphere are also taken into account (2). As peat accumulates, the ecosystem becomes independent of the groundwater influence and the vegetation becomes more nutrient limited. This gradual succession from minerotrophic fen to Sphagnum-dominated ombrotrophic bog ecosystem is the general peatland development pattern. Because the growth and decomposition rates of Sphagnum mosses are greatly responsible for the C biosequestration in peatlands, the ecology of Sphagnum mosses is of particular interest. Recent studies have shown that Sphagnum mosses have an association with methanotrophic bacteria that leads to a reduction in methane (CH4) emissions to the atmosphere and the provision of additional carbon dioxide (CO2) source for the host plants (3, 4). However, the growth of Sphagnum mosses in peatlands is often N limited, at least under low atmospheric N deposition (5), so biological fixation of atmospheric N2, i.e., the biological conversion of dinitrogen to plant-available ammonium, may stimulate moss growth (6). Under low atmospheric N deposition, moss-associated cyanobacteria have been shown to play an important role in the N budget in forests (7, 8) and peatlands (9), where N2 fixation is favored by moist conditions. In peatlands, however, molecular analyses of genes that encode nitrogenase reductase proteins (nifH) in Sphagnum mosses have indicated that moss-associated N2 fixers (diazotrophs) belong mainly to the metabolically diverse class Alphaproteobacteria (10), which includes phototrophic, heterotrophic, and methanotrophic genera.
In previous studies, N2 fixation rates in peatlands have been found to correlate with the minerotrophy of peatlands, in particular with the level of phosphorus (P) (11), but the nutrient controls of N2 fixation have not been linked to peatland succession toward the bog stage. Further, in these studies, N2 fixation has been measured by using acetylene reduction assay (12), which does not provide a quantitative measure of N added to the system, because it inhibits the activity of many noncyanobacterial diazotrophs, but specifically methanotrophic bacteria by inactivating the essential methane monooxygenase enzyme (13). This inhibition is a serious drawback, because a broad range of methanotrophic bacteria contains genes that code for the N2 fixation pathway and shows nitrogenase activity (11, 14, 15). Because of this methodological problem, the role of methanotrophic N2 fixation and the relationship of N2 fixation with C cycling have not previously been evaluated at an ecosystem scale. The elucidation of the linkage between methanotrophy and the overall N cycle in peatlands becomes feasible by the application of stable isotope (15N2) techniques (8, 16).
We studied N2 fixation and CH4 oxidation in the dominant flark and hummock vegetation of 12 pristine peatlands, which varied in age from 200 to 2,500 y due to still ongoing postglacial rebound on the coast of Bothnian Bay, Finland (Fig. 1 and Table S1). Together with the Hudson Bay Lowlands in Canada, the Bothnian Bay of the Baltic Sea between Finland and Sweden is the region where the rebound after the pressure of ice mass and the consequent formation of new land from the sea is most rapid. This chronosequence of peatlands offers an exceptional opportunity to study the links between N and C cycling over an undisturbed peatland gradient.
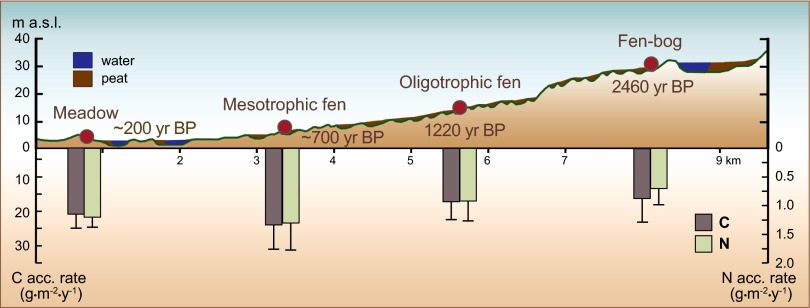
Fig. 1.
Carbon (left axis downward) and nitrogen (right axis downward) accumulation rates during the 2,500-y primary succession of Siikajoki peatlands from meadows, mesotrophic fens, and oligotrophic fens toward fen-bog transitions on the land-uplift coast of the Bothnian Bay (age cohorts SJ2–SJ5; ref. 23). The error bars are SD. The estimates of site age means are based on the land-uplift rate (meadows and mesotrophic fens) or on radiocarbon dating (oligotrophic fens and fen-bog transitions); m a.s.l., meters above sea level; BP, before present.
In young peatlands, unstable hydrological conditions are likely to result in low CH4 emissions and, thereby, small methanotrophic communities (17). As the hydrological conditions become more stable in the later successional stages with increasing peat depths, CH4 emissions increase (18). High CH4 emissions have been linked to minerotrophic fen stages, in which the dense sedge vegetation readily provides substrate for methanogenesis, whereas in ombrotrophic bogs characterized by Sphagnum mosses and dwarf shrubs, the rates of decomposition and substrate supply for the CH4 production are slow (19). However, the ranges for Sphagnum hosted CH4 oxidation rates in minerotrophic fens and ombrotrophic bogs have been found to overlap (4). It has been hypothesized that despite the low inorganic N concentration in bogs, CH4 oxidation is not N limited because methanotrophic N2 fixation may compensate for the N requirement of methanotrophic bacteria (20).
With reference to these factors, we hypothesized that (i) CH4 induces moss-associated N2 fixation, which would be most pronounced in the late stages of peatland succession and (ii) the overall N2 fixation rates along the peatland succession gradient are governed by gradually changing environmental factors, such as water table depth, availability of CH4 in the pore water, and concentrations of P, iron (Fe), or molybdenum (Mo). The rationale for studying the role of Fe and Mo was that the two methane monooxygenases (e.g., ref. 21) and all three nitrogenase isoenzymes contain Fe, and one of the nitrogenase isoenzymes contains Mo as a necessary cofactor (22). We tested the two hypotheses in a 15N2 and 13CH4 pulse-labeling experiment, where moss samples collected from flark and hummock habitats of four successional peatland stages were incubated in situ with and without CH4 addition, each under prevailing light conditions and in the dark. These treatments were used to reveal whether N2 fixation was attributed to photosynthetic, heterotrophic, or methanotrophic activity.
Results and Discussion
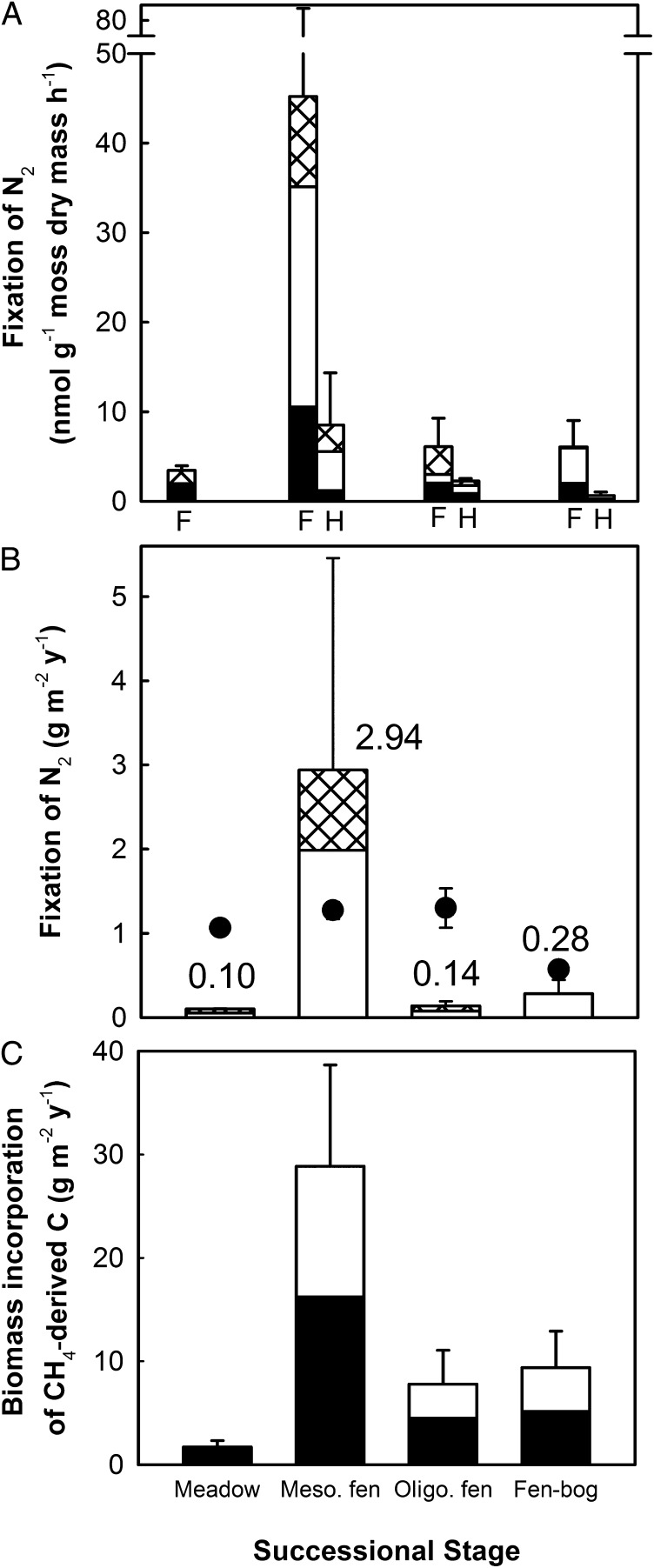
Fixation of N2 was found in the live parts of the Sphagnum mosses in all of the studied moss patches. Although Sphagnum-associated N2 fixation was observed in all of the study peatlands of different successional stages, the process was significantly higher (five- to ninefold) in the wet depressions (flarks) of the midsuccessional mesotrophic fens compared with the other successional stages, which consisted of younger meadows, older oligotrophic fens and fen-bog transitions (Fig. 2A and Tables S2 and S3). When the summertime N2 fixation rates (0–126 nmol⋅g−1 of moss biomass⋅h−1) were converted to annual areal values (0.1–2.9 g of N⋅m−2⋅y−1; Fig. 2B), they were up to 10 times greater than the current inorganic N deposition rates (0.3 g⋅m−2⋅y−1) for the region. These annual values indicate that Sphagnum-associated N2 fixation is a major N input to boreal peatlands and, in conjunction with atmospheric N deposition, explains the long-term annual N accumulation rate (0.6–1.3 g⋅m−2; ref. 23) in the studied peatland ecosystems (Fig. 2B). Our areal estimations for fen stages and fen-bog transition are within the wide range of the admittedly few estimates available for Sphagnum-associated N2 fixation in temperate, boreal, and subarctic ecosystems. These range from 0.1–6.4 g of N⋅m−2⋅y−1 for fens (11, 16, 24) and 0.1–1 g⋅m−2⋅y−1 for bogs (9, 25). Nevertheless, our estimates may be rough because of the large SEs and the extrapolation over time: We assume a 6-mo active season, but substantial heterotrophic microbial activity in addition to phototrophic activity could occur during winter. At these sites, 30–65% of annual moss growth occurs during the October–April period, outside the traditionally defined growing season (26).
Fig. 2.
Sphagnum moss-associated N2 fixation and CH4 oxidation (biomass incorporation of CH4-derived C) in 12 peatlands of the peatland chronosequence (meadows, mesotrophic and oligotrophic fens, and fen-bog transitions) based on the stable isotope pulse-labeling experiment. The error bars are SEM (n = 3 peatlands in each stage). (A) Contribution of dark (heterotrophic, black), light-induced (phototrophic, white), and CH4-induced (methanotrophic, cross-hatched) N2 fixation in flark (F) and hummock (H) vegetation. The meadows have only flark Sphagnum. (B) The contribution of CH4-induced N2 fixation (cross-hatched) to Sphagnum-associated N2 fixation. Data are averages of light and dark incubations in 15N2 and 15N2+13CH4 treatments, respectively, weighted with the proportions of flark and hummock microhabitats in each successional stage. Long-term average peat N accumulation rates for the sites (total accumulation divided by the year since peatland initiation; ref. 23) is shown with filled circles. (C) Incorporation of 13CH4-C into the biomass (moss + microbes) based on the weighted averages of flark and hummock microhabitats in each successional stage. In each bar, the filled area indicates the incorporation of 13CH4-derived C in the dark (i.e., incorporation of CH4 into methanotroph biomass) and the open area indicates the average additional incorporation of 13CH4-derived C under prevailing light conditions (i.e., incorporation of CO2 emitted by methanotrophs into autotrophic plant or microbial biomass via photosynthesis).
In all studied peatland stages except wet meadows, incubation under prevailing light conditions resulted in enhanced N2 fixation, on average threefold in comparison with the dark treatments, which suggests that phototrophic organisms may be the most active N2 fixers or that photosynthesis provides carbohydrates to fuel heterotrophic N2 fixers (Fig. 2A). Methane-induced N2 fixation contributed approximately 40% (33–47%) of the N2 fixation in the three younger peatland stages, but was negligible in the fen-bog transition stage (Fig. 2 A and B). Thus, the hypothesis of a larger methanotrophic contribution to N2 fixation in late successional stages was not supported. The rate of the biomass incorporation of 13CH4-derived C indicated that moss-associated methanotrophy was also highest in mesotrophic fens and continued at moderate rates in the flarks of the older stages (Fig. 2C), where CH4 addition did not enhance N2 fixation. The meadows showed significantly lower CH4 oxidation rates compared with the other stages (Table S2). A comparison of the successional patterns of N accumulation and N2 fixation (Fig. 2B) provides further evidence of the ecological and biogeochemical importance of N2 fixation and methanotrophic N2 fixation during peatland development. Based on peat profiles from our sites, 70% of N and 40% of C accumulated during the first 1,000 y of the 2,500-y period (23), a period during which N2 fixation peaked and had the strongest response to CH4 addition. This pattern implies that methanotrophic N2 fixation contributes to rapid N accumulation in the fen stages. The predicted warming conditions in the northern latitudes (27) may impact on boreal peatland development in two ways: At the southern limit of permafrost, melting is promoting a reverse succession from ombrotrophic bog to fen ecosystem, whereas at the southern border of the fen region (aapamires, wet fen-dominated peatland complexes) drainage due to increased evapotranspiration may accelerate ombrotrophication, i.e., succession toward bog ecosystem (28). Our results indicate that these successional changes are likely to lead to changes in N2 dynamics.
Based on the growth rates of Sphagnum species at our sites (26) and site-specific N content of the Sphagnum species (Table S4), we estimate that N obtained by N2 fixation could correspond to an average 37 ± 18% (mean ± SEM, range 4–58%, n = 6) of the moss biomass N increment. This proportion is in agreement with the recent estimate of 35% for Sphagnum riparium, inferred from N content and growth rate in a 2-mo laboratory experiment (6). Despite the low absolute areal rate of N2 fixation in the older mainly rainwater-fed stages (fen-bog transitions), where moss growth is more nutrient limited, the comparison of Sphagnum growth rates (26) and N contents further suggests that the proportion of fixed N of the new biomass N increment may increase to 58%. The rest of the N is, we presume, being taken up as inorganic ions and organic N, or recycled to new growth from older parts of the moss shoot (29). The time scale at which N fixed in the moss becomes available for the moss host and for other plants may range from fast exchange over a time span of days (6, 11) to slower nutrient release from decomposing Sphagnum litter over a period of years.
Our results showed faster CH4-C biomass incorporation in light than in dark (Fig. 2C), which suggests that mosses fixed additional CH4-derived CO2 during photosynthesis. The contribution of CH4-derived C was 26% in light, but 10% in dark (calculated as above for N) for Sphagnum C in the flarks. In hummocks, where CH4 and CO2 concentrations are at atmospheric levels, CH4-derived C contributed to only 0–3% of the incorporated C in light and dark. These findings indicate that CH4 can be a significant C source for submerged Sphagnum, supporting the results of previous studies (3, 4).
The moss ratios of N:P, C:N, and C:P, integrated indices of nutrient availability, showed decreasing ratios with increasing N2 fixation activity (Spearman ρ −0.32, −0.61, and −0.54, for N:P, C:N, and C:P, respectively, P < 0.003). The successional pattern in N2 fixation rates was best explained by P availability (Fig. 3 and Tables S4 and S5). The highest rates of N2 fixation were associated with the lowest moss N:P ratios, being lower than the threshold for N limitation in soil microbes (N:P < 7:1; ref. 30). Thus, N2 fixers were able to respond to N demand relative to P supply, as shown (e.g., ref. 31). In the older stages, N:P ratios indicated that plant growth was limited by P (32), yet active moss associated N2 fixation persisted there as well (Figs. 2 and 3).
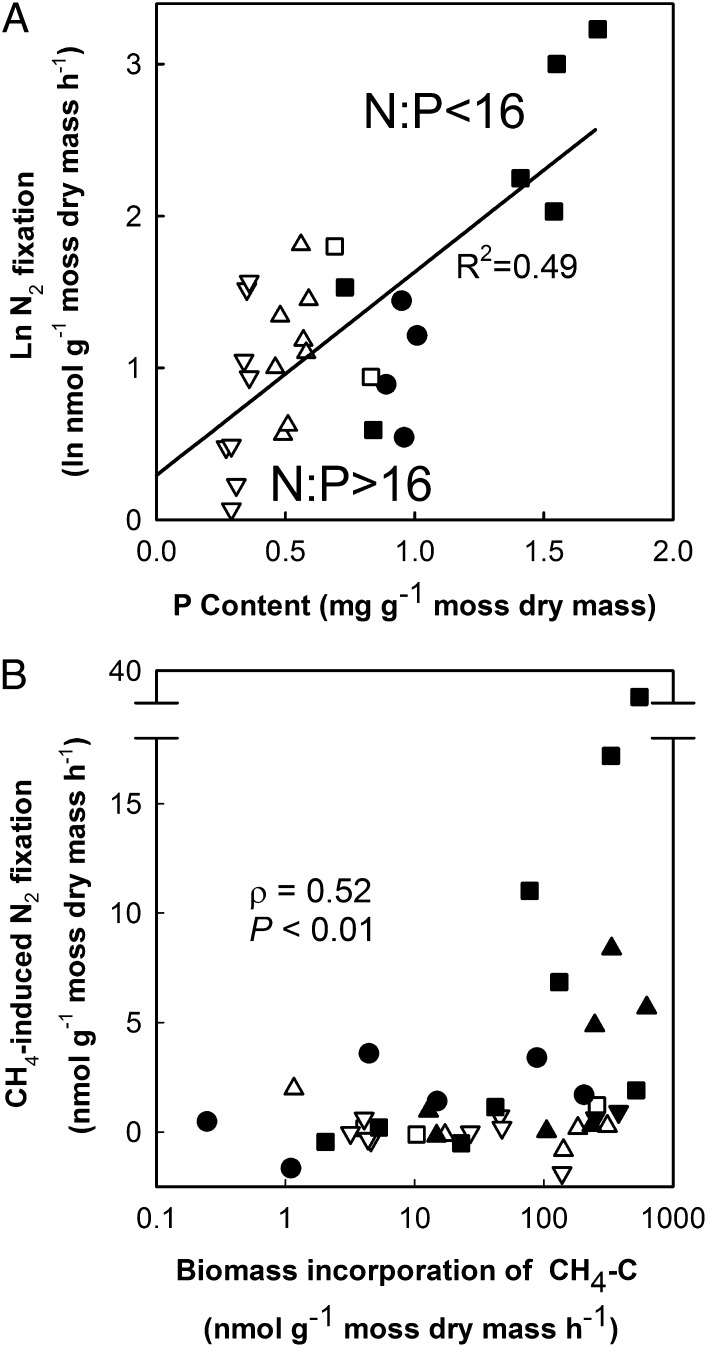
Fig. 3.
The relationships between the rates of N2 fixation, CH4 oxidation and phosphorus content in Sphagnum moss samples of different successional stages of peatlands (●, meadow; ■, mesotrophic fen; ▲, oligotrophic fen, and ▼, fen-bog transition). (A) The relationship between N2 fixation (ln transformed) and moss P content (ln N2 fixation = 1.25 ± 0.14[P content]+0.36 ± 0.13 (coefficient ± SE), R2 = 0.49, df reg, res 1,82; Table S5). The moss N:P ratios >16 indicating P limitation (32) are shown with open symbols. Averages for all four treatments per microhabitat and stage are shown (n = 84 incubations). (B) The Spearman rank correlation between the rates of CH4 oxidation (measured as biomass incorporation of CH4-derived C) and CH4-induced N2 fixation (calculated as the difference in N2 fixation between 15N2+13CH4 and 15N2 only treatments). The moss P content <0.5 mg⋅g−1 is indicated by open symbols (n = 42 incubations).
Although the moss P content was the sole significant predictor of N2 fixation in the light treatment without CH4, the contribution of CH4-induced N2 fixation was best explained by the combination of the moss P and Fe contents (Table S5). Among the sampled microhabitats, moss Fe content correlated strongly with the water table depth below the moss capitula and pH (Table S4). Thus, provided that P supply was sufficient, CH4-induced N2 fixation correlated with CH4 oxidation (hypothesis 2; Fig. 3), but the rate of CH4 oxidation as such was not the primary factor controlling CH4-induced N2 fixation in these sites. The CH4 oxidation was, in turn, best explained by the moss Fe content that depended on the water table level (Fig. S1). Concurrent CH4 concentration in pore water did not significantly correlate with N2 fixation (Spearman rank correlation P = 0.96, n = 84) or biomass incorporation of CH4-derived C (P = 0.16, n = 42).
Overall, our results demonstrate that methanotrophy and N2 fixation are tightly linked in the wet fen depressions and that methanotrophic activity enhances N2 fixation (hypothesis 1). However, because the interactions within the endosymbiotic microbial communities complicate the analysis, we do not directly interpret the experimentally observed dark, light-induced, and CH4-induced rates as heterotrophic, phototrophic, and methanotrophic N2 fixation. The experimental approach cannot distinguish whether the CH4-induced N2 fixation equals methanotrophic N2 fixation, because the observed CH4 induction may be, at least partly, indirect. The higher CO2 concentrations that result from CH4 oxidation inside the Sphagnum cells would enhance phototrophic N2 fixation (Fig. 4). Cell-level identification of the correct nutritional mode of the N2 fixing organisms may require single cell analysis, which is possible by using secondary ion mass spectrometry. Similarly, dark and light-induced N2 fixation may not equal phototrophic and heterotrophic N2 fixation, because light can increase the cellular concentration of C substrates and oxygen, which are to some extent essential to N2 fixation of aerobic heterotrophs.
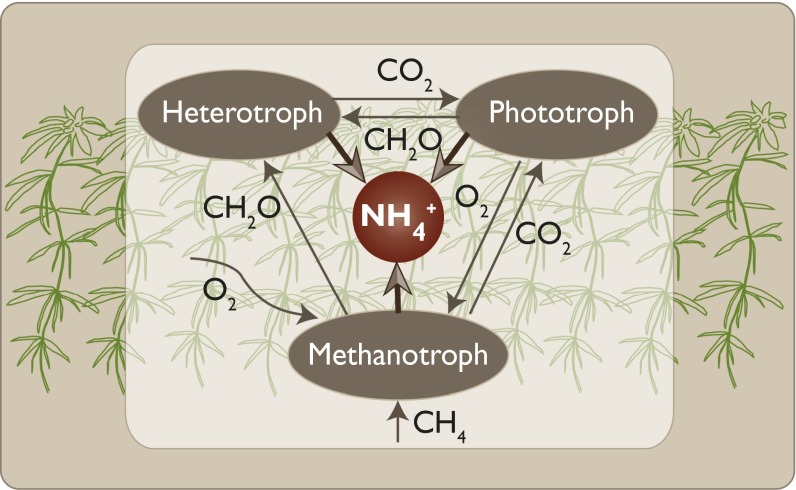
Fig. 4.
The interplay of N2-fixing bacteria in the upper moss vegetation. Phototrophic, heterotrophic, and methanotrophic bacteria all take part in the fixation of atmospheric N2 to plant available N (NH4+). The supply of CH4 may support the direct methanotrophic N2 fixation, but it may also provide additional CO2 for phototrophic microbes, as well as for the moss itself. Both phototrophs and methanotrophs provide organic molecules (CH2O) to heterotrophic N2 fixers. Methanotrophic activity may also decrease the oxygen tension, providing a more suitable microenvironment for the oxygen-sensitive nitrogenase enzyme complex.
In all, our findings imply that interlinks of N2 fixation and CH4 cycling constitute an important mechanism that fulfils the need of new N and explains the rapid peat and N accumulation rates during the fen stages of peatland development. Our results further support the idea that N2 fixation is the primary mechanism linking N and P availability, as suggested for terrestrial and aquatic ecosystems (e.g., refs. 31 and 33). Both the N2 fixation rates and the methanotrophic contribution to N2 fixation along the peatland succession gradient were primarily governed by P availability. The successional patterns of different microbial groups involved in N2 fixation were further regulated by water table level and Fe content, which controlled both the N2 fixation and CH4 oxidation activities of the plant-associated bacteria. In conclusion, moss-associated microbes may explain the dominance of Sphagnum mosses in nutrient-poor peatlands: besides providing an additional in-cell CO2 source for photosynthesis, these microbial communities provide new N in the cold and acidic environment where decomposition and nutrient recycling are otherwise slow.
Materials and Methods
Field Site.
The Siikajoki peatland chronosequence is a replicated primary successional series of peatland ecosystems in the land-uplift coast of Bothnian Bay, Finland (64° 45′N, 24° 42′E; refs. 23 and 34). Our study sites at Siikajoki consisted of 12 peatlands that represent four successional stages, which vary in age from 200 to 2,500 y (Fig. 1). Each stage had three replicates (Table S1). The sites were located within 8 km on the coast of Bothnian Bay, where the rate of land uplift is approximately 8 mm⋅y−1 due to ongoing postglacial rebound (35). The mean annual temperature is 2.3 °C, and the mean annual precipitation 521 mm. The inorganic N deposition rate in the region is 0.3 g⋅m−2⋅y−1 (36).
The peatlands, 0.5–1.5 ha in size, have developed in depressions between sand dune formations with similar underlying soil (18, 37, 38). Along the chronosequence, the vegetation changes from flooded Carex nigra and Agrostis canina dominated meadows in the early stages to Carex-dominated mesotrophic and oligotrophic fens and to fen-bog transitions where the bog vegetation is characterized by an increasing cover of dwarf shrubs in hummock surfaces. The moss layer in the meadows is sparse (total moss cover < 20%) and dominated by Warnstorfia spp. mosses, with a Sphagnum cover of approximately 2%. In the fen and bog stages, Sphagnum mosses cover 50–70% of the peat surface (total moss cover 55–90%): The fen stages show a distinct development of microtopography with Sphagnum subsecundum and Sphagnum majus dominated flarks (wet depressions) followed by Sphagnum fimbriatum and then Sphagnum papillosum dominated hummocks which lead in the oldest stages to colonization by Sphagnum fuscum, a species characteristic of bog hummocks.
Field Experiment.
In early June 2010, we collected the dominant Sphagnum mosses from both flark and hummock habitats at each site. Moss sampling was based on a vegetation survey made earlier at the sites (23). We incubated the moss samples in situ for 2 d under three treatments (A 15N2+13CH4, B 15N2, and C unlabeled control), each under prevailing light conditions (day length 20 h) and in the dark. We hypothesized that in the 15N2+13CH4 treatment under prevailing light conditions, all potential diazotrophs (cyanobacteria, heterotrophs, methanotrophs) can be active. In the 15N2 treatment under prevailing light, cyanobacteria and heterotrophs can actively fix N2. The 15N2 treatment in the dark will provide information on the activity of heterotrophs other than obligate methanotrophs. Additionally, the 13CH4 treatments will determine the incorporation of CH4-derived C in to the microbial biomass. Under prevailing light, this treatment will also show the potential of mosses to use CH4-derived CO2 in photosynthesis. Mosses (1 g in dry mass) were incubated in situ in 120-mL glass vials. Only the upper, live parts were used in the incubation, and 10 mL of water from the sampling site was added into the vials to keep the samples moist. A volume of 20 mL of air was removed, and 20 mL of 15N2 tracer gas [98% (vol/vol) enriched; Cambridge Isotope Laboratories] and 1.2 mL of 13CH4 tracer gas [99% (vol/vol) enriched; CK Gas Products] were injected into the vials to reach headspace enrichment levels of 21% (vol/vol) and 99% (vol/vol) for 15N2 and 13CH4, respectively. Control samples were incubated in the ambient air, and the dark-treated samples were covered with aluminum foil. The vials were placed where the samples were collected, so that half of the vial remained above the water level. Incubations were terminated after 45 h by opening the vials, emptying them of water, and freezing them at −20 °C. Then the samples were dried at +50 °C to a constant mass and analyzed for their bulk δ15N and δ13C values and C and N contents using a stable isotope ratio mass spectrometer as described in ref. 8. The internal precision (SD of replicate standards) of the isotope analysis was always better than 1.5‰ for δ15N and 0.1 for δ13C.
During the field incubations, the temperature in the moss layer (measured by using iButton; Maxim) averaged 14.7 °C, with a range of 5–30 °C, and did not differ significantly among the sites. In each microhabitat, we measured the water table depth within a pipe well and drew pore water samples to determine pH and dissolved CH4 concentrations. The CH4 concentration in water samples of 10 mL was determined by using the headspace equilibration technique (39) by following ref. 4. The concentration of CH4 was analyzed in the gas headspace by using a gas chromatograph equipped with flame ionization detector (Agilent 7890A; Agilent Technologies). Dissolved CH4 concentrations were calculated from headspace concentrations according to Henry’s law by using the values after ref. 40. We took volumetric samples of each moss species from the uppermost 10 cm to determine density. After removing debris and other mosses, volumetric samples of Sphagnum were dried at 60 °C for 48 h and weighed to convert the incubation results to N2 fixation and CH4-C biomass incorporation (CH4 oxidation) rates per peatland surface area (m2). A subset of plots (n = 1 in each stage) was surveyed for the relative proportions of flark and hummock habitats by measuring the water table depth in a network of 8–12 wells. Phosphorus, Fe, and Mo (below detection limit) contents in the tissue of the incubated Sphagnum samples were extracted with NHO3 by using wet digestion (EPA-3051) and measured by using a plasma emission spectrometer (ICP-OES; IRIS Intrepid ll XSP).
Data Analyses.
The rates of N2 fixation and CH4 oxidation measured as CH4-C incorporation in to the moss biomass were calculated as enrichment values and thereafter as fixation rates (nmol⋅g−1 of dry moss⋅h−1) by following ref. 8. The values represent the actual 15N and 13C uptake in each sample during the 45-h incubation. The rates of N2 fixation, CH4-induced N2 fixation, and CH4-C biomass incorporation were natural-log (ln) transformed to meet the requirements of normality. The effects of terrestrial age and treatments on N2 fixation rates were analyzed with four-way nested analysis of covariance (ANCOVA) with successional stage, site nested within the successional stage, light treatment, and CH4 treatment as the four factors. Water table depth was used as a covariate to take in to account microtopography. The effects of terrestrial age and light on the rates of biomass incorporation of CH4-derived C were analyzed with three-way nested ANCOVA with successional stage, site nested within the successional stage and light treatment as the three factors and water table depth as a covariate. Pair-wise differences in successional stages were tested by using Bonferroni post hoc tests. The relationships between environmental variables (nutrient contents, water table depth, water pH, and CH4 concentration) and N2 fixation, CH4-induced N2 fixation, and the biomass incorporation of CH4-derived C were analyzed by using Spearman rank correlations and stepwise regressions.
Supplementary Material
Acknowledgments
We thank L. Kuusisto for nutrient analyses, T. Sinisalo for help in the stable isotope mass spectrometer analyses, I. Murtovaara for designing the schematic figures, and D. Wilson for English revision. Funding was provided by Academy of Finland Projects 121535 (to T.L.), 131409 and 218101 (to E.-S.T.), and 260797 (to M.T.), and by Biological Interactions Graduate School (S.M.L.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1314284111/-/DCSupplemental.
References
- 1.Gorham E. Northern peatlands: Role in the carbon cycle and probable responses to climatic warming. Ecol Appl. 1991;1(2):182–195. doi: 10.2307/1941811. [DOI] [PubMed] [Google Scholar]
- 2.Tarnocai C, et al. Soil organic carbon pools in the northern circumpolar permafrost region. Global Biogeochem Cycles. 2009;23 doi: 10.1029/2008GB003327. [DOI] [Google Scholar]
- 3.Raghoebarsing AA, et al. Methanotrophic symbionts provide carbon for photosynthesis in peat bogs. Nature. 2005;436(7054):1153–1156. doi: 10.1038/nature03802. [DOI] [PubMed] [Google Scholar]
- 4.Larmola T, et al. The role of Sphagnum mosses in the methane cycling of a boreal mire. Ecology. 2010;91(8):2356–2365. doi: 10.1890/09-1343.1. [DOI] [PubMed] [Google Scholar]
- 5.Aerts R, Wallen B, Malmer N. Growth-limiting nutrients in Sphagnum-dominated bogs subject to low and high atmospheric nitrogen supply. J Ecol. 1992;80(1):131–140. [Google Scholar]
- 6.Berg A, Danielsson Å, Svensson BH. Transfer of fixed-N from N2-fixing cyanobacteria associated with the moss Sphagnum riparium results in enhanced growth of the moss. Plant Soil. 2013;362(1-2):271–278. [Google Scholar]
- 7.DeLuca TH, Zackrisson O, Nilsson M-C, Sellstedt A. Quantifying nitrogen-fixation in feather moss carpets of boreal forests. Nature. 2002;419(6910):917–920. doi: 10.1038/nature01051. [DOI] [PubMed] [Google Scholar]
- 8.Leppänen SM, Salemaa M, Smolander A, Mäkipää R, Tiirola M. Nitrogen fixation in forest mosses along a N deposition gradient. Environ Exp Bot. 2013;90:62–69. [Google Scholar]
- 9.Granhall U, Selander H. Nitrogen fixation in a subarctic mire. Oikos. 1973;24(1):8–15. [Google Scholar]
- 10.Bragina A, Berg C, Müller H, Moser D, Berg G. Insights into functional bacterial diversity and its effects on Alpine bog ecosystem functioning. Sci Rep. 2013;3:1955. doi: 10.1038/srep01955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Basilier K, Granhall U, Stenstrom TA. Nitrogen fixation in wet minerotrophic moss communities of a subarctic mire. Oikos. 1978;31(2):236–246. [Google Scholar]
- 12.Hardy RWF, Holsten RD, Jackson EK, Burns RC. The acetylene-ethylene assay for n(2) fixation: Laboratory and field evaluation. Plant Physiol. 1968;43(8):1185–1207. doi: 10.1104/pp.43.8.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Flett RJ, Rudd JWM, Hamilton RD. Acetylene reduction assays for nitrogen fixation in freshwaters: A note of caution. Appl Microbiol. 1975;29(5):580–583. doi: 10.1128/am.29.5.580-583.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Davis JB, Coty VF, Stanley JP. ATMOSPHERIC NITROGEN FIXATION BY METHANE-OXIDIZING BACTERIA. J Bacteriol. 1964;88(2):468–472. doi: 10.1128/jb.88.2.468-472.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Auman AJ, Speake CC, Lidstrom ME. nifH sequences and nitrogen fixation in type I and type II methanotrophs. Appl Environ Microbiol. 2001;67(9):4009–4016. doi: 10.1128/AEM.67.9.4009-4016.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gavazov KS, Soudzilovskaia NA, van Logtestijn RSP, Braster M, Cornelissen JHC. Isotopic analysis of cyanobacterial nitrogen fixation associated with subarctic lichen and bryophyte species. Plant Soil. 2010;333(1-2):507–517. [Google Scholar]
- 17.Juottonen H, et al. Methane-cycling microbial communities and methane emission in natural and restored peatlands. Appl Environ Microbiol. 2012;78(17):6386–6389. doi: 10.1128/AEM.00261-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leppälä M, Oksanen J, Tuittila E-S. Methane flux dynamics during mire succession. Oecologia. 2011;165:489–499. doi: 10.1007/s00442-010-1754-6. [DOI] [PubMed] [Google Scholar]
- 19.Nykänen H, Alm J, Silvola J, Tolonen K, Martikainen P. Methane fluxes on boreal peatlands of different fertility and the effect of long-term experimental lowering of the water table on flux rates. Global Biogeochem Cycles. 1998;12(1):53–69. [Google Scholar]
- 20.Bodelier PLE, Laanbroek HJ. Nitrogen as a regulatory factor of methane oxidation in soils and sediments. FEMS Microbiol Ecol. 2004;47(3):265–277. doi: 10.1016/S0168-6496(03)00304-0. [DOI] [PubMed] [Google Scholar]
- 21.Chowdbury TR, Dick RP. Ecology of aerobic methanotrophs in controlling methane fluxes from wetlands. Appl Soil Ecol. 2013;65:8–22. [Google Scholar]
- 22.Zehr JP, Jenkins BD, Short SM, Steward GF. Nitrogenase gene diversity and microbial community structure: a cross-system comparison. Environ Microbiol. 2003;5(7):539–554. doi: 10.1046/j.1462-2920.2003.00451.x. [DOI] [PubMed] [Google Scholar]
- 23.Tuittila E-S, et al. Wetland chronosequence as a model of peatland development: Vegetation succession, peat and carbon accumulation. Holocene. 2013;23(1):25–35. [Google Scholar]
- 24.Waughman GJ, Bellamy DJ. Nitrogen fixation and the nitrogen balance in peatland ecosystems. Ecology. 1980;61(5):1185–1198. [Google Scholar]
- 25.Chapman RR, Hemond HF. Dinitrogen fixation by surface peat and Sphagnum in an ombrotrophic bog. Can J Bot. 1982;60(5):538–543. [Google Scholar]
- 26.Laine AM, Juurola E, Hájek T, Tuittila E-S. Sphagnum growth and ecophysiology during mire succession. Oecologia. 2011;167(4):1115–1125. doi: 10.1007/s00442-011-2039-4. [DOI] [PubMed] [Google Scholar]
- 27. Intergovernmental Panel on Climate Change (2007): The Physical Science Basis. Working Group I Contribution to the IPCC Fourth Assessment Report, eds Solomon S, Qin D, Manning M, Chen Z, Marquis M, Averyt KB, Tignor M, Miller HL (Cambridge Univ Press, Cambridge, UK)
- 28.Tahvanainen T. Abrupt ombrotrophication of a boreal aapa mire triggered by hydrological disturbance in the catchment. J Ecol. 2011;99(2):404–415. [Google Scholar]
- 29.Aldous AR. Nitrogen translocation in Sphagnum mosses: effects of atmospheric nitrogen deposition. New Phytol. 2002;156(2):241–253. doi: 10.1046/j.1469-8137.2002.00518.x. [DOI] [PubMed] [Google Scholar]
- 30.Cleveland CC, Liptzin D. C:N:P stoichiometry in soil: Is there a “Redfield ratio” for the microbial biomass? Biogeochemistry. 2007;85(3):235–252. [Google Scholar]
- 31.Houlton BZ, Wang Y-P, Vitousek PM, Field CB. A unifying framework for dinitrogen fixation in the terrestrial biosphere. Nature. 2008;454(7202):327–330. doi: 10.1038/nature07028. [DOI] [PubMed] [Google Scholar]
- 32.Reich PB, Oleksyn J. Global patterns of plant leaf N and P in relation to temperature and latitude. Proc Natl Acad Sci USA. 2004;101(30):11001–11006. doi: 10.1073/pnas.0403588101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schindler DW. Evolution of phosphorus limitation in lakes. Science. 1977;195(4275):260–262. doi: 10.1126/science.195.4275.260. [DOI] [PubMed] [Google Scholar]
- 34.Merilä P, et al. Methanogen communities along a primary succession transect of mire ecosystems. FEMS Microbiol Ecol. 2006;55(2):221–229. doi: 10.1111/j.1574-6941.2005.00030.x. [DOI] [PubMed] [Google Scholar]
- 35.Ekman M. A consistent map of the postglacial uplift of Fennoscandia. Terra Nova. 1996;8(2):158–165. [Google Scholar]
- 36.Mustajärvi K, et al. Fluxes of dissolved organic and inorganic nitrogen in relation to stand characteristics and latitude in Scots pine and Norway spruce stands in Finland. Bor. Environ. Res. 2008;13(suppl.B):3–21. [Google Scholar]
- 37.Leppälä M, Kukko-Oja K, Laine J, Tuittila E-S. Seasonal dynamics of CO2 exchange during primary succession of boreal mires as controlled by phenology of plants. Ecoscience. 2008;15(4):460–471. [Google Scholar]
- 38.Leppälä M, Laine AM, Seväkivi M-L, Tuittila E-S. Differences in CO2 dynamics between successional mire plant communities during wet and dry summers. J Veg Sci. 2011;22(2):357–366. [Google Scholar]
- 39.McAuliffe CC. GC determination of solutes by multiple phase equilibration. Chem Technol. 1971;1:46–51. [Google Scholar]
- 40.Lide R, Fredrikse HPR, editors. CRC Handbook of Chemistry and Physics. 76th Ed. Boca Raton, FL: CRC; 1995. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.