Abstract
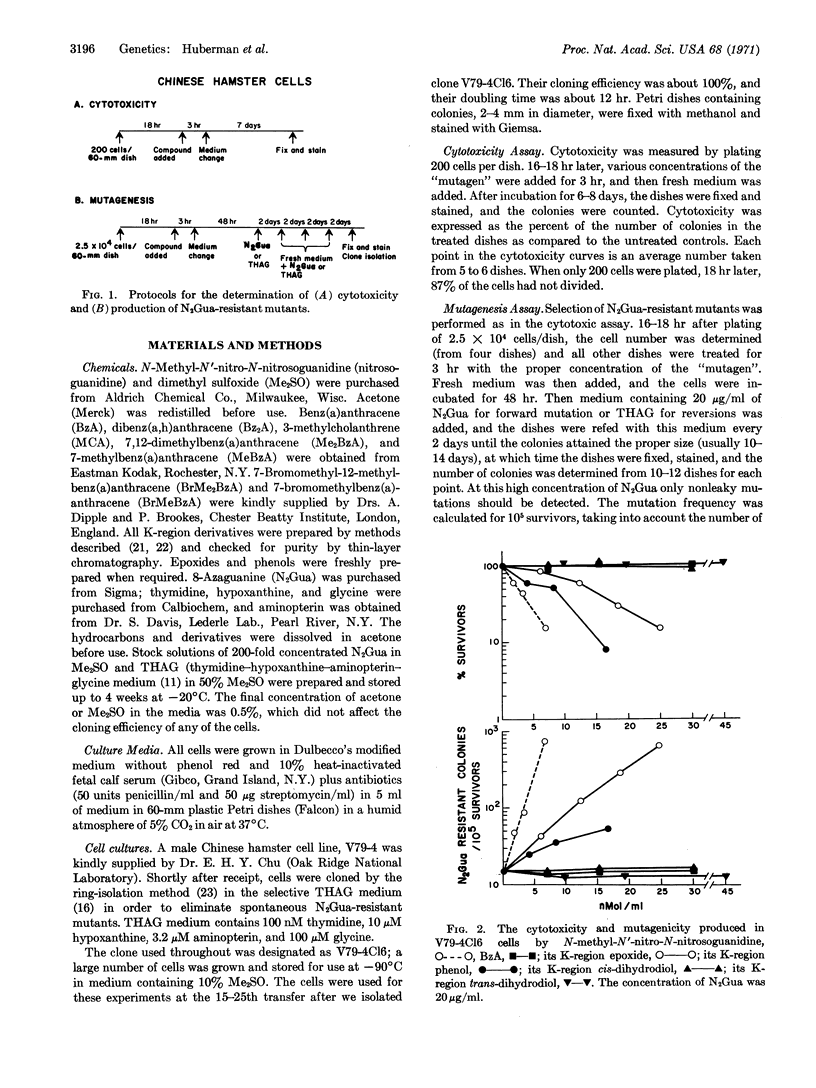
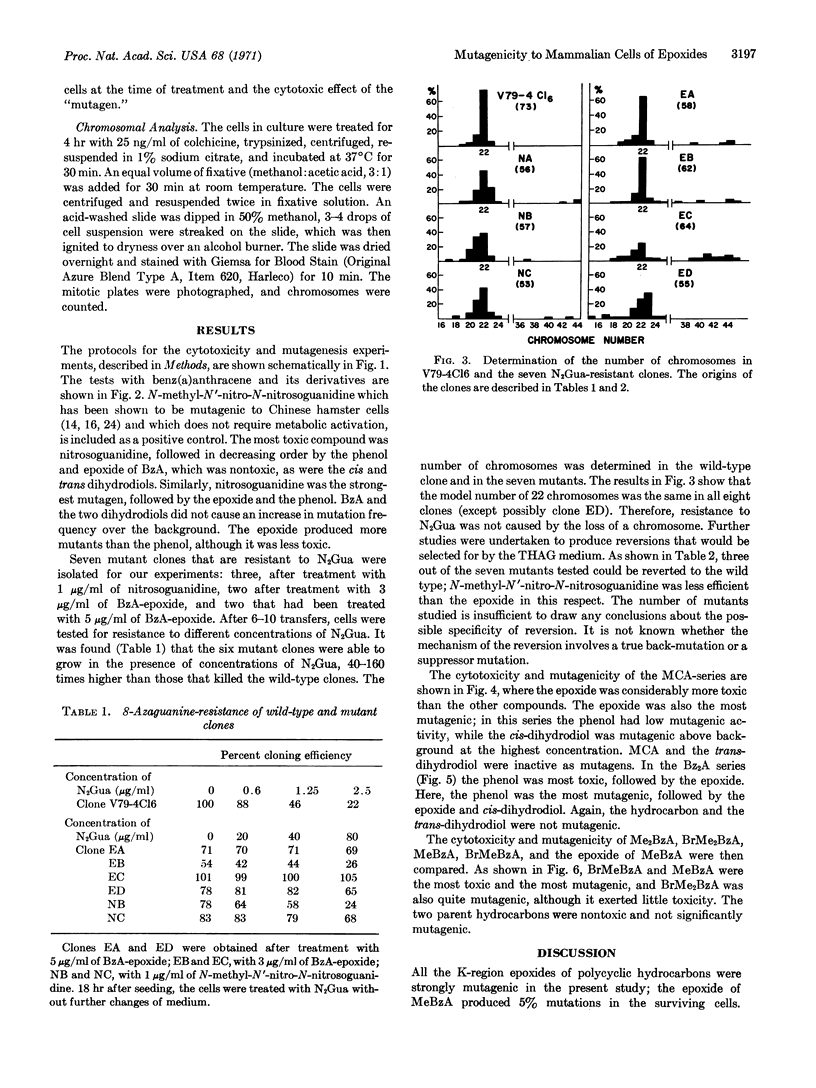
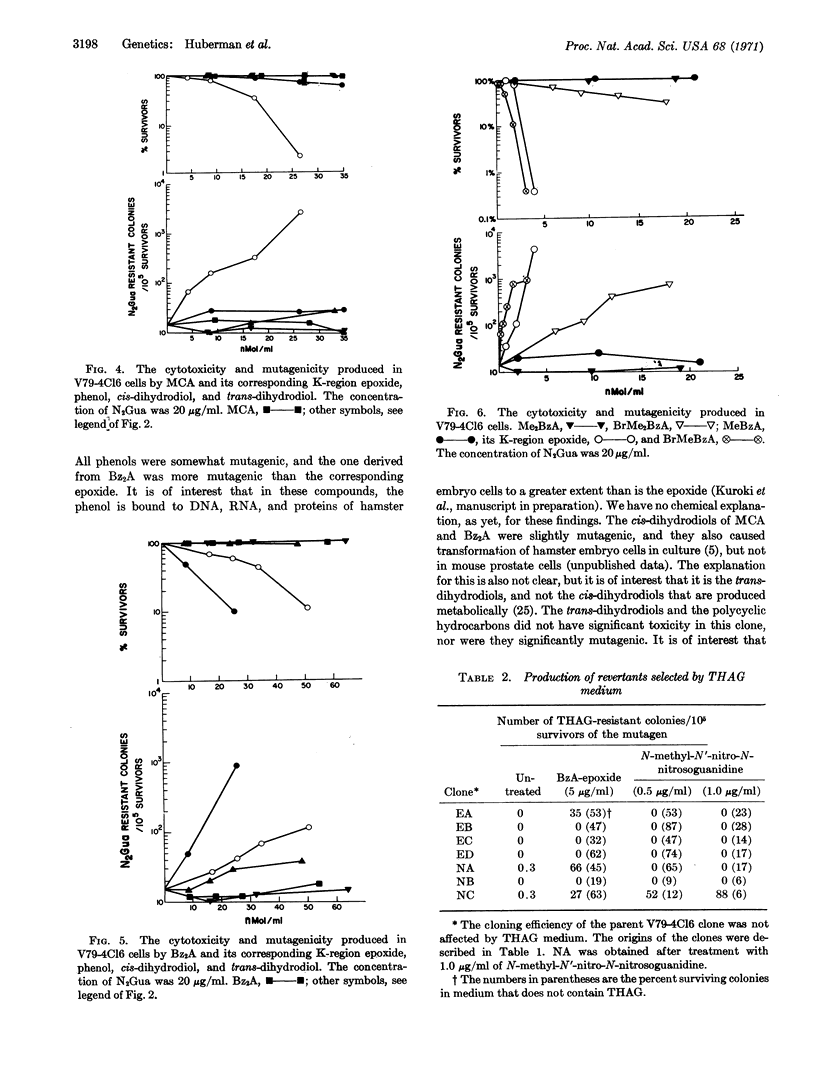
The cytotoxicity and mutagenicity of several polycyclic hydrocarbons and their K-region derivatives were tested in a clone of Chinese hamster cells; the production of clones resistant to 8-azaguanine was used as the marker for mutagenesis. In the series related to benz(a)anthracene, the K-region epoxide was highly mutagenic, and the phenol was less mutagenic; the hydrocarbon and cis and trans-dihydrodiols were not mutagenic. Seven resistant clones were isolated and retained their drug-resistance; three of these could be reverted to the wild type. There was no difference in the chromosome numbers among the parent and mutant clones. The results in the methylcholanthrene series were similar to those for benz(a)anthracene. However, in the dibenz(a,h)anthracene series, the phenol was more mutagenic than the epoxide. 7-Methylbenz(a)anthracene epoxide and 7-bromomethylbenz(a)anthracene were highly mutagenic, 7-bromomethyl-12-methylbenz(a)anthracene was less mutagenic, and the parent hydrocarbons were inactive. These results demonstrate that metabolic activation of polycyclic hydrocarbon is required for mutagenic activity in mammalian cells.
Keywords: tissue culture, carcinogens
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albertini R. J., Demars R. Diploid azaguanine-resistant mutants of cultured human fibroblasts. Science. 1970 Jul 31;169(3944):482–485. doi: 10.1126/science.169.3944.482. [DOI] [PubMed] [Google Scholar]
- Chu E. H., Brimer P., Jacobson K. B., Merriam E. V. Mammalian cell genetics. I. Selection and characterization of mutations auxotrophic for L-glutamine or resistant to 8-azaguanine in Chinese hamster cells in vitro. Genetics. 1969 Jun;62(2):359–377. doi: 10.1093/genetics/62.2.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu E. H., Malling H. V. Mammalian cell genetics. II. Chemical induction of specific locus mutations in Chinese hamster cells in vitro. Proc Natl Acad Sci U S A. 1968 Dec;61(4):1306–1312. doi: 10.1073/pnas.61.4.1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu E. H. Mammalian cell genetics. 3. Characterization of x-ray-induced forward mutations in Chinese hamster cell cultures. Mutat Res. 1971 Jan;11(1):23–34. doi: 10.1016/0027-5107(71)90029-7. [DOI] [PubMed] [Google Scholar]
- Corbett T. H., Heidelberger C., Dove W. F. Determination of the mutagenic activity to bacteriophage T4 of carcinogenic and noncarcinogenic compounds. Mol Pharmacol. 1970 Nov;6(6):667–679. [PubMed] [Google Scholar]
- DE MARS R., HOOPER J. L. A method of selecting for auxotrophic mutants of HeLa cells. J Exp Med. 1960 Apr 1;111:559–572. doi: 10.1084/jem.111.4.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dipple A., Lawley P. D., Brookes P. Theory of tumour initiation by chemical carcinogens: dependence of activity on structure of ultimate carcinogen. Eur J Cancer. 1968 Oct;4(5):493–506. doi: 10.1016/0014-2964(68)90005-4. [DOI] [PubMed] [Google Scholar]
- FORD D. K., YERGANIAN G. Observations on the chromosomes of Chinese hamster cells in tissue culture. J Natl Cancer Inst. 1958 Aug;21(2):393–425. [PubMed] [Google Scholar]
- Grover P. L., Sims P., Huberman E., Marquardt H., Kuroki T., Heidelberger C. In vitro transformation of rodent cells by K-region derivatives of polycyclic hydrocarbons. Proc Natl Acad Sci U S A. 1971 Jun;68(6):1098–1101. doi: 10.1073/pnas.68.6.1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grover P. L., Sims P. Interactions of the K-region epoxides of phenanthrene and dibenz (a,h)anthracene with nucleic acids and histone. Biochem Pharmacol. 1970 Jul;19(7):2251–2259. doi: 10.1016/0006-2952(70)90124-3. [DOI] [PubMed] [Google Scholar]
- Jerina D. M., Daly J. W., Witkop B., Zaltzman-Nirenberg P., Udenfriend S. 1,2-naphthalene oxide as an intermediate in the microsomal hydroxylation of naphthalene. Biochemistry. 1970 Jan 6;9(1):147–156. doi: 10.1021/bi00803a019. [DOI] [PubMed] [Google Scholar]
- Kao F. T., Puck T. T. Genetics of somatic mammalian cells, VII. Induction and isolation of nutritional mutants in Chinese hamster cells. Proc Natl Acad Sci U S A. 1968 Aug;60(4):1275–1281. doi: 10.1073/pnas.60.4.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao F. T., Puck T. T. Genetics of somatic mammalian cells. IX. Quantitation of mutagenesis by physical and chemical agents. J Cell Physiol. 1969 Dec;74(3):245–258. doi: 10.1002/jcp.1040740305. [DOI] [PubMed] [Google Scholar]
- Miller J. A. Carcinogenesis by chemicals: an overview--G. H. A. Clowes memorial lecture. Cancer Res. 1970 Mar;30(3):559–576. [PubMed] [Google Scholar]
- PUCK T. T., MARCUS P. I., CIECIURA S. J. Clonal growth of mammalian cells in vitro; growth characteristics of colonies from single HeLa cells with and without a feeder layer. J Exp Med. 1956 Feb 1;103(2):273–283. doi: 10.1084/jem.103.2.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puck T. T., Kao F. T. Genetics of somatic mammalian cells. V. Treatment with 5-bromodeoxyuridine and visible light for isolation of nutritionally deficient mutants. Proc Natl Acad Sci U S A. 1967 Sep;58(3):1227–1234. doi: 10.1073/pnas.58.3.1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salzmann J., DeMars R., Benke P. Single-allele expression at an X-linked hyperuricemia locus in heterozygous human cells. Proc Natl Acad Sci U S A. 1968 Jun;60(2):545–552. doi: 10.1073/pnas.60.2.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seegmiller J. E., Rosenbloom F. M., Kelley W. N. Enzyme defect associated with a sex-linked human neurological disorder and excessive purine synthesis. Science. 1967 Mar 31;155(3770):1682–1684. doi: 10.1126/science.155.3770.1682. [DOI] [PubMed] [Google Scholar]
- Selkirk J. K., Huberman E., Heidelberger C. An epoxide is an intermediate in the microsomal metabolism of the chemical carcinogen, dibenz(a,h)anthracene. Biochem Biophys Res Commun. 1971 Jun 4;43(5):1010–1016. doi: 10.1016/0006-291x(71)90562-6. [DOI] [PubMed] [Google Scholar]
- Sims P. The metabolism of some aromatic hydrocarbons by mouse embryo cell cultures. Biochem Pharmacol. 1970 Jan;19(1):285–297. doi: 10.1016/0006-2952(70)90348-5. [DOI] [PubMed] [Google Scholar]


