Abstract
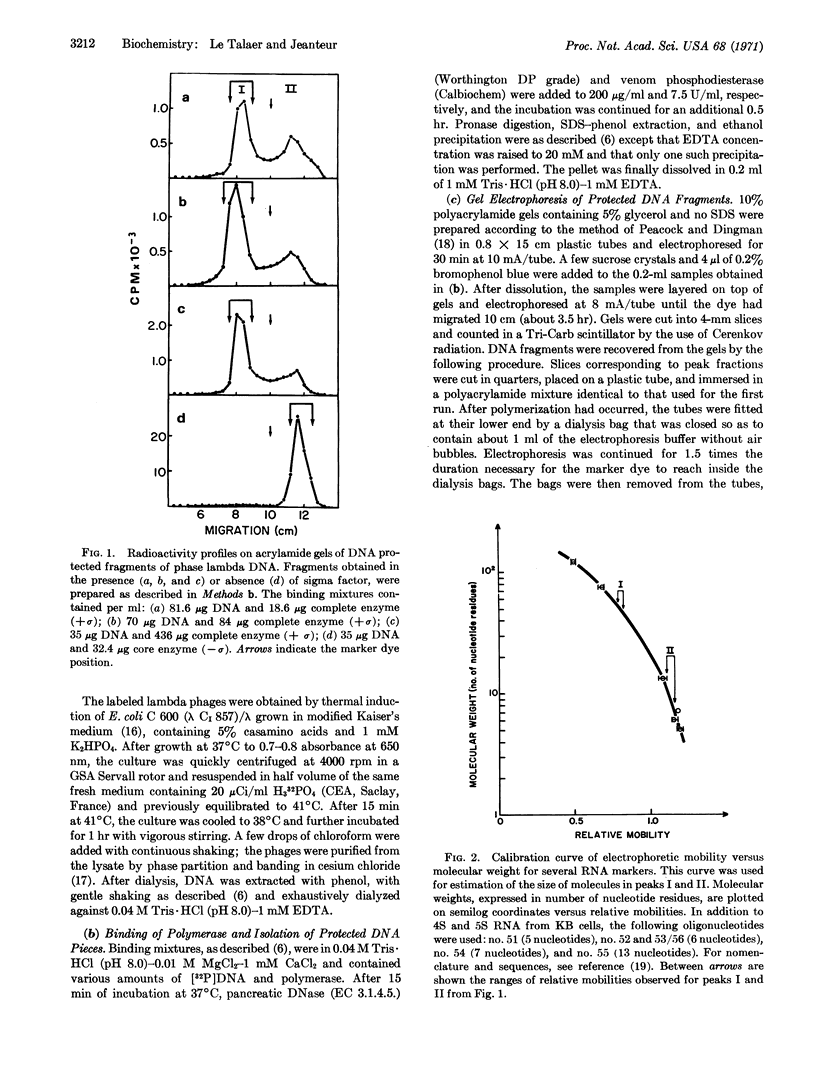
RNA-polymerase of Escherichia coli was allowed to bind to DNA of phage lambda in the absence of precursors. The resulting complex was excised by nuclease digestion and the protected DNA was recovered by phenol-extraction and ethanol precipitation. Acrylamide gel electrophoresis of protected DNA fragments reveals the existence of two distinct oligonucleotide peaks corresponding, respectively, to 45-52 and 7-10 nucleotide residues along with species of intermediate sizes. Peak I molecules have two properties: (a) their existence is dependent on the presence of sigma factor during the initial binding step, and (b) they are considerably enriched in A-T (up to 67%). On the contrary, peak II molecules have the same base composition as DNA of phage lambda, whether obtained in the presence or absence of sigma factor. Peak I molecules are thus believed to contain DNA sequences involved in promoter recognition, whether they are the promoters themselves, adjacent, or related sequences.
Keywords: E. coli, RNA polymerase, sigma factor
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alberts B. M., Amodio F. J., Jenkins M., Gutmann E. D., Ferris F. L. Studies with DNA-cellulose chromatography. I. DNA-binding proteins from Escherichia coli. Cold Spring Harb Symp Quant Biol. 1968;33:289–305. doi: 10.1101/sqb.1968.033.01.033. [DOI] [PubMed] [Google Scholar]
- Babinet C. A new method for the purification of RNA-polymerase. Biochem Biophys Res Commun. 1967 Mar 21;26(6):639–644. doi: 10.1016/s0006-291x(67)80119-0. [DOI] [PubMed] [Google Scholar]
- Bautz E. K., Bautz F. A. Initiation of RNA synthesis: the function of sigma in the binding of RNA polymerase to promoter sites. Nature. 1970 Jun 27;226(5252):1219–1222. doi: 10.1038/2261219a0. [DOI] [PubMed] [Google Scholar]
- Bishop D. H., Claybrook J. R., Spiegelman S. Electrophoretic separation of viral nucleic acids on polyacrylamide gels. J Mol Biol. 1967 Jun 28;26(3):373–387. doi: 10.1016/0022-2836(67)90310-5. [DOI] [PubMed] [Google Scholar]
- Bram S. Secondary structure of DNA depends on base composition. Nat New Biol. 1971 Aug 11;232(2):174–176. doi: 10.1038/newbio232174a0. [DOI] [PubMed] [Google Scholar]
- Burgess R. R. A new method for the large scale purification of Escherichia coli deoxyribonucleic acid-dependent ribonucleic acid polymerase. J Biol Chem. 1969 Nov 25;244(22):6160–6167. [PubMed] [Google Scholar]
- Burgess R. R., Travers A. A., Dunn J. J., Bautz E. K. Factor stimulating transcription by RNA polymerase. Nature. 1969 Jan 4;221(5175):43–46. doi: 10.1038/221043a0. [DOI] [PubMed] [Google Scholar]
- Dubert J. M., Hermier B., Babinet C. Essai de caractérisation immunochimique des fractions présentes dans une prépration de RNA polymérase d'E. coli. Biochimie. 1971;53(2):185–193. doi: 10.1016/s0300-9084(71)80050-0. [DOI] [PubMed] [Google Scholar]
- Fisher M. P., Dingman C. W. Role of molecular conformation in determining the electrophoretic properties of polynucleotides in agarose-acrylamide composite gels. Biochemistry. 1971 May 11;10(10):1895–1899. doi: 10.1021/bi00786a026. [DOI] [PubMed] [Google Scholar]
- Forget B. G., Weissman S. M. Nucleotide sequence of KB cell 5S RNA. Science. 1967 Dec 29;158(3809):1695–1699. doi: 10.1126/science.158.3809.1695. [DOI] [PubMed] [Google Scholar]
- Girard M., Marty L. Séparation électrophorétique en gels de polyacrylamide des formes mono, bi, et pluricaténaires de l'acide ribonucléique du poliovirus. Bull Soc Chim Biol (Paris) 1969 Jun 4;51(1):31–45. [PubMed] [Google Scholar]
- Hayward W. S., Green M. H. Effect of the lambda repressor on the binding of RNA polymerase to DNA. Proc Natl Acad Sci U S A. 1969 Nov;64(3):962–969. doi: 10.1073/pnas.64.3.962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones O. W., Berg P. Studies on the binding of RNA polymerase to polynucleotides. J Mol Biol. 1966 Dec 28;22(2):199–209. doi: 10.1016/0022-2836(66)90126-4. [DOI] [PubMed] [Google Scholar]
- KAISER A. D. The production of phage chromosome fragments and their capacity for genetic transfer. J Mol Biol. 1962 Apr;4:275–287. doi: 10.1016/s0022-2836(62)80005-9. [DOI] [PubMed] [Google Scholar]
- Le Talaer J. Y., Jeanteur P. Preferential binding of E. coli RNA-polymerase to A-T rich sequences of bacteriophage lambda DNA. FEBS Lett. 1971 Jan 30;12(5):253–256. doi: 10.1016/0014-5793(71)80190-4. [DOI] [PubMed] [Google Scholar]
- LePecq J. B., Baldwin R. L. The starting point and direction of lambda DNA replication. Cold Spring Harb Symp Quant Biol. 1968;33:609–620. doi: 10.1101/sqb.1968.033.01.067. [DOI] [PubMed] [Google Scholar]
- Lin S. Y., Riggs A. D. Lac repressor binding to DNA not containing the lac operator and to synthetic poly dAT. Nature. 1970 Dec 19;228(5277):1184–1186. doi: 10.1038/2281184a0. [DOI] [PubMed] [Google Scholar]
- Lubin M. Observations on the structure of RNA polymerase and its attachmnt to DNA. J Mol Biol. 1969 Jan 14;39(1):219–233. doi: 10.1016/0022-2836(69)90343-x. [DOI] [PubMed] [Google Scholar]
- Matsukage A., Murakami S., Kameyama T. The isolation and the characterization of DNA region bound to Escherichia coli RNA polymerase. Biochim Biophys Acta. 1969 Mar 18;179(1):145–157. doi: 10.1016/0005-2787(69)90130-0. [DOI] [PubMed] [Google Scholar]
- Nakano E., Sakaguchi K. Isolation and base composition of sites on lambda phage DNA which bind with RNA polymerase of Escherichia coli. J Biochem. 1969 Jan;65(1):147–150. [PubMed] [Google Scholar]
- Novak R. L. Deoxyribonuclease resistance of DNA-RNA polymerase complexes. Biochim Biophys Acta. 1967 Dec 19;149(2):593–595. doi: 10.1016/0005-2787(67)90189-x. [DOI] [PubMed] [Google Scholar]
- Peacock A. C., Dingman C. W. Resolution of multiple ribonucleic acid species by polyacrylamide gel electrophoresis. Biochemistry. 1967 Jun;6(6):1818–1827. doi: 10.1021/bi00858a033. [DOI] [PubMed] [Google Scholar]
- Ptashne M. Specific binding of the lambda phage repressor to lambda DNA. Nature. 1967 Apr 15;214(5085):232–234. doi: 10.1038/214232a0. [DOI] [PubMed] [Google Scholar]
- Riggs A. D., Suzuki H., Bourgeois S. Lac repressor-operator interaction. I. Equilibrium studies. J Mol Biol. 1970 Feb 28;48(1):67–83. doi: 10.1016/0022-2836(70)90219-6. [DOI] [PubMed] [Google Scholar]
- Sentenac A., Ruet A., Fromageot P. Excision de la region du DNA liee a la RNA polymerase in vitro. FEBS Lett. 1968 Nov;2(1):53–56. doi: 10.1016/0014-5793(68)80099-7. [DOI] [PubMed] [Google Scholar]
- Shishido K., Ikeda Y. Preferential binding of RNA polymerase to the thymidylic acid-rich fragments obtained from bacteriophage fl DNA. J Biochem. 1970 Dec;68(6):881–884. doi: 10.1093/oxfordjournals.jbchem.a129427. [DOI] [PubMed] [Google Scholar]


