Abstract
The plasma membrane transporters for the neurotransmitter glutamate belong to the solute carrier 1 (SLC1) family. They are secondary active transporters, taking up glutamate into the cell against a substantial concentration gradient. The driving force for concentrative uptake is provided by the cotransport of Na+ ions and the countertransport of one K+ in a step independent of the glutamate translocation step. Due to eletrogenicity of transport, the transmembrane potential can also act as a driving force. Glutamate transporters are expressed in many tissues, but are of particular importance in the brain, where they contribute to the termination of excitatory neurotransmission. Glutamate transporters can also run in reverse, resulting in glutamate release from cells. Due to these important physiological functions, glutamate transporter expression and, therefore, the transport rate, are tightly regulated. This review summarizes recent literature on the functional and biophysical properties, structure-function relationships, regulation, physiological significance, and pharmacology of glutamate transporters. Particular emphasis is on the insight from rapid kinetic and electrophysiological studies, transcriptional regulation of transporter expression, and reverse transport and its importance for pathophysiological glutamate release under ischemic conditions.
The SLC1 transporter family
Glutamate transporters belong to a large class of secondary-active transport proteins, which use the free energy stored in the transmembrane concentration gradient of co/counter-transported ionic species for uphill transport of the specific amino acid substrates. Thus, glutamate transporters are able to generate and sustain glutamate concentration gradients across membranes [228], which are essential for the functioning of organs such as the intestine [91], the kidney [11] and the brain [35,68].
In the brain, glutamate transporters are expressed in neurons and in astrocytes [89,144]. One of the major functions of the transporters in the brain is the removal of released glutamate from the synaptic cleft (reviewed in [35]), as well as the initiation of the recycling cascade (glutamate-glutamine cycle, Fig. 1B), which eventually restores released glutamate in synaptic vesicles [25]. Because of this central role in glutamatergic signaling, it is not surprising that glutamate transporter malfunction has been implicated in a number of nervous system diseases, ranging from Alzheimer’s disease [122] to neuro-degenerative diseases, such as amyotrophic lateral sclerosis (ALS) [165], and also conditions brought about by oxygen deprivation, such as stroke [178].
Figure 1.
Excitatory amino acid transporter (EAAT) stoichiometry (A) and illustration of the glutamate-glutamine cycle (B) (adapted from [68]). (C) Potential multistep kinetic mechanism of glutamate transport.
Five glutamate transporter subtypes that belong to the solute carrier 1 (SLC1) family of transmembrane proteins have been cloned [145,192,91,48,8]. The first three clones, which were identified almost simultaneously, were named GLAST1 (Glutamate Aspartate Transporter 1, [192]) and GLT1 (Glutamate Transporter 1, [145]) from rat brain, and EAAC1 (Excitatory Amino Acid Carrier 1, [90]) from rabbit small intestine. When the human homologues of these three glutamate transporter subtypes were cloned [9], a standardized nomenclature was introduced using the acronym EAAT (Excitatory Amino Acid Transporter). Based on sequence similarities, EAAT1 is the human homologue of GLAST1, EAAT2 of GLT1, and EAAT3 of EAAC1. In addition, subtypes EAAT4 and EAAT5 were identified, with EAAT5 predominately expressed in the retina. The SLC1 family also contains two neutral amino acid transporters, which share high sequence homology with the EAATs, which are the Alanine Serine Cysteine Transporters 1 and 2 (ASCT1 and 2, [207]). These transporters accept glutamate as a substrate only at low pH, but are otherwise specific for polar neutral amino acids, including the physiologically important glutamine in the case of ASCT2 [24].
Molecular transport mechanism
Insight into the molecular mechanism of glutamate transport has been obtained through functional studies, as well as through the determination of the structures of the archeal aspartate transporter from Pyrococcus horikoshii, GltPh in different conformations [158,211,226]. In addition, molecular dynamics (MD) simulations based on these structures have been valuable in understanding transport mechanism [38,74,80-81,110,185,191]. This section will focus on evidence from functional studies, while structural insight will be discussed in the next section. Transport mechanisms are generally based on the alternating access hypothesis [84]. Such mechanisms assume that the transporter cycles through at least two discrete conformational states, one of them allowing access of substrate to its binding site from the extracellular side, the other one allowing access from the cytoplasm [84]. For Na+-driven secondary-active co-transporters, alternating access is controlled by the co-translocation of Na+ ion(s), which regulate external/internal accessibility of the substrate binding site based on the Na+ concentration gradient across the membrane. The postulated kinetic transport mechanism for the mammalian glutamate transporters is illustrated in Fig. 1C.
After cloning of the glutamate transporters, it was immediately recognized that transport is electrogenic, i.e. associated with net charge transport across the membrane [91]. Since external glutamate application to glutamate transporter expressing Xenopus oocytes induced inward current, it was concluded that more than one Na+ ion is co-transported with glutamate, to account for the inward movement of positive charge [91]. Detailed studies on the coupling stoichiometry of the transporter subtypes EAAT1-3 later revealed that one glutamate anion is co-transported with three Na+ ions and one proton, in exchange for one K+ ion leaving the cell [137,228] (Fig. 1A). This stoichiometry results in a total of two positive charges entering the cell for each glutamate, consistent with the observed inward current and the Hill coefficients determined from glutamate dose response curves.
Important insight was obtained by pre-steady-state analysis of transporter function. Here, an existing steady state is rapidly perturbed by changing either the glutamate concentration (or that of one of the co- or counter-transported cations), or the transmembrane potential. Voltage jumps applied to the glutamate-free transporter induced Na+-dependent transient currents that relaxed within the millisecond time scale [214,219]. These currents were interpreted as caused by Na+ binding to the glutamate-free transporter, or conformational changes associated with this binding, as has been demonstrated for other Na+-coupled, secondary-active transporters [117-118]. Interestingly, transient currents were also observed in the sole presence of K+ on both the intracellular and extracellular side of the membrane, indicating that K+ binding and/or translocation is/are also electrogenic [72] (Fig. 2B, see Fig. 2A for an illustration of the concept of this single-turnover experiment). However, in contrast to Na+ application, the K+-induced transient currents were generated by movement of negative charge within the membrane dielectric, suggesting that negatively-charged cation binding sites create the charge movement, rather than the movement of the positively charged cation itself. These results directly indicate the existence of a charge compensation mechanism, in which negative charge of the binding sites overcompensates for the positive charge of the single, bound K+, but only partially compensates for the positive charges of the three co-transported Na+ ions [72]. Poisson-Boltzmann electrostatic calculations based on structures of the inward- and outward-facing configurations of EAAT3 homology models yielded a valence of the unloaded transporter of approximately −1.2, which was predicted to be reduced, but not eliminated, by K+ association to −0.6. As shown in Fig. 2C, intrinsic charges of the transporter binding sites move substantially within the predicted constraints of the transmembrane electric field (see solid lines in Fig. 2C), directly suggesting charge movement associated with the conformational change. Based on this limited predicted movement of the charges along the normal of the membrane, partial valencies that are less than unity are expected because the unit charges never fully traverse 100 % of the transmembrane electric field.
Figure 2.
(A) Concept of the single-turnover K+ exchange experiment to determine electrical events associates with K+ relocation and/or binding. (B) Outward current induced by external K+ application (grey bar), due to negative charge of the reorienting binding site(s). (C-D) Iso-potential planes for hypothesized outward-facing and inward-facing structures. The protein is shown in blue (slice at the depth of the substrate binding site in the left subunit). Conserved, potentially charged residues are overlaid. D454 is omitted due to the possibility of its side chain being neutral. The figure is adapted from [72].
Consistent with the charge compensation mechanism described in the previous paragraph, inward charge movement was observed in response to rapid glutamate concentration jumps, with an apparent valence of about +0.45, consistent with Poisson-Boltzmann calculations of the valence of +0.2 to +0.4, depending on the protonation state of the highly-conserved D454 residue [72,133]. Interestingly, transient currents in response to glutamate concentration jumps decay with two exponential components [128,219], both of which are also present when the transporter is restricted to access states associated with translocation, in the Na+/glutamate-exchange mode. In the exchange mode, Na+/glutamate concentrations are used that prevent Na+/glutamate dissociation to both the intra- and extracellular solutions, while allowing relaxation of the translocation equilibrium. The existence of two exponential components of this relaxation suggests that the translocation process consists of at least two separable steps [128], consistent with the existence of intermediate/s along the translocation pathway. This intermediate could possibly be an occluded state, as will be discussed in the following section. The significant temperature dependence (activation energies of 100-120 kJ/mol) of these two relaxation processes would be in agreement with conformational changes as the underlying factor [128].
Overall, functional studies provide evidence for a sequential, multistep transport mechanism, as shown in Fig. 1C, in which substrate/cation binding/release steps are coordinated with conformational changes to allow for the proposed alternating access functionality. Interestingly, voltage dependence appears to be distributed over many different binding/translocation steps [72,133], including Na+ dissociation on the intracellular side [231], resulting in a relatively shallow voltage dependence of steady state glutamate transport, considering the large number of positive charges co-transported with glutamate (3 Na+, 1 H+). If these charges were transported across the membrane in a single step, transport would be strongly inhibited upon depolarization.
The mechanism of proton co-transport is not fully understood. Structure-function studies indicate that protonation of the transporter occurs at the acidic glutamate side chain in position 373 (EAAC1 numbering) [70]. The proton was proposed to bind to the empty transporter, before glutamate association [221]. However, the sequence of events with respect to protonation and Na+ binding is not known. Therefore, protonation steps have been omitted from the kinetic scheme shown in Fig. 1C.
In addition to charge transport due to electrogenic glutamate movement across the membrane, which is coupled stoichiometrically to glutamate transport, the transporters facilitate anion flux across the membrane [213,44]. Anion current is kinetically, but not stoichiometrically coupled to glutamate transport [18,71,135]. The molecular mechanism of the anion current is as yet poorly understood. It may be mediated by an anion channel [109,143] or by anion uniport. Anions are conducted at specific steps in the transport cycle (see also Fig. 1C, potential anion-conducting states are indicated by the asterisk [71,215,219-220]). Therefore, the anion current carried by this conductance is a convenient tool to dissect these specific transporter reaction steps, because in the presence of highly permeant anions, such as SCN-, the anion current is much larger than the transport current [17,44]. Anions are mainly conducted when the transporter resides in Na+-bound states [199,219]. These are the Na+-bound empty transporter (in the absence of glutamate), which mediates the leak anion conductance, as well as the fully Na+/glutamate loaded state (Fig. 1C). In a mutant transporter (D439N, EAAC1 numbering), the transported acidic amino acid actually inhibits the anion conductance, as is also found for competitive inhibitors, such as dl-threo-β-benzyloxyaspartic acid (TBOA) [199]. However, in contrast to TBOA, which presumably prevents Na+ binding to the TBOA-bound transporter (most likely to the Na2 binding site, [20]), this site can still be occupied in the mutant transporter, although Na+ binding is very slow. This delayed Na+ binding step following glutamate binding results in the activation of the anion conductance, leading to the hypothesis that the anion conductance is Na+-gated, but not glutamate gated [219]. In a recent report, an intermediate between the outward-facing and inward-facing conformations was identified in an asymmetric trimer of GltPh. This intermediate conformation was proposed to represent the anion conducting state [211]. This interpretation is consistent with previous functional analysis, in which the anion conducting states were linked to steps in the glutamate translocation pathway [128,215,219]. The requirement for bound Na+ for anion permeation is consistent with the large negative electrostatic potential in the transport domain, as evidenced by the negative valence of the Na+-free transporter. Thus, binding of Na+ ions has to neutralize some of the negative charge to prevent repulsion of the negatively-charged anion.
The physiological importance of the glutamate transporter anion conductance is currently unclear. Possible functions would be that the inwardly-directed anion flux balances the inflow of positively charged sodium ions during glutamate inward transport, keeping the membrane potential at a hyperpolarized level that favors Na+ entry and glutamate uptake [189], or by directly influencing neuronal excitability. Thus, glutamate transporters would function as glutamate-dependent, inhibitory receptors, counteracting the well-established excitatory glutamatergic effects. At least for a retinal glutamate transporter, presumably EAAT5, such an inhibitory function appears plausible, because two reports highlight the existence of a pre-synaptic inhibitory glutamate receptor in retinal rod bipolar cells with pharmacological properties matching those of glutamate transporters, rather than those of other chloride channels [212,222]. The anion channel function would be consistent with the exceedingly slow activation of the anion conductance, which precludes significant transporter function due to low turnover [55].
Structure-function relationships and computational analysis
Our understanding of structure-function relationships of glutamate transporters was dramatically improved by the identification of a crystal structure of the archeal glutamate transporter homologue, GltPh [226]. In contrast to the mammalian EAATs, GltPh does not countertransport K+ and is insensitive to H+, but the interaction with the organic substrate and Na+ ions appears to be surprisingly conserved [73,169]. In agreement with biochemical studies, the structure shows a homo-trimeric assembly of three identical subunits, each harboring substrate and Na+ binding sites [20]. Several functional studies have provided evidence that the three subunits operate independently from one another [67,111,101]. For example, transport currents from glutamate transporters with a mutant isoform that is sensitive to glutamine instead of glutamate, were purely additive, in contrast to what would be expected on the basis of a cooperative model [67], although inter-subunit interaction was proposed for EAAT4 [204]. Interestingly, EAAT3 and EAAT4 were found to be able to co-assemble as heteromultimers [134], affecting localization of the heteromeric transporters in epithelial cells.
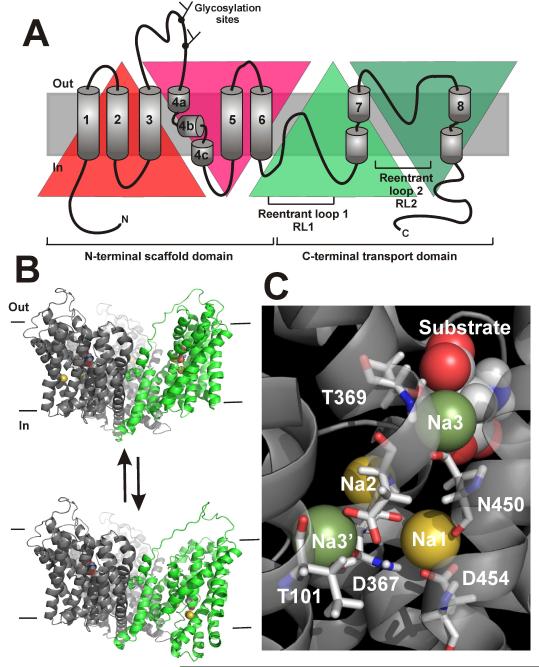
Each of the three monomers in the functional assembly is comprised of a transport domain and a scaffold domain [20-21,226]. Both of these domains consist of an inverted repeat structure (Fig. 3A), which is not evident from analysis of the amino acid sequence, but which was discovered by analysis of the three-dimensional structure, as it was the case in several other, structurally unrelated, membrane transport proteins (reviewed, for example, in [50]). The functionally important transport domain is a C-terminal peptide, which is inserted into a N-terminal barrel (scaffold domain) formed by transmembrane domains (TMDs) 1-6 [226]. This C-terminal peptide is composed of two helical transmembrane domains (TMDs 7 and 8) with helix breaks in the central core of the protein, and two reentrant loop structures (RL 1 and 2), which dip into the transmembrane segment of the transporter from opposite sides of the membrane [226] (Figs. 3A and B). As will be discussed below, these reentrant loops are proposed to function as intracellular and extracellular gates that close off the substrate binding site from the aqueous solution phase.
Figure 3.
(A) Inverted repeat topology (highlighted by colored triangles) of the SLC1 family members. (B) Proposed major structural change leading to alternating access of the substrate binding site. (C) Structural evidence predicts two Na+ binding sites in mammalian EAATs, Na1 and Na2 (yellow). Computational analysis of Na+ binding by MD simulations, as well as mutational studies, provided evidence for two other potential Na+ binding sites, termed Na3 and Na3′.
The substrate and Na+ binding sites are located in the C-terminal transport domain. A GltPh crystal structure with the substrate, aspartate, bound shows that the β-carboxylate of the ligand is coordinated by the side chain of the highly-conserved R397 residue (GltPh numbering), located in TM8 [20]. This interaction points to a salt bridge stabilization of the bound substrate, as had been suggested based on mutational analysis of the homologous amino acid residue in mammalian transporters, the neutralization of which renders the binding site specific for neutral instead of acidic amino acids [14,67]. Other amino acid side chains implicated in substrate binding are D394, N401 and T314 (all GltPh numbering).
GltPh was crystallized in complex with two Tl+ ions, which were proposed to bind to Na+ binding sites, termed Na1 and Na2 (Fig. 3C, [20]). The Na1 site is buried deeply within the membrane. In the crystal structure, the side chain of the highly-conserved aspartate residue D405 (GltPh numbering) contributes oxygen ligands to this binding site [20]. However, mutation of the homologous D454 to asparagine had little effect on the affinity of interaction between the mammalian EAAC1 counterpart and Na+ [201], and did not significantly affect the kinetics of Na+/glutamate exchange [133], raising questions about the details of the involvement of D454 in Na+ coordination. However, estimations of the pKa value of this aspartate, which is much higher than the pKa in aqueous solution, suggested that the carboxylate may be protonated and, thus, neutral. This could explain why the neutral amide side chain of the asparagine substitution did not result in substantial functional differences versus the wild-type transporter [133]. Alternatively, the D454 to asparagine mutation may affect a Na+ binding site other than the one responsible for activating the anion conductance, which was used as a readout for measuring Na+ affinity.
The Na2 site is formed by the tip of RL2 and the region of TM7, in which the α-helix structure is interrupted [20]. Coordination is mainly through main-chain carbonyl oxygen atoms. Therefore, this binding site is much harder to study by site directed mutagenesis, and, to our knowledge, no structure function analysis has been performed on this site. Since the Na+:glutamate coupling stoichiometry of the mammalian transporters, as well as GltPh [73] is 3:1, it is likely that a third Na+ binding site must exist that is not observed in the crystal structure. Two potential binding sites were discussed in the literature, both supported by results from molecular modeling and site-directed mutagenesis, named Na3 and Na3′ (Fig. 3C). The hypothesized Na3 site is formed by the GltPh T314 side chain, as well as oxygen ligands form the bound aspartate molecule [110]. A direct coordination of Na+ by aspartate would explain the observed effects of the nature of the bound cation (Na+ vs Li+) on the affinity and specificity of the transported substrate [125]. The proposed Na3′ site is formed, among other ligands, by the highly conserved aspartate residue 312 (TM7), as well as T92 in TM3 [81,12]. Mutation of both of these homologous residues in mammalian EAAC1 results in drastic reduction of the apparent Na+ affinity of the transporter [200-201]. MD simulations also provide strong evidence for the existence of the Na3′ site, as Na+ was found to be highly stable in this site in long-time range MD simulations [81,133].
GltPh was first crystallized in an “outward-occluded” state, in which the substrate is occluded by a closed external gate, but the aspartate binding site is close to the extracellular solution [226]. Substrate movement then occurs by a large structural change of the transport domain, which brings the substrate binding site closer to the intracellular solution, in the background of relatively small motions of the scaffold domain (Fig. 3B) [34,158]. This structural change of the transport domain is remarkably analogous to traditionally-proposed carrier models, with a motion that has been described as “elevator-like” [162,186] because the bound substrate moves up and down along the bilayer normal, like an occupied elevator would move between two stories of a building (Fig. 3B). Direct evidence for this motion comes from an “inward occluded” crystal structure [158]. The inward occluded conformation, in which the binding site is close to the intracellular solution, but the substrate is locked in by the internal gate, was stabilized by Hg2+ crosslinking of residues K55C and A364C, which have been previously found to be close in space by functional crosslinking studies of a homologous mammalian transporter [170]. Simultaneously, the general features of the inward occluded conformation were captured by symmetry-based homology modeling of each domain of the transporter, based on the structure of the sequence- and structure-related inverted domain [34]. Evidence for collective large-scale, global motions of the transport domain was also obtained from computational analysis using normal mode computations [88]. Finally, electron paramagnetic resonance (EPR) spectroscopy provided evidence for heterogeneity of transporter conformations [77].
Recently a third conformational state was identified, in which the transport domain appears to adopt a configuration in-between the outward- and inward-occluded states [211]. This configuration may represent an intermediate on the translocation pathway, which has also been postulated based on MD simulations [191]. Single molecule analysis based on fluorescence resonance energy transfer in GltPh trimers specifically labeled with fluorescence dyes revealed the existence of quiescent periods interrupted by bursts of transitions between inward and outward-facing states [3]. This behavior was explained by assuming that the structurally-observed intermediate state represents a configuration, in which the transport domain has become dislodged from the scaffold domain. This transition may be slow, occurring on the minute timescale, while the translocation of the dislodged transport domain may be fast [3]. It should be noted that two phases of the current relaxation were observed in transient kinetic experiments on mammalian glutamate transporters [219]. It is possible that the slow phase of these currents represents the proposed dislodging event, although it occurs several orders of magnitude faster than the slow phase observed in the GltPh single molecule studies. However, since the turnover rate of mammalian transporters is about 5000-fold faster than that of GltPh [3,71], it would not be surprising that such individual reaction steps are also dramatically faster in the mammalian transporters.
Overall, the following picture emerges from structural and computational analysis: In the apo-form of the transporter (no substrate/ions bound), the extracellular gate (RL2) is open to the extracellular side, allowing access of the substrate to the binding site [80-81,185]. Before the substrate binds, either one or two Na+ ions associate with the transporter, presumably at the Na1 and the Na3′ sites [81,100,219]. In the next step, glutamate binds, resulting in closure of the external gate (RL1). Once RL1 is closed, the Na2 binding site is formed, which then is occupied by binding of extracellular Na+. Once the fully loaded complex is formed with RL2 closed (outward occluded state, [20]), the elevator-like motion of the transport domain is facilitated, which, possibly through intermediates, results in formation of the inward occluded form [158,202]. Once the internal gate (RL1) opens, possibly driven by dissociation of Na+ from the Na2 site, the substrate can dissociate, eventually also leading to the release of the remaining Na+ ions [38]. It should be noted that a crystal structures in the inward-facing apo-form, or in the blocker-bound state are not available. Therefore, our knowledge of the structural dynamics of the intracellular gate is derived only from MD simulations.
The sequence of events described above is remarkably consistent with data from functional studies (mainly electrophysiology), from which the sequence of extracellular binding events was predicted before the structure was known [219]. In addition, pre-steady-state analysis of translocation events with high time resolution directly point to translocation being a two-step process, supporting the structural evidence for the existence of an intermediate on the translocation pathway [128].
Regulation of glutamate transporter function
CNS Glutamate transporter activity is not constant, but is subject to regulation, not only during development, but also in the adult and aged brain. It appears that glutamate transporters, like other proteins, are modulated on virtually any step of gene expression, from transcriptional initiation, to RNA processing, and to post-translational modifications of the transporter protein. Mechanisms including transcript/protein-expression, such as long term memory formation, generally are time-consuming (hours up to days), while synaptic events, or more general, synaptic plasticity, require modulation and interaction of relevant transporters and receptors on a time scale of minutes. Post translational regulatory mechanisms of glutamate transporters mediate these rapid modulatory effects (e.g. membrane trafficking, see below) which are the basis of fast synaptic transmission. Several recent reviews highlighted both the slow transcriptional/translational and rapid post translational regulatory mechanism acting on glutamate transporters [59,36,58,64,13,181]. Here, we provide an update on most recent progress made on transcriptional, translational and post translational regulation of glutamate transporters and will add/provide recent insights into epigenetic effects on glutamate transporters.
Transcriptional Regulation: EAAT Promoter studies
Bulk glutamate uptake in the CNS is facilitated by glial glutamate transporters [78,40,177,154,151,36,54]. It has been suggested that glial GLT1/EAAT2 accounts for about 90% of total glutamate uptake in the brain, and thus, it is considered as the most important glutamate transporter subtype in the CNS [198,36]. GLT1/EAAT2 is predominantly but not exclusively expressed in astrocytes [54]. During early stages of brain development and in disease GLT1/EAAT2 is also expressed in neurons [36]. A prominent example for neuronal expression of GLT1/EAAT2 in adult and healthy CNS is the mammalian retina [156,151,153]. Independent of glial or neuronal expression, however, GLT1/EAAT2 appears to play a significant role in maintaining the physiological functioning of the brain, and thus, is believed to be a major player in acute and chronic brain diseases (for review [58,181]).
Numerous studies have addressed the mechanisms regulating expression of GLT1/EAAT2 [57,59,160,132,227,138] indicating GLT1 mRNA transcription and protein expression is down regulated by the pro-inflammatory cytokine, tumor necrosis factor α (TNFα) and endothelins [193,168,188,22,115,4,61] and upregulated by cyclic adenosine monophosphate (cAMP), epidermal growth factor (EGF), transforming growth factor α (TGFα), and pituitary adenylate cyclase-activating polypeptide (PACAP) [196,175,227,49,188].
In general, little is known about the mechanism underlying transcriptional regulation of the different glutamate transporter subtypes. Early studies on the promoter sequence of glutamate transporters revealed for the mouse GLAST1/Slc1a3 gene 5′-untranslated region with regulatory elements [75], and were initially not successful for the human EAAT2/Slc1a2 gene [127]. Using a sequential progressive genomic scanning cloning approach, [193] Su et al. were able to isolate the putative human EAAT2/Slc1a2 promoter, followed by the identification of the human and rodent EAAT1/GLAST/Slc1a3 promoter sequence [97,206], and the GLT1 promoter from rat [5]. A ≈2.5 kb 5′-upstream region, immediately flanking the transcription start site of GLT1/EAAT2, has been identified as putative full-length promoter of GLT1/EAAT2 [193,5]. Sequence analysis demonstrated the presence of highly conserved regions on both the rat and human GLT1/EAAT2 promoter which are not only essential for constitutive GLT1/EAAT2 expression but also for EGF, TNFα, and PACAP-induced transporter transcription. The rat and human promoter sequence of GLT1/EAAT2 share functional and structural similarities highlighted by identical consensus sites for the transcription factors N-myc proto-oncogene protein (n-Myc), C/EBP homologous protein (CHOP-C), nuclear factor kappa B/c-rel (NF-κB), cAMP-response element-binding protein (CREB), and Specificity Protein 1 (SP-1) [193,5,96]. Among these transcription factors, NF-κB is required for both basal GLT1/EAAT2 expression as well as for induced activation or repression of the GLT1/EAAT2 promoter and appears to be the key player in regulating the transcription of GLT1/EAAT2 [188]. While NF-κB mediates cAMP-, TGFα-, and EGF-induced GLT1/EAAT2 promoter activation, simultaneous binding of both n-Myc and NF-κB appear to be required for TNFα-mediated promoter repression. However, a rat GLT1-promoter construct missing the n-Myc and NF-κB consensus sites still mediated TNFα-dependent inhibition of GLT1 expression in rat, suggesting a remarkable difference of transcriptional regulation between the highly related rodent and human GLT1/EAAT2 promoter regions [193,188,5]. In contrast to the structural similarities of the rodent and human GLT1/EAAT2 promoter sequences, the GLAST/EAAT1 promoter region from both species lack sequence homology [97,206]. Like the putative GLT1/EAAT2 promoter, the full-length promoters of GLAST/EAAT1 lack well defined cis-acting elements such as TATA boxes, CCAAT boxes or initiator elements (Inr). The absence of TATA box or Inr elements in the EAAT-promoters identified so far is not unusual. Previously identified core promoter elements including the TATA box and the initiator (Inr) are not present in a large number of genes in the mammalian genome [203,195,130], and it has been suggested that TATA-less promoters regulate tissue-specific genes while TATA-containing promoters drive transcription of ubiquitously expressed genes [130].
Despite the lack of sequence homology, the GLAST/EAAT1 promoters from rat and human share various common putative transcription factor binding sites - NF-κB, CART, CEBP, GATA, HAML, MZFI, NKXH, Oct1, SP1 and YY1F -,suggesting that constitutive GLAST/EAAT1 expression in both species is controlled by similar mechanism [206].
Surprisingly Kim et al. (2003) and Unger et al. (2012) found that known activators of GLAST/EAAT2 expression, such as dbcAMP, PACAP, EGF, and TGFα, failed to further increase reporter gene activity in astrocytes transfected with the full-length rat or human GLAST/EAAT2 promoter. However using instead reporter constructs containing in addition to the human or rat full length promoter the 3′-untranslated region (3′-UTR) of the human GLAST/EAAT1 gene restored the stimulatory effect of dbcAMP, PACAP, EGF, and TGFα on GLAST/EAAT1 expression and additionally revealed that the human 3′-UTR acts as a transcriptional repressor of constitutive GLAST/EAAT1 transcription. In contrast, the rat 3′-UTR further enhanced GLAST/EAAT1 expression and mediates only the stimulatory influence of cAMP, but not that of PACAP, EGF, and TGFα [206]. Taken together, promoter studies of the glutamate transporter subtypes GLT1/EAAT2 and GLAST/EAAT1 revealed similarities in some transcriptional regulatory pathways, but at the same time these studies stress species specific differences between rodents and humans which are especially pronounced in GLAST/EAAT1.
β-Lactam antibiotics, such as ceftriaxone, were identified as potent modulators of glutamate transport through NF-κB-mediated EAAT2 promoter activation [114] and the ceftriaxone-mediated upregulation of EAAT2 seems to be neuroprotective in vitro when used in animal models of ischaemic injury, motor neuron degeneration, and amyotrophic lateral sclerosis (ALS) [166], emphasizing the potential importance of animal models in analyzing the molecular background of human disease pathways and potential therapeutics. However, animal models show often significant differences from human pathology not only because of species specific transcriptional regulation mechanism as shown for glutamate transporters. Thus, most animal models are hampered by not adequately representing human pathophysiology. For example, the antibiotic minocycline, a second-generation tetracycline, actually decreased survival time of amyotrophic lateral sclerosis patients in a recent clinical study, but in mouse models, which even contained the human mutated SOD1 gene from patients; it improved longevity [103,65].
Comparing the promoter regions of human EAAT1 and EAAT2, and the neuronal EAAT3 genes, Ma et al., 2006 [119] found that the promoter region of EAAT3 but not the promoter regions of EAAT1 or EAAT2 contained a binding sequence for regulatory factor X1 (RFX1). Consistent with this finding, the transcription factor RFX1 enhanced the expression of EAAT3 but not that of the glial glutamate transporters indicating that the effects of RFX1 on EAAT3 are specific.
As shown by Escartin et al. (2011), the transcription factor nuclear factor erythroid 2-related factor 2 upregulates the expression of EAAT3 in neurons. EAAT3 is transcriptionally upregulated through the nuclear factor erythroid 2-related factor 2 (Nrf2)-antioxidant responsive element (ARE) pathway and the resultant increase in EAAT3 expression promotes neuronal glutathione (GSH) synthesis by the amplified uptake of cysteine via EAAT3. At the same time activation of the Nrf2-ARE pathway by oxidative stress promotes astrocytic GSH release by increasing the expression of enzymes and transporters involved in GSH metabolism. These findings identify a trans-activating mechanism that couples astrocytic GSH release with neuronal GSH synthesis on transcriptional level.
In retinal Müller glial cells a coupled transcriptional regulation of GLAST and glutamine synthetase, the key proteins of the so called glutamate-glutamine cycle [78], via glucocorticoid-mediated mechanisms has been suggested which are able to coordinate glutamate uptake and glutamate degradation to improve transmitter clearance and transmitter recycling in retinal tissue [155].
Less is known about the transcriptional regulation of the glutamate transporter subtypes EAAT4 and EAAT5. At the moment, EAAT4 promoter studies are restricted to anatomical expression studies in vivo using EAAT4-eGFP BAC reporter transgenic mice which indicate predominant expression of EAAT4 in neuronal cells with highest density of expression in cerebellar Purkinje cells [60,37].
Posttranscriptional Regulation: Alternative Splicing
While the main regulation of gene expression is considered to happen on transcriptional level, with major roles for transcription factor recruitment at promoter elements, the functions of protein non-coding mRNA regions (UTRs) for gene expression were initially underestimated, but are now accepted to play similar important roles in the regulation of gene expression. UTRs of the vast majority of genes have been shown as important regulatory elements with a strong impact on the post-transcriptional regulation of gene expression. Together with the complex of different RNA-interacting factors, UTRs regulate mRNA stability, nuclear export to the cytoplasm, alternative splicing, sub-cellular localization and translation efficiency to influence the total amount of synthesized protein (for review see [32,105,124,197]). Small noncoding RNAs (microRNAs or miRNAs) binding sites are mostly localized within the 3′ UTRs and are rarely identified in the mRNA coding sequence and the 5′-UTR and act by promoting the destabilization/degradation of mRNA and/or inhibition of translation on protein expression [6].
The GLT1/EAAT2 coding region spans more than 100 kb, contains 11 exons, and encodes an unusually long transcript of 10-12 kb [145,9,99,208,127,43,98,193,5]. However, the actual protein coding sequence is only 1.7 kb [145], suggesting the presence of a large untranslated region (UTR) of 9-10 kb in EAAT2 mRNA. Multiple GLT1/EAAT2 mRNA transcripts have been identified in vitro and in vivo and these transcripts give rise to differential GLT1/EAAT2 splice variants [208,126,131,156,194,16,139]. In the 3′ coding region of the GLT1/EAAT2 gene, alternative exon usage can result in the generation of at least 3 distinct C-termini and 4 potential distinct N-termini by differential exon usage at the 5′ end of the gene (Table 1).
TABLE 1. GLT1/EAAT2 splice variants (adapted from [69]).
| N-terminal Amino Acid Sequence | Species/Name/ Reference | C-terminal Amino Acid Sequence |
|---|---|---|
| MASTEGANNMPK | rat, GLT1 [145]; mouse, mGLT1 [208]; human, EAAT2 [9] |
DECKVTLAANGKSADCSVEEEPWKREK |
| MASTEGANNMPK | rat, GLT1v [176]; rat, GLT1b [31] |
DECKVPFPFLDIETCI |
| MASTEGANNMPK | rat & mouse, GLT1cA [156] |
DECKSLHYVEYQSWV |
| MVSANNMPK | mouse, GLT1cA (Rauen, unpublished) |
DECKSLHYVEYQSWV |
| MVSANNMPK | mouse, mGLT1-A [208] | DECKVTLAANGKSADCSVEEEPWKREK |
| MVSANNMPK | mouse, mGLT1-B [208] | DECKVPFPFLDIETC |
| MPK | mouse [131]; rat [167] | Not determined |
| MKRPKEHSIQRSANNMPK | rat [167] | Not determined |
The first identified GLT1/EAAT2 variant from rat [145], mouse [208], and human [9] is classified by the N-terminal starting sequence MASTEG- and C-terminal sequence VTLAANGKSADCSVEEEPWKREK. Additionally, Utsunomiya-Tate et al. (1997) identified in mouse two additional splice variants, termed mGLT1-A and mGLT1-B (Table 1) which are recognized by their short alternately spliced N-terminal sequence MVS and in the case of mGLT1-B the alternately spliced C-terminal VPFPFLDIETC-sequence. [176] and [31] identified a hybrid GLT1 splice variant in rat, which possessed the C-terminal splicing of mGLT1-B, but retained the original GLT1 N-terminal sequence. Not yet known is if protein expression of the suggested GLT1 variants MPK-KREK, MPK-DIETC or MKRP takes place in the CNS and what the functional differences of these GLT1-variants are. [139] Peacey et al. (2009) showed that some of the above mentioned N- and C-terminal splice variants of GLT1 form cell surface homomeric and heteromeric assemblies in heterologous expression systems, but these GLT1 splice variants did not differ functionally, regarding glutamate uptake or transport kinetics. Correspondingly, GLT1v (MASTEG-DIETC) exhibits kinetics indistinguishable of those of GLT1 (MASTEG-KREK) suggesting that alternate C-terminal splicing of GLT-1 does not have significant effects on its glutamate transport properties [156]. This result is also consistent the work presented in [208] and in [31] and is also valid for another GLT1-splice variant called GLT1c which is predominantly expressed in rat and human retina in photoreceptor terminals [156]. Both N-terminal variants (MASTEG and MVS) of GLT1c are found in rat and mouse retina (unpublished, Rauen). The carboxy terminal amino-acid sequence of GLT1c – SWV – suggests a class-I PDZ binding motif which might tailor this GLT1 variant for its particular purpose of spatially clustering and anchoring within specific subcellular domains in neurons. Taken together, instead of showing different functional properties, different GLT1 splice variants are localized in distinct cellular and subcellular distribution patterns in the CNS [156]. Thus, heteromeric assembly of different GLT1 splice variants might be the exception of the rule because expression, as seen e.g. for GLT1 and GLT1v, in one and the same cell showed that each of the splice variants of these transporters are either segregated in small membrane “rafts” or that each splice variant is targeted to distinct membrane domains [194].
Epigenetics
Epigenetic mechanisms convert environmental conditions and physiological stresses into long-term changes in gene expression and translation [173]. Epigenetic changes are heritable and potentially reversible and do not alter the nucleotide sequence within the genome.
Little is known about the epigenetics of glutamate transporter transcription processing. However, knowledge of such mechanisms would be promising for better understanding of glutamate transporter related pathology and the development of new strategies for its treatment. Three main epigenetic mechanisms are discussed: (1) DNA methylation, (2) histone protein modifications (acetylation/deacetylation), and (3) the effects of microRNAs [173].
Interestingly, all these GLT1/EAAT2 mRNA transcripts have a uniquely long 3′-UTR (9.7kb) [97], implicating potential 3′-UTR-dependent regulation, especially through microRNA-mediated translational inhibition or mRNA degradation. MicroRNAs (miRNAs) are endogenous ≈22 nucleotide noncoding RNAs that regulate the expression of complementary messenger RNA [230].
Morel et al. (2013) [129] investigated miRNA-dependent regulation of GLT1 expression in vitro and in vivo using the miRNA miR-124a which is completely conserved at the nucleotide level from worms to humans and is estimated to be the most abundant miRNA in the brain, accounting for 25-48% of all brain miRNAs [107]. Surprisingly, miR-124a significantly and consistently increases GLT1 protein expression levels in cultured astrocytes and mice, although miRNA action is expected to promote the destabilization/degradation of mRNA and/or inhibit of mRNA translation, and thus, downregulation of GLT1 protein expression. The authors concluded that miR-124a does not directly interact with GLT1 mRNA 3′UTR und does not modulate its translation/degradation. However, Morel et al. (2013) [144] discovered a highly interesting mechanism of neuron-glia interaction which is mediated by the exchange of exosomes. Exosomes are 40-to 100-nm membrane vesicles of endocytic origin secreted by most cell types and contain various active biomolecules, including mRNAs, proteins, lipids, and miRNAs. Exosomes can be secreted into the extracellular space and then fused to neighboring cells to release their contents, thus serving as a novel discovered intercellular regulatory mechanism. Such exosome-mediated intercellular transfer of miR-124a from neurons to astrocytes is able to regulate GLT1 expression in glial cells by neurons, an exciting intercellular miRNA signaling pathway.
GLT1/EAAT2-promoter DNA-methylation inhibits GLT1/EAAT2 expression [232,141]. A striking feature of the GLT1/EAAT2 promoter is the occurrence of numerous Sp1 sites and other GC-rich repeats [193]. Sequence inspection identified a classical CpG island at the most proximal part of the GLT1/EAAT2 promoter. Although, objective definitions for CpG islands are limited, the usual formal definition of a CpG island is a region with at least 200 bp, and a GC percentage that is greater than 50%, and with an observed-to-expected CpG ratio that is greater than 60% [174]. The state of CpG methylation regulates and stabilizes chromatin structure, perhaps regulating accessibility of the transcription machinery to regions of DNA. Thus, whereas methylated CpGs might prevent the recruitment of transcription factors important for either basal expression or activation of gene transcription, unmethylated CpGs in the vicinity of a gene allow that gene to be expressed. Interestingly, valproic acid (VPA, Valproate) clinically used as anticonvulsant and mood-stabilizing psychoactive drug in the treatment of epilepsy and bipolar disorders, exerted strong influence on chromatin remodeling events in astrocytes involving CpG demethylation and histone modifications targeting the GLT1/EAAT2 gene. Distinct parts of the GLT-1 promoter were demethylated and enriched in acetylated histone H4 after VPA treatment whereas the CpG island in proximity of the translational start site was completely unmethylated, concomitantly, the activity of the GLT1/EAAT2 gene was profoundly increased [142]. In line with this Yang et al., 2010 [225] showed that hypermethylation on selective CpG sites of the GLT1 promoter is involved in repression of GLT1 promoter activation, but this regulation does not play a role in astroglial dysfunction of EAAT2 expression in patients with ALS.
Posttranslational Modifications: Rapid Membrane Trafficking
Synaptic transmission is fast (≈ 1 ms), even synaptic plasticity involving the alteration of the number of receptors and/or transporters located at a synapse is much faster (≈ minutes) than transcriptional and translational processes synthesizing mRNA and finally protein (≈ hours/days). Glutamate transporters are fast in removing their substrate from the synaptic cleft [71,136]. However, impact on synaptic transmission of glutamate transporters does not only require rapid substrate turnover rates but also high density expression at synapses (for review see [69]). Actually, glutamate transporters are expressed at high densities of 15.000-20.000/μm2 in the vicinity of glutamatergic synapses [36]. Thus, the large number of transporters present at the synapse may significantly and rapidly “buffer” glutamate that binds to and dissociates from the transporter binding sites multiple times before it is either translocated across the membrane or diffuses out of the synaptic cleft. Due to the rapid buffering of glutamate, excitatory amino acid transporters may shape the temporal profile of the synaptic glutamate concentration, keep the signal local, prevent spillover, such that low-affinity AMPA (2-amino-3-(3-hydroxy-5-methyl-isoxazol-4-yl)propanoic acid) receptors experience only a very short burst of high glutamate concentration, and high-affinity NMDA (N-methyl-d-aspartic acid) receptors are then activated by a prolonged low glutamate concentration within the synaptic cleft. In other words, the density of glutamate transporters in synaptic membranes and its regulation plays a crucial role in regulating synaptic transmission in the CNS. In fact, glutamate transporter plasma membrane expression appears to be as dynamic [58] as that of AMPA and NMDA receptors [171,190]. A typical example for regulated plasma membrane expression among the different glutamate transporter subtypes is EAAC1/EAAT3 and this process also called membrane trafficking has been extensively reviewed in [58,161,64,181]. Cell surface trafficking of glutamate transporters is generally independent of protein synthesis, and is not only activated by kinases/phosphatases and scaffolding proteins, but also regulated by a variety of other factors including e.g. endothelin B, vasoactive intestinal peptide, pituitary adenylate cyclase-activating peptide, platelet-derived growth factor, thyroid hormone and trophic factors that appear to affect trafficking to and from the plasma membrane to intracellular pools involving lipid rafts and caveolae; see for review [13].
Poitry-Yamate et al. (2002) [147] showed that GLT1 and GLAST are clustered on astrocytic processes and that their distribution is regulated in the time scale of minutes by neuronal activity. GLT1 and GLAST function was activated by neurons and this effect was mimicked by pre-incubation of astrocytes with micromolar concentrations of glutamate or by the synaptic release of glutamate from neurons. Thus, increase in transport activity is dependent on neuronal release of glutamate, is associated with the local redistribution (clustering) of GLT1 and GLAST but is independent of transporter synthesis and of glutamate receptor activation. In line with this, blockage of neuronal activity reduces both the density and perisynaptic localization of GLT1 clusters in situ (organotypic hippocampal slice cultures), whereas conversely enhanced neuronal activity increases the size of GLT1 clusters and their proximity to synapses [15]. The underlying mechanisms may be dependent on GLT1 ubiquitation [62,179]. GLT1 is constitutively endocytosed into the recycling endosome via a clathrin-dependent pathway, a process that is dependent on the ubiquitination of lysines located in the cytoplasmic C-terminus (lysines 517 and 526) of the transporter [121]. This finding points to the involvement of cytoplasmic C-terminal domains in the trafficking processes of different glutamate transporter subtypes (see above GLT1/EAAT2 alternative splicing and [180]). The return translocation of GLT1 from the recycling endosome to the plasma membrane was blocked by specifically inhibiting the deubiquitinating enzyme (DUB) ubiquitin C-terminal hydrolase-L1 (UCH-L1), supporting the existence of an ubiquitination/deubiquitination cycle that ensures the correct concentration of GLT1 at the cell surface [121]. Garcia-Tardon et al. (2012) [56] identified the ubiquitin ligase Nedd4-2 as a mediator of the GLT1 endocytosis that is triggered by protein kinase C (PKC) activation. PKC promotes the phosphorylation of Nedd4-2, its association with GLT-1, and the subsequent ubiquitination of the transporter that precedes its endocytosis.
Endocytosis and exocytosis of glutamate transporters have initially been thought to account alone for this cell surface trafficking process. However, membrane proteins also traffic through surface lateral diffusion in the plasma membrane. Proteoliposome reconstitution experiments have shown that cholesterol is required for Na+-dependent [3H]-glutamate uptake [184] and Na+-dependent [3H]-glutamate uptake is significantly reduced by long term treatment with a cholesterol-depleting agent [26], suggesting plasma membrane organization in or outside of lipid rafts may be another means of regulating glutamate transporter function.
Lipid rafts are lipid-protein microdomains of the plasma membrane that are enriched in cholesterol and glycosphingolipids [187]. These lipid raft microdomains have been implicated in regulating the trafficking and clustering of membrane-associated proteins as well as their intracellular signaling molecules. Lipid rafts are present on the surfaces of neurons as well as glial cells and Butchbach et al. (2004) [27] suggest that membrane cholesterol is important for the function of glutamate transporters.
However, EAAT1, EAAT3 and EAAT4 are less strongly associated with lipid raft-containing microdomains than EAAT2. GLT1/EAAT2 appears to be located in non-caveolar lipid rafts [233]. Gonzales et al. (2007), however, conclude that caveolin facilitates both the constitutive delivery and internalization of EAAC1/EAAT3 and that caveolin interacts with EAAC1, suggesting that this interaction may participate in the regulation of EAAC1 trafficking by caveolin. [63].
It has been reported that the organization of lipid rafts is disrupted in Alzheimer’s disease brains because Cholesterol-24S-Hydroxylase (CYP46), a key enzyme in maintenance of cholesterol homeostasis in the brain, is markedly increased in astrocytes but decreased in neurons [112]. Tian et al. (2010) demonstrated that increased expression of CYP46 in primary astrocytes results in a reduction of membrane cholesterol levels and leads to the dissociation of EAAT2 from lipid rafts and the loss of EAAT2 including glutamate uptake function.
Cholesterol Effects
Apparently glutamate transporters interact with cholesterol and forward glutamate transport appears to be promoted in the presence of cholesterol. As thermodynamic machines, however, these transporters can also run in reverse, releasing glutamate into the extracellular space (reviewed in [68]). Because glutamate is excitotoxic, this transporter-mediated release is detrimental to the health of neurons and glial cells [68]. In stroke, cerebral hypoxia/ischemia, and traumatic brain injury, transporter-mediated glutamate homeostasis fails dramatically, instead of removing extracellular glutamate to protect neurons, transporters release glutamate, triggering neuronal death [83,163]. Under these conditions, neuroprotection by lowering the plasma membrane cholesterol content is expected to reduce transporter-mediated glutamate release, and thus, might protect neurons and glial cells from excitotoxicity [102]. Nevertheless, regulating the brain cholesterol content might be a tightrope performance, fighting rapidly spreading excitotoxicity with slowly acting drugs lowering plasma membrane cholesterol, particularly regarding the fatal consequences of low cholesterol on glutamate uptake in healthy brain areas.
Involvement in disease
Considering the almost ubiquitous expression of glutamate transporters in the CNS as well as in peripheral tissue, it is not surprising that the EAAT gene family has been suggested to be involved in a wide range of diseases, such as alcohol dependence [149], Alzheimer disease (AD) [41,157], amyotrophic lateral sclerosis (ALS) [164], autism [216], depression [108], diabetes [85], epilepsy [198,33], HIV-1-induced excitotoxicity [148], Huntington’s disease [47], ischemia [150,68,95], manganism [113], multiple sclerosis [10], neuropathic pain [120], obesity [209]; obsessive compulsive disorder [7], Parkinson’s disease [146,123], schizophrenia [93-94], Tourette syndrome [2], West Nile virus infection [19]. A remarkably long list of potential glutamate transporter related disorders, but by far not complete. However, the question of cause and effect is still open, asking whether these diseases are caused by glutamate transporter dysfunction or rather the disease alters the expression and function of glutamate transporters.
In the case of Slc1a3, encoding the glial glutamate transporter GLAST/EAAT1, a point mutation of this glutamate transporter gene appears to be the genetic basis of episodic ataxia, hemiplegia and seizures [87]. Episodic ataxia is a disorder characterized by sporadic attacks of severe uncoordination and imbalance. There are 6, possibly 7, types recognized and ataxia can be provoked by stress, startle, or heavy exertion such as exercise. Whereas episodic ataxia types 1, 2 and 5 are caused by mutations in genes encoding voltage-gated cation channels, the genetic basis of episodic ataxia types 3 and 4 has not been defined [86]. The rare episodic ataxia type 6 (EA6) differs clinically from other episodic ataxia forms in long duration of attacks (hours – days), epilepsy and the absent myokymia, nystagmus and tinnitus [86]. EA6 was identified in a single child through a candidate gene approach identifying a de novo mutation in the Slc1a3 gene predicting a substitution of proline by arginine (P290R) in the fifth transmembrane domain of GLAST/EAAT1 [87]. The missense mutation P290R decreased the cell surface expression of GLAST/EAAT1, and consequently reduces the cellular glutamate uptake capacity. When co-expressed, the mutant EAAT1-P290R decreased the activity of wild-type GLAST/EAAT1 but not that of GLT1/EAAT2 or EAAC3/EAAT3, suggesting that EAAT1-P290R specifically multimerizes with wild-type GLAST/EAAT1 to exert its dominant negative effect. Jen et al. (2005) concluded that a heterozygous mutation in GLAST/EAAT1 can lead to decreased glutamate uptake, which can contribute to neuronal hyperexcitability to cause seizures, hemiplegia, and episodic ataxia [87].
However, the clinical phenotype of this particular patient with episodic ataxia type 6 differs from the neurological symptoms observed in animal models lacking GLAST/EAAT1 [218] suggesting that P290R might not only reduce the glutamate transport, but also affect other functions of GLAST/EAAT1 [224]. And indeed, Winter et al. (2012) reported that the EAAT1 mutation P290R not only decreases glutamate transport rates and surface expression, but also increases EAAT1 associated anion currents as a novel pathomechanism associated with episodic ataxia 6.
Animal models lacking certain glutamate transporter subtypes usually do not provide clear cut correlations between the missing transporter and disorders. Instead, glutamate transporter knockout models do not even show robust phenotypes and are difficult to interpret regarding glutamate transporter pathophysiology as long as stress situations (e.g. ischemic conditions, drug-induced seizures etc.) are omitted [92,116,93,172,205,76,217-218,140]. The mild phenotype of single glutamate transporter subtype knockouts might be interpreted as an adaptive process (e.g. upregulation of other glutamate transporting proteins) and might propose a robust fail-save backup system to prevent glutamate excitotoxicity. The nature of the molecular mechanisms underlying glutamate transporter related disorders are possibly better represented by inducible knockout models which exclude developmental adaption, and thus, allow the real-time detection of the effect of the disturbance without masking it by compensatory processes. However, the question of cause and effect remains valid and still has to be kept in mind.
Function under physiological and pathophysiological conditions
Glutamate transporters contribute to many physiological processes in the kidney [11], the intestine [91], the heart [106], and the brain (reviewed in [36]). In the brain, glutamate transporters contribute to the termination of excitatory synaptic neurotransmission and regulation of the GABA supply of neurons, as well as the glutamate-glutamine cycle [25]. The glial glutamate transporter subtypes GLT1/EAAT2 and GLAST/EAAT1 are thought to be responsible for the majority of the uptake under physiological conditions [36,79], since they are expressed at high levels (up to 10,000 per μm2 for GLT1 [36]). In addition, astrocytes are more resistant to depolarization than neurons, thus allowing glutamate transporters to work at the maximum possible rate. Because turnover of the transporters is relatively slow, it was hypothesized that glutamate transporters can buffer glutamate on a fast timescale, before actual translocation across the membrane takes place [39]. This buffering effect is compatible with the kinetic properties of the transporters, which bind glutamate with an almost diffusion-controlled rate constant [71,128]. Considering the intrinsic affinity of the transporters for glutamate in comparison to those of AMPA and NMDA receptors, the buffering effect would allow the transporters to set glutamate concentrations for rapid deactivation of AMPA receptors, while allowing prolonged activation of NMDA receptors [69]. Thus, the kinetic parameters of glutamate binding/translocation by the EAATs may be intimately linked to the spatiotemporal profile of glutamate receptor activation.
In terms of glutamate transporter function under pathophysiological conditions, it is rather well established that glutamate reverse transport is at least partially responsible for the rise of extracellular glutamate concentration [83,163], when membrane depolarization occurs and extracellular [K+] increases. Relatively quickly following hypoxia, neurons start to depolarize and extracellular K+ levels begin to rise [104]. Assuming a relatively moderate depolarization from −80 mV to −40 mV (in the presence of initially normal (physiological) Na+ and K+ concentration gradients across the membrane) the steady-state release rate of glutamate (1.2 molecules/transporter/s) is predicted to increase 20-fold at short times after the depolarization, as can be predicted from simulations of reverse transport based on established kinetic parameters [68]. This release will be significantly increased as extracellular [K+] increases and extracellular [Na+] drops. For example, in the later phases of hypoxia, [K+]o rises to 100 mM and depolarization is virtually complete [104]. Together with Na+ influx into neurons, the extracellular glutamate concentration is expected to increase significantly, considering that the glutamate concentration in neurons is in the range of 5 mM, and that the driving force for glutamate uptake is almost completely lost.
Glutamate transporter pharmacology
Glutamate transporters are specific for acidic amino acids, which are glutamate and aspartate, as the natural substrates. Some of the most important transportable substrates and competitive and non-competitive inhibitors are illustrated in Fig. 4. Whereas aspartate is transported in both the l- and the d- form, only the l- form is transported for glutamate [90,152]. Cysteine is also a transported substrate of glutamate transporters [229], but it is not clear whether cysteine is transported in its neutral, protonated form, or as the anion [70].
Figure 4.
Representative structures of the most important classes of transported substrates and inhibitors of glutamate transporters.
Current inhibitors for glutamate transporters can be divided into two classes: Competitive inhibitors that bind to the amino acid substrate binding site, and non-substrate inhibitors, which are thought to interact with an allosteric site that is independent of the substrate site. Initial studies focused on the first class of compounds, which were mainly glutamate and aspartate analogs. Glutamate and aspartate have been modified at the side-chain carboxylate position, as well as using substituents on the side chain. For example, oxidation products of cysteine, such as cysteic acid and cysteine sulfinic acid are transported substrates [223], as is the serine derivative serine-O-sulfate [210]. Direct glutamate analogs include L-glutamate-ο-hydroxamate [159].
Conformationally-restricted substrate analogs were tested, which included cyclic molecules, such as L-trans-2,5-pyrrolidinedicarboxylic acid (PDC,[152]), as well as kainate, which shows preference for EAAT2 [9]. These molecules belong to the general class of pyrrolidine dicarboxylates, for which a number of compounds with more complex substitution patterns have been characterized, including the bicyclic methanopyrrolidine dicarboxylates (MPDC derivatives, [23]). While kainate is a blocker, PDC is a transportable inhibitor, blocking glutamate transport by being transported in itself across the membrane. Transportable inhibitors can be differentiated from non-transportable blockers by their ability to induce glutamate release from cells through an exchange mechanism.
Competitive blockers
A breakthrough in our understanding of glutamate transporter pharmacology came with the development of a hydroxyaspartate (THA, [45]) analog with a bulky substituent attached to the hydroxy group. The resulting dl-threo-β-benzyloxyaspartic acid (TBOA) was found to be a potent, although subtype unspecific, competitive inhibitor with a low μM affinity [182]. In contrast, THA is a transportable substrate. This led to the conclusion that hydrophobic bulk is important for blocker-type behavior, as well as for high affinity interaction. The Shimamoto group later extended the library of TBOA-like compounds by modifying the hydrophobic substituent, resulting in the potent inhibitor (3S)-3-[[3-[[4-(Trifluoromethyl)benzoyl]amino]phenyl]methoxy]-L-aspartic acid (TFB-TBOA), which inhibits EAATs with a 20-300 nM affinity (depending on the subtype and the assay used), an about 100-fold improvement in affinity over TBOA [183].
Another class of competitive, non-transportable, inhibitors is based on aromatic amide derivatives of asparagine, as well as di-amino propionic acid analogues [66,42]. These compounds have the interesting property of lacking a negative charge on the side chain. Despite the lack of electrostatic interaction with the positively-charged arginine side chain in the binding site, some of the compounds bind with very high affinity (for example Ki = 35 nM for the fluorene derivative WAY-213613, [42]). These results indicate that a distal hydrophobic pocket exists, necessitating extension of the pharmacophore predicted on the basis of previous aspartate and glutamate analogs.
Oxazole- and oxazoline-based ring systems in the side chain have been applied to increase rigidity of the system [29]. Some of the compounds with the carboxy group directly attached to the ring system demonstrate substrate-like behavior, but fail to interact with ionotropic glutamate receptors – an advantage to other known substrates. A set of other compounds, in which both carboxylates are attached to the ring and which do not possess an amino group, were found to be non-transportable blockers. This finding suggests that interaction of the transporter binding site with a positively charged amino function is not required for high affinity binding [29-30].
Non-competitive inhibitors
Inhibitors that interact with the glutamate transporter at a site different than the substrate binding site have recently been developed. Interestingly, glutamate-like bicyclic compounds, such as the hydroxyisoxazolines HIP-A and HIP-B [53] were found to inhibit glutamate uptake with a Ki that is independent of the glutamate concentration, suggesting a lack of competition with substrate binding [28]. Furthermore, HIP-B was unable to displace the competitive inhibitor TBOA from its EAAC1 binding site, and inhibition was not affected by a mutation to the substrate binding site that eliminates glutamate, as well as TBOA binding. Together, these results suggest that HIP-B binds at an allosteric binding site [28].
Another example of compounds displaying non-competitive inhibition kinetics are coumarine analogs that have been identified in a screen from a library of 3040 drug-like compounds. UCPH-101, which is the compound with the highest apparent affinity, not only inhibits through binding at a hydrophobic pocket at the subunit interface, but this pocket is also subtype specific [46]. This allows the inhibitor to be specific for EAAT1, while displaying virtually no interaction with the subtypes EAAT2 and 3 [82,46]. This was an important development, since up to this study compounds with moderate specificity existed only for EAATs 2 and 3. Recently, site directed mutagenesis allowed the identification of a putative binding site for this non-competitive inhibitor. Interestingly, this site is proposed to be located in the trimerization domain as a hydrophobic pocket [1].
Activators
An important goal has been the identification of small-molecule compounds that can upregulate glutamate transporter function. Such molecules should be neuroprotective, since several neurological diseases involving glutamatergic dysfunction are associated with loss of glutamate transport. Several compounds have been developed that increase glutamate transporter function, mainly by indirectly increasing expression levels. β-lactam antibiotics, such as Ceftriaxone, for example, enhance glutamate uptake by EAAT2 2-5 fold through a transcriptional mechanism ([166], see section on regulation). In addition, a spider toxin, parawixin, was isolated that increases transporter turnover by possibly increasing the rate of relocation of the K+-bound transporter [51-52]. Continuing development of activators represents an important goal, due to the potential neuroprotective effects of such compounds, which could speed the removal of the excitotoxin glutamate from the extracellular space in the brain.
Future perspectives
Our knowledge of the structure and function of plasma membrane glutamate transporters continues to increase due to the recent advancements in structural and computational biology, but also due to the large number of regulatory pathways that have been identified to control transporter expression levels. In addition, much advancement in the development of pharmacological tools have contributed, in particular the recent discoveries of non-competitive inhibitors, that potentially open new avenues for the modulation of transporter function, through the targeting of sites other than the glutamate binding site.
From the perspective of molecular mechanism, integration between the large number of functional data and the structural picture is underway. However, this effort is somewhat compromised by the lack of crystal structures of the mammalian transporters, as well as the lack of functional data on the archeal homologue, GltPh. This is of particular relevance because GltPh is insensitive to K+ and H+, thus differing in mechanism from the mammalian glutamate transporters. In addition, only few of the many states implicated in the whole transport cycle have been structurally identified. For example, the structure of the apo state has only been implicated from MD simulations, but no direct structural information is available. Finally, available crystal structures, most importantly those of intermediates, need to be further verified using biophysical/biochemical methods. For these reasons, functional biophysical studies continue to play important roles in the elucidation of transport mechanism.
Our understanding of processes involved in the regulation of transporter expression levels has been bolstered by recent advances in the identification of transcription regulation (discovery of promoter sequences), as well as trafficking and cell surface targeting. Establishing regulatory pathways that can be targeted to increase glutamate transporter expression in the plasma membrane, as well as pharmacological tools to directly upregulate transporter turnover, may be useful for increasing glutamate uptake in vivo, a desirable intervention under conditions in which glutamate uptake is impaired. These advancements may lead to novel therapeutic strategies that directly target glutamate transporters in a variety of nervous system disorders.
Acknowledgements
This work was supported by the National Institutes of Health Grant 2R01NS049335-06A1 awarded to CG, and a Binational Science Foundation (BSF), Grant 2007051 awarded to CG and B. I. Kanner.
References
- 1.Abrahamsen B, Schneider N, Erichsen MN, Huynh TH, Fahlke C, Bunch L, Jensen AA. Allosteric modulation of an excitatory amino acid transporter: the subtype-selective inhibitor UCPH-101 exerts sustained inhibition of EAAT1 through an intramonomeric site in the trimerization domain. J Neurosci. 2013;33(3):1068–1087. doi: 10.1523/JNEUROSCI.3396-12.2013. doi:33/3/1068 [pii] 10.1523/JNEUROSCI.3396-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adamczyk A, Gause CD, Sattler R, Vidensky S, Rothstein JD, Singer H, Wang T. Genetic and functional studies of a missense variant in a glutamate transporter, SLC1A3, in Tourette syndrome. Psychiatric genetics. 2011;21(2):90–97. doi: 10.1097/YPG.0b013e328341a307. doi:10.1097/YPG.0b013e328341a307. [DOI] [PubMed] [Google Scholar]
- 3.Akyuz N, Altman RB, Blanchard SC, Boudker O. Transport dynamics in a glutamate transporter homologue. Nature. 2013 doi: 10.1038/nature12265. doi:nature12265 [pii] 10.1038/nature12265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Allritz C, Bette S, Figiel M, Engele J. Endothelin-1 reverses the histone deacetylase inhibitor-induced increase in glial glutamate transporter transcription without affecting histone acetylation levels. Neurochem Int. 2009;55(1-3):22–27. doi: 10.1016/j.neuint.2008.12.020. doi:10.1016/j.neuint.2008.12.020. [DOI] [PubMed] [Google Scholar]
- 5.Allritz C, Bette S, Figiel M, Engele J. Comparative structural and functional analysis of the GLT-1/EAAT-2 promoter from man and rat. J Neurosci Res. 2010;88(6):1234–1241. doi: 10.1002/jnr.22303. doi:10.1002/jnr.22303. [DOI] [PubMed] [Google Scholar]
- 6.Ambros V. The functions of animal microRNAs. Nature. 2004;431(7006):350–355. doi: 10.1038/nature02871. doi:10.1038/nature02871. [DOI] [PubMed] [Google Scholar]
- 7.Arnold PD, Sicard T, Burroughs E, Richter MA, Kennedy JL. Glutamate transporter gene SLC1A1 associated with obsessive-compulsive disorder. Archives of general psychiatry. 2006;63(7):769–776. doi: 10.1001/archpsyc.63.7.769. doi:10.1001/archpsyc.63.7.769. [DOI] [PubMed] [Google Scholar]
- 8.Arriza JL, Eliasof S, Kavanaugh MP, Amara SG. Excitatory amino acid transporter 5, a retinal glutamate transporter coupled to a chloride conductance. Proc Natl Acad Sci U S A. 1997;94(8):4155–4160. doi: 10.1073/pnas.94.8.4155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arriza JL, Fairman WA, Wadiche JI, Murdoch GH, Kavanaugh MP, Amara SG. Functional comparisons of three glutamate transporter subtypes cloned from human motor cortex. J Neurosci. 1994;14(9):5559–5569. doi: 10.1523/JNEUROSCI.14-09-05559.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Azami Tameh A, Clarner T, Beyer C, Atlasi MA, Hassanzadeh G, Naderian H. Regional regulation of glutamate signaling during cuprizone-induced demyelination in the brain. Annals of anatomy = Anatomischer Anzeiger: official organ of the Anatomische Gesellschaft. 2013 doi: 10.1016/j.aanat.2013.03.004. doi:10.1016/j.aanat.2013.03.004. [DOI] [PubMed] [Google Scholar]
- 11.Bailey CG, Ryan RM, Thoeng AD, Ng C, King K, Vanslambrouck JM, Auray-Blais C, Vandenberg RJ, Broer S, Rasko JE. Loss-of-function mutations in the glutamate transporter SLC1A1 cause human dicarboxylic aminoaciduria. J Clin Invest. 2011;121(1):446–453. doi: 10.1172/JCI44474. doi:44474 [pii] 10.1172/JCI44474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bastug T, Heinzelmann G, Kuyucak S, Salim M, Vandenberg RJ, Ryan RM. Position of the third Na+ site in the aspartate transporter GltPh and the human glutamate transporter, EAAT1. PLoS One. 2012;7(3):e33058. doi: 10.1371/journal.pone.0033058. doi:10.1371/journal.pone.0033058 PONE-D-11-21038 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beart PM, O’Shea RD. Transporters for L-glutamate: an update on their molecular pharmacology and pathological involvement. Br J Pharmacol. 2007;150(1):5–17. doi: 10.1038/sj.bjp.0706949. doi:10.1038/sj.bjp.0706949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bendahan A, Armon A, Madani N, Kavanaugh MP, Kanner BI. Arginine 447 plays a pivotal role in substrate interactions in a neuronal glutamate transporter. J Biol Chem. 2000;275(48):37436–37442. doi: 10.1074/jbc.M006536200. doi:10.1074/jbc.M006536200 M006536200 [pii] [DOI] [PubMed] [Google Scholar]
- 15.Benediktsson AM, Marrs GS, Tu JC, Worley PF, Rothstein JD, Bergles DE, Dailey ME. Neuronal activity regulates glutamate transporter dynamics in developing astrocytes. Glia. 2012;60(2):175–188. doi: 10.1002/glia.21249. doi:10.1002/glia.21249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Berger UV, DeSilva TM, Chen W, Rosenberg PA. Cellular and subcellular mRNA localization of glutamate transporter isoforms GLT1a and GLT1b in rat brain by in situ hybridization. J Comp Neurol. 2005;492(1):78–89. doi: 10.1002/cne.20737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bergles DE, Tzingounis AV, Jahr CE. Comparison of coupled and uncoupled currents during glutamate uptake by GLT-1 transporters. J Neurosci. 2002;22(23):10153–10162. doi: 10.1523/JNEUROSCI.22-23-10153.2002. doi:22/23/10153 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Billups B, Rossi D, Attwell D. Anion conductance behavior of the glutamate uptake carrier in salamander retinal glial cells. J Neurosci. 1996;16(21):6722–6731. doi: 10.1523/JNEUROSCI.16-21-06722.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Blakely PK, Kleinschmidt-DeMasters BK, Tyler KL, Irani DN. Disrupted glutamate transporter expression in the spinal cord with acute flaccid paralysis caused by West Nile virus infection. Journal of neuropathology and experimental neurology. 2009;68(10):1061–1072. doi: 10.1097/NEN.0b013e3181b8ba14. doi:10.1097/NEN.0b013e3181b8ba14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boudker O, Ryan RM, Yernool D, Shimamoto K, Gouaux E. Coupling substrate and ion binding to extracellular gate of a sodium-dependent aspartate transporter. Nature. 2007;445(7126):387–393. doi: 10.1038/nature05455. doi:nature05455 [pii] 10.1038/nature05455. [DOI] [PubMed] [Google Scholar]
- 21.Boudker O, Verdon G. Structural perspectives on secondary active transporters. Trends Pharmacol Sci. 2010;31(9):418–426. doi: 10.1016/j.tips.2010.06.004. doi:S0165-6147(10)00110-0 [pii] 10.1016/j.tips.2010.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boycott HE, Wilkinson JA, Boyle JP, Pearson HA, Peers C. Differential involvement of TNF alpha in hypoxic suppression of astrocyte glutamate transporters. Glia. 2008;56(9):998–1004. doi: 10.1002/glia.20673. doi:10.1002/glia.20673. [DOI] [PubMed] [Google Scholar]
- 23.Bridges RJ, Lovering FE, Koch H, Cotman CW, Chamberlin AR. A conformationally constrained competitive inhibitor of the sodium-dependent glutamate transporter in forebrain synaptosomes: L-anti-endo-3,4-methanopyrrolidine dicarboxylate. Neurosci Lett. 1994;174(2):193–197. doi: 10.1016/0304-3940(94)90019-1. [DOI] [PubMed] [Google Scholar]
- 24.Broer A, Wagner C, Lang F, Broer S. Neutral amino acid transporter ASCT2 displays substrate-induced Na+ exchange and a substrate-gated anion conductance. Biochem J. 2000;346:705–710. [PMC free article] [PubMed] [Google Scholar]
- 25.Broer S, Brookes N. Transfer of glutamine between astrocytes and neurons. J Neurochem. 2001;77(3):705–719. doi: 10.1046/j.1471-4159.2001.00322.x. [DOI] [PubMed] [Google Scholar]
- 26.Butchbach ME, Guo H, Lin CL. Methyl-beta-cyclodextrin but not retinoic acid reduces EAAT3-mediated glutamate uptake and increases GTRAP3-18 expression. J Neurochem. 2003;84(4):891–894. doi: 10.1046/j.1471-4159.2003.01588.x. [DOI] [PubMed] [Google Scholar]
- 27.Butchbach ME, Tian G, Guo H, Lin CL. Association of excitatory amino acid transporters, especially EAAT2, with cholesterol-rich lipid raft microdomains: importance for excitatory amino acid transporter localization and function. J Biol Chem. 2004;279(33):34388–34396. doi: 10.1074/jbc.M403938200. [DOI] [PubMed] [Google Scholar]
- 28.Callender R, Gameiro A, Pinto A, De Micheli C, Grewer C. Mechanism of inhibition of the glutamate transporter EAAC1 by the conformationally constrained glutamate analogue (+)-HIP-B. Biochemistry. 2012;51(27):5486–5495. doi: 10.1021/bi3006048. doi:10.1021/bi3006048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Campiani G, De Angelis M, Armaroli S, Fattorusso C, Catalanotti B, Ramunno A, Nacci V, Novellino E, Grewer C, Ionescu D, Rauen T, Griffiths R, Sinclair C, Fumagalli E, Mennini T. A rational approach to the design of selective substrates and potent nontransportable inhibitors of the excitatory amino acid transporter EAAC1 (EAAT3) J Med Chem. 2001;44(16):2507–2510. doi: 10.1021/jm015509z. doi:jm015509z [pii] [DOI] [PubMed] [Google Scholar]
- 30.Campiani G, Fattorusso C, De Angelis M, Catalanotti B, Butini S, Fattorusso R, Fiorini I, Nacci V, Novellino E. Neuronal high-affinity sodium-dependent glutamate transporters (EAATs): targets for the development of novel therapeutics against neurodegenerative diseases. Curr Pharm Des. 2003;9(8):599–625. doi: 10.2174/1381612033391261. [DOI] [PubMed] [Google Scholar]
- 31.Chen W, Aoki C, Mahadomrongkul V, Gruber CE, Wang GJ, Blitzblau R, Irwin N, Rosenberg PA. Expression of a variant form of the glutamate transporter GLT1 in neuronal cultures and in neurons and astrocytes in the rat brain. J Neurosci. 2002;22(6):2142–2152. doi: 10.1523/JNEUROSCI.22-06-02142.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Conne B, Stutz A, Vassalli JD. The 3′ untranslated region of messenger RNA: A molecular ‘hotspot’ for pathology? Nat Med. 2000;6(6):637–641. doi: 10.1038/76211. doi:10.1038/76211. [DOI] [PubMed] [Google Scholar]
- 33.Crino PB, Jin H, Shumate MD, Robinson MB, Coulter DA, Kayal AR. Increased expression of the neuronal glutamate transporter (EAAT3/EAAC1) in hippocampal and neocortical epilepsy. Epilepsia. 2002;43(3):211–218. doi: 10.1046/j.1528-1157.2002.35001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Crisman TJ, Qu S, Kanner BI, Forrest LR. Inward-facing conformation of glutamate transporters as revealed by their inverted-topology structural repeats. Proc Natl Acad Sci U S A. 2009;106(49):20752–20757. doi: 10.1073/pnas.0908570106. doi:0908570106 [pii] 10.1073/pnas.0908570106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Danbolt NC. Glutamate uptake. Prog Neurobiol. 2001;65(1):1–105. doi: 10.1016/s0301-0082(00)00067-8. doi:S0301-0082(00)00067-8 [pii] [DOI] [PubMed] [Google Scholar]
- 36.Danbolt NC. Glutamate uptake. Prog Neurobiol. 2001;65(1):1–105. doi: 10.1016/s0301-0082(00)00067-8. [DOI] [PubMed] [Google Scholar]
- 37.de Vivo L, Melone M, Bucci G, Rothstein JD, Conti F. Quantitative analysis of EAAT4 promoter activity in neurons and astrocytes of mouse somatic sensory cortex. Neurosci Lett. 2010;474(1):42–45. doi: 10.1016/j.neulet.2010.03.003. doi:10.1016/j.neulet.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 38.DeChancie J, Shrivastava IH, Bahar I. The mechanism of substrate release by the aspartate transporter GltPh: insights from simulations. Mol Biosyst. 2011;7(3):832–842. doi: 10.1039/c0mb00175a. doi:10.1039/c0mb00175a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Diamond JS, Jahr CE. Transporters buffer synaptically released glutamate on a submillisecond time scale. J Neurosci. 1997;17(12):4672–4687. doi: 10.1523/JNEUROSCI.17-12-04672.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Drejer J, Larsson OM, Schousboe A. Characterization of L-glutamate uptake into and release from astrocytes and neurons cultured from differnt brain regions. ExpBrain Res. 1982;47:259–269. doi: 10.1007/BF00239385. [DOI] [PubMed] [Google Scholar]
- 41.Duerson K, Woltjer RL, Mookherjee P, Leverenz JB, Montine TJ, Bird TD, Pow DV, Rauen T, Cook DG. Detergent-insoluble EAAC1/EAAT3 aberrantly accumulates in hippocampal neurons of Alzheimer’s disease patients. Brain pathology. 2009;19(2):267–278. doi: 10.1111/j.1750-3639.2008.00186.x. doi:10.1111/j.1750-3639.2008.00186.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dunlop J, McIlvain HB, Carrick TA, Jow B, Lu Q, Kowal D, Lin S, Greenfield A, Grosanu C, Fan K, Petroski R, Williams J, Foster A, Butera J. Characterization of novel aryl-ether, biaryl, and fluorene aspartic acid and diaminopropionic acid analogs as potent inhibitors of the high-affinity glutamate transporter EAAT2. Mol Pharmacol. 2005;68(4):974–982. doi: 10.1124/mol.105.012005. doi:mol.105.012005 [pii] 10.1124/mol.105.012005. [DOI] [PubMed] [Google Scholar]
- 43.Eliasof S, Arriza JL, Leighton BH, Kavanaugh MP, Amara SG, Amara SG. Excitatory amino acid transporters of the salamander retina: identification, localization, and function. J Neurosci. 1998;18(2):698–712. doi: 10.1523/JNEUROSCI.18-02-00698.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Eliasof S, Jahr CE. Retinal glial cell glutamate transporter is coupled to an anionic conductance. Proc Natl Acad Sci U S A. 1996;93(9):4153–4158. doi: 10.1073/pnas.93.9.4153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Eliasof S, Werblin F. Characterization of the glutamate transporter in retinal cones of the tiger salamander. J Neurosci. 1993;13(1):402–411. doi: 10.1523/JNEUROSCI.13-01-00402.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Erichsen MN, Huynh TH, Abrahamsen B, Bastlund JF, Bundgaard C, Monrad O, Bekker-Jensen A, Nielsen CW, Frydenvang K, Jensen AA, Bunch L. Structure-activity relationship study of first selective inhibitor of excitatory amino acid transporter subtype 1: 2-Amino-4-(4-methoxyphenyl)-7-(naphthalen-1-yl)-5-oxo-5,6,7,8-tetrahydro-4H-chrom ene-3-carbonitrile (UCPH-101) J Med Chem. 2010;53(19):7180–7191. doi: 10.1021/jm1009154. doi:10.1021/jm1009154. [DOI] [PubMed] [Google Scholar]
- 47.Estrada-Sanchez AM, Rebec GV. Corticostriatal dysfunction and glutamate transporter 1 (GLT1) in Huntington’s disease: interactions between neurons and astrocytes. Basal ganglia. 2012;2(2):57–66. doi: 10.1016/j.baga.2012.04.029. doi:10.1016/j.baga.2012.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fairman WA, Vandenberg RJ, Arriza JL, Kavanaugh MP, Amara SG. An excitatory amino-acid transporter with properties of a ligand-gated chloride channel. Nature. 1995;375(6532):599–603. doi: 10.1038/375599a0. [DOI] [PubMed] [Google Scholar]
- 49.Figiel M, Engele J. Pituitary adenylate cyclase-activating polypeptide (PACAP), a neuron-derived peptide regulating glial glutamate transport and metabolism. J Neurosci. 2000;20(10):3596–3605. doi: 10.1523/JNEUROSCI.20-10-03596.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Focke PJ, Wang X, Larsson HP. Neurotransmitter transporters: structure meets function. Structure. 2013;21(5):694–705. doi: 10.1016/j.str.2013.03.002. doi:S0969-2126(13)00082-8 [pii] 10.1016/j.str.2013.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fontana AC, de Oliveira Beleboni R, Wojewodzic MW, Ferreira Dos Santos W, Coutinho-Netto J, Grutle NJ, Watts SD, Danbolt NC, Amara SG. Enhancing glutamate transport: mechanism of action of Parawixin1, a neuroprotective compound from Parawixia bistriata spider venom. Mol Pharmacol. 2007;72(5):1228–1237. doi: 10.1124/mol.107.037127. doi:mol.107.037127 [pii] 10.1124/mol.107.037127. [DOI] [PubMed] [Google Scholar]
- 52.Fontana AC, Guizzo R, de Oliveira Beleboni R, Meirelles ESAR, Coimbra NC, Amara SG, dos Santos WF, Coutinho-Netto J. Purification of a neuroprotective component of Parawixia bistriata spider venom that enhances glutamate uptake. Br J Pharmacol. 2003;139(7):1297–1309. doi: 10.1038/sj.bjp.0705352. doi:10.1038/sj.bjp.0705352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Funicello M, Conti P, De Amici M, De Micheli C, Mennini T, Gobbi M. Dissociation of [3H]L-glutamate uptake from L-glutamate-induced [3H]D-aspartate release by 3-hydroxy-4,5,6,6a-tetrahydro-3aH-pyrrolo[3,4-d]isoxazole-4-carboxylic acid and 3-hydroxy-4,5,6,6a-tetrahydro-3aH-pyrrolo[3,4-d]isoxazole-6-carboxylic acid, two conformationally constrained aspartate and glutamate analogs. Mol Pharmacol. 2004;66(3):522–529. doi: 10.1124/mol.66.3.. doi:10.1124/mol.66.3. 66/3/522 [pii] [DOI] [PubMed] [Google Scholar]
- 54.Furness DN, Dehnes Y, Akhtar AQ, Rossi DJ, Hamann M, Grutle NJ, Gundersen V, Holmseth S, Lehre KP, Ullensvang K, Wojewodzic M, Zhou Y, Attwell D, Danbolt NC. A quantitative assessment of glutamate uptake into hippocampal synaptic terminals and astrocytes: new insights into a neuronal role for excitatory amino acid transporter 2 (EAAT2) Neuroscience. 2008;157(1):80–94. doi: 10.1016/j.neuroscience.2008.08.043. doi:10.1016/j.neuroscience.2008.08.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gameiro A, Braams S, Rauen T, Grewer C. The discovery of slowness: low-capacity transport and slow anion channel gating by the glutamate transporter EAAT5. Biophys J. 2011;100(11):2623–2632. doi: 10.1016/j.bpj.2011.04.034. doi:S0006-3495(11)00480-2 [pii] 10.1016/j.bpj.2011.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Garcia-Tardon N, Gonzalez-Gonzalez IM, Martinez-Villarreal J, Fernandez-Sanchez E, Gimenez C, Zafra F. Protein kinase C (PKC)-promoted endocytosis of glutamate transporter GLT-1 requires ubiquitin ligase Nedd4-2-dependent ubiquitination but not phosphorylation. J Biol Chem. 2012;287(23):19177–19187. doi: 10.1074/jbc.M112.355909. doi:10.1074/jbc.M112.355909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gegelashvili G, Danbolt NC, Schousboe A. Neuronal soluble factors differentially regulate the expression of the GLT1 and GLAST glutamate transporters in cultured astroglia. J Neurochem. 1997;69(6):2612–2615. doi: 10.1046/j.1471-4159.1997.69062612.x. [DOI] [PubMed] [Google Scholar]
- 58.Gegelashvili G, Robinson MB, Trotti D, Rauen T. Regulation of glutamate transporters in health and disease. Prog Brain Res. 2001;132:267–286. doi: 10.1016/S0079-6123(01)32082-4. doi:S0079-6123(01)32082-4 [pii] 10.1016/S0079-6123(01)32082-4. [DOI] [PubMed] [Google Scholar]
- 59.Gegelashvili G, Schousboe A. High affinity glutamate transporters: regulation of expression and activity. Mol Pharmacol. 1997;52(1):6–15. doi: 10.1124/mol.52.1.6. [DOI] [PubMed] [Google Scholar]
- 60.Gincel D, Regan MR, Jin L, Watkins AM, Bergles DE, Rothstein JD. Analysis of cerebellar Purkinje cells using EAAT4 glutamate transporter promoter reporter in mice generated via bacterial artificial chromosome-mediated transgenesis. Exp Neurol. 2007;203(1):205–212. doi: 10.1016/j.expneurol.2006.08.016. [DOI] [PubMed] [Google Scholar]
- 61.Glisic D, Lehmann C, Figiel M, Odemis V, Lindner R, Engele J. A novel cross-talk between endothelin and ErbB receptors controlling glutamate transporter expression in astrocytes. J Neurochem. 2012;122(4):844–855. doi: 10.1111/j.1471-4159.2012.07819.x. doi:10.1111/j.1471-4159.2012.07819.x. [DOI] [PubMed] [Google Scholar]
- 62.Gonzalez-Gonzalez IM, Garcia-Tardon N, Gimenez C, Zafra F. PKC-dependent endocytosis of the GLT1 glutamate transporter depends on ubiquitylation of lysines located in a C-terminal cluster. Glia. 2008;56(9):963–974. doi: 10.1002/glia.20670. doi:10.1002/glia.20670. [DOI] [PubMed] [Google Scholar]
- 63.Gonzalez MI, Krizman-Genda E, Robinson MB. Caveolin-1 regulates the delivery and endocytosis of the glutamate transporter, excitatory amino acid carrier 1. J Biol Chem. 2007;282(41):29855–29865. doi: 10.1074/jbc.M704738200. doi:M704738200 [pii] 10.1074/jbc.M704738200. [DOI] [PubMed] [Google Scholar]
- 64.Gonzalez MI, Robinson MB. Protein KINASE C-Dependent Remodeling of Glutamate Transporter Function. Mol Intervent. 2004;4(1):48–58. doi: 10.1124/mi.4.1.48. [DOI] [PubMed] [Google Scholar]
- 65.Gordon PH, Moore DH, Miller RG, Florence JM, Verheijde JL, Doorish C, Hilton JF, Spitalny GM, MacArthur RB, Mitsumoto H, Neville HE, Boylan K, Mozaffar T, Belsh JM, Ravits J, Bedlack RS, Graves MC, McCluskey LF, Barohn RJ, Tandan R, Western ALSSG. Efficacy of minocycline in patients with amyotrophic lateral sclerosis: a phase III randomised trial. Lancet Neurol. 2007;6(12):1045–1053. doi: 10.1016/S1474-4422(07)70270-3. doi:10.1016/S1474-4422(07)70270-3. [DOI] [PubMed] [Google Scholar]
- 66.Greenfield A, Grosanu C, Dunlop J, McIlvain B, Carrick T, Jow B, Lu Q, Kowal D, Williams J, Butera J. Synthesis and biological activities of aryl-ether-, biaryl-, and fluorene-aspartic acid and diaminopropionic acid analogs as potent inhibitors of the high-affinity glutamate transporter EAAT-2. Bioorg Med Chem Lett. 2005;15(22):4985–4988. doi: 10.1016/j.bmcl.2005.08.003. doi:S0960-894X(05)01017-6 [pii] 10.1016/j.bmcl.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 67.Grewer C, Balani P, Weidenfeller C, Bartusel T, Tao Z, Rauen T. Individual subunits of the glutamate transporter EAAC1 homotrimer function independently of each other. Biochemistry. 2005;44(35):11913–11923. doi: 10.1021/bi050987n. doi:10.1021/bi050987n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Grewer C, Gameiro A, Zhang Z, Tao Z, Braams S, Rauen T. Glutamate forward and reverse transport: from molecular mechanism to transporter-mediated release after ischemia. IUBMB Life. 2008;60(9):609–619. doi: 10.1002/iub.98. doi:10.1002/iub.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Grewer C, Rauen T. Electrogenic glutamate transporters in the CNS: molecular mechanism, pre-steady-state kinetics, and their impact on synaptic signaling. J Membr Biol. 2005;203(1):1–20. doi: 10.1007/s00232-004-0731-6. doi:10.1007/s00232-004-0731-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Grewer C, Watzke N, Rauen T, Bicho A. Is the glutamate residue Glu-373 the proton acceptor of the excitatory amino acid carrier 1? J Biol Chem. 2003;278(4):2585–2592. doi: 10.1074/jbc.M207956200. doi:10.1074/jbc.M207956200 M207956200 [pii] [DOI] [PubMed] [Google Scholar]
- 71.Grewer C, Watzke N, Wiessner M, Rauen T. Glutamate translocation of the neuronal glutamate transporter EAAC1 occurs within milliseconds. Proc Natl Acad Sci U S A. 2000;97(17):9706–9711. doi: 10.1073/pnas.160170397. doi:10.1073/pnas.160170397 160170397 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Grewer C, Zhang Z, Mwaura J, Albers T, Schwartz A, Gameiro A. Charge compensation mechanism of a Na+-coupled, secondary active glutamate transporter. J Biol Chem. 2012;287(32):26921–26931. doi: 10.1074/jbc.M112.364059. doi:M112.364059 [pii] 10.1074/jbc.M112.364059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Groeneveld M, Slotboom DJ. Na(+):aspartate coupling stoichiometry in the glutamate transporter homologue Glt(Ph) Biochemistry. 2010;49(17):3511–3513. doi: 10.1021/bi100430s. doi:10.1021/bi100430s. [DOI] [PubMed] [Google Scholar]
- 74.Gu Y, Shrivastava IH, Amara SG, Bahar I. Molecular simulations elucidate the substrate translocation pathway in a glutamate transporter. Proc Natl Acad Sci U S A. 2009;106(8):2589–2594. doi: 10.1073/pnas.0812299106. doi:0812299106 [pii] 10.1073/pnas.0812299106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hagiwara T, Tanaka K, Takai S, Maeno-Hikichi Y, Mukainaka Y, Wada K. Genomic organization, promoter analysis, and chromosomal localization of the gene for the mouse glial high-affinity glutamate transporter Slc1a3. Genomics. 1996;33(3):508–515. doi: 10.1006/geno.1996.0226. [DOI] [PubMed] [Google Scholar]
- 76.Hamann M, Rossi DJ, Marie H, Attwell D. Knocking out the glial glutamate transporter GLT-1 reduces glutamate uptake but does not affect hippocampal glutamate dynamics in early simulated ischaemia. The European journal of neuroscience. 2002;15(2):308–314. doi: 10.1046/j.0953-816x.2001.01861.x. [DOI] [PubMed] [Google Scholar]
- 77.Hanelt I, Wunnicke D, Bordignon E, Steinhoff HJ, Slotboom DJ. Conformational heterogeneity of the aspartate transporter Glt(Ph) Nat Struct Mol Biol. 2013;20(2):210–214. doi: 10.1038/nsmb.2471. doi:nsmb.2471 [pii] 10.1038/nsmb.2471. [DOI] [PubMed] [Google Scholar]
- 78.Hertz L. Functional interactions between neurons and astrocytes. I. Turnover and metabolism of putative amino acid transmitters. ProgNeurobiol. 1979;13:277–323. doi: 10.1016/0301-0082(79)90018-2. [DOI] [PubMed] [Google Scholar]
- 79.Holmseth S, Dehnes Y, Huang YH, Follin-Arbelet VV, Grutle NJ, Mylonakou MN, Plachez C, Zhou Y, Furness DN, Bergles DE, Lehre KP, Danbolt NC. The density of EAAC1 (EAAT3) glutamate transporters expressed by neurons in the mammalian CNS. J Neurosci. 2012;32(17):6000–6013. doi: 10.1523/JNEUROSCI.5347-11.2012. doi:32/17/6000 [pii] 10.1523/JNEUROSCI.5347-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Huang Z, Tajkhorshid E. Dynamics of the extracellular gate and ion-substrate coupling in the glutamate transporter. Biophys J. 2008;95(5):2292–2300. doi: 10.1529/biophysj.108.133421. doi:S0006-3495(08)78377-2 [pii] 10.1529/biophysj.108.133421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Huang Z, Tajkhorshid E. Identification of the third Na+ site and the sequence of extracellular binding events in the glutamate transporter. Biophys J. 2010;99(5):1416–1425. doi: 10.1016/j.bpj.2010.06.052. doi:S0006-3495(10)00795-2 [pii] 10.1016/j.bpj.2010.06.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Huynh TH, Shim I, Bohr H, Abrahamsen B, Nielsen B, Jensen AA, Bunch L. Structure-activity relationship study of selective excitatory amino acid transporter subtype 1 (EAAT1) inhibitor 2-amino-4-(4-methoxyphenyl)-7-(naphthalen-1-yl)-5-oxo-5,6,7,8-tetrahydro-4H-chrom ene-3-carbonitrile (UCPH-101) and absolute configurational assignment using infrared and vibrational circular dichroism spectroscopy in combination with ab initio Hartree-Fock calculations. J Med Chem. 2012;55(11):5403–5412. doi: 10.1021/jm300345z. doi:10.1021/jm300345z. [DOI] [PubMed] [Google Scholar]
- 83.Jabaudon D, Scanziani M, Gähwiler BH, Gerber U. Acute decrease in net glutamate uptake during energy deprivation. Proc Natl Acad Sci USA. 2000;97:5610–5615. doi: 10.1073/pnas.97.10.5610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Jardetzky O. Simple allosteric model for membrane pumps. Nature. 1966;211(5052):969–970. doi: 10.1038/211969a0. [DOI] [PubMed] [Google Scholar]
- 85.Jayanarayanan S, Smijin S, Peeyush KT, Anju TR, Paulose CS. NMDA and AMPA receptor mediated excitotoxicity in cerebral cortex of streptozotocin induced diabetic rat: ameliorating effects of curcumin. Chemico-biological interactions. 2013;201(1-3):39–48. doi: 10.1016/j.cbi.2012.11.024. doi:10.1016/j.cbi.2012.11.024. [DOI] [PubMed] [Google Scholar]
- 86.Jen JC, Graves TD, Hess EJ, Hanna MG, Griggs RC, Baloh RW, investigators C. Primary episodic ataxias: diagnosis, pathogenesis and treatment. Brain: a journal of neurology. 2007;130(Pt 10):2484–2493. doi: 10.1093/brain/awm126. doi:10.1093/brain/awm126. [DOI] [PubMed] [Google Scholar]
- 87.Jen JC, Wan J, Palos TP, Howard BD, Baloh RW. Mutation in the glutamate transporter EAAT1 causes episodic ataxia, hemiplegia, and seizures. Neurology. 2005;65(4):529–534. doi: 10.1212/01.wnl.0000172638.58172.5a. doi:10.1212/01.wnl.0000172638.58172.5a. [DOI] [PubMed] [Google Scholar]
- 88.Jiang J, Shrivastava IH, Watts SD, Bahar I, Amara SG. Large collective motions regulate the functional properties of glutamate transporter trimers. Proc Natl Acad Sci U S A. 2011;108(37):15141–15146. doi: 10.1073/pnas.1112216108. doi:1112216108 [pii] 10.1073/pnas.1112216108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kanai Y, Hediger MA. Primary structure and functional characterization of a high-affinity glutamate transporter. Nature. 1992;360(6403):467–471. doi: 10.1038/360467a0. doi:10.1038/360467a0. [DOI] [PubMed] [Google Scholar]
- 90.Kanai Y, Hediger MA. Primary Structure and Functional Characterization of a High-Affinity Glutamate Transporter. Nature. 1992;360(6403):467–471. doi: 10.1038/360467a0. [DOI] [PubMed] [Google Scholar]
- 91.Kanai Y, Nussberger S, Romero MF, Boron WF, Hebert SC, Hediger MA. Electrogenic properties of the epithelial and neuronal high affinity glutamate transporter. J Biol Chem. 1995;270(28):16561–16568. doi: 10.1074/jbc.270.28.16561. [DOI] [PubMed] [Google Scholar]
- 92.Karlsson RM, Adermark L, Molander A, Perreau-Lenz S, Singley E, Solomon M, Holmes A, Tanaka K, Lovinger DM, Spanagel R, Heilig M. Reduced alcohol intake and reward associated with impaired endocannabinoid signaling in mice with a deletion of the glutamate transporter GLAST. Neuropharmacology. 2012;63(2):181–189. doi: 10.1016/j.neuropharm.2012.01.027. doi:10.1016/j.neuropharm.2012.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Karlsson RM, Tanaka K, Heilig M, Holmes A. Loss of glial glutamate and aspartate transporter (excitatory amino acid transporter 1) causes locomotor hyperactivity and exaggerated responses to psychotomimetics: rescue by haloperidol and metabotropic glutamate 2/3 agonist. Biological psychiatry. 2008;64(9):810–814. doi: 10.1016/j.biopsych.2008.05.001. doi:10.1016/j.biopsych.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Karlsson RM, Tanaka K, Saksida LM, Bussey TJ, Heilig M, Holmes A. Assessment of glutamate transporter GLAST (EAAT1)-deficient mice for phenotypes relevant to the negative and executive/cognitive symptoms of schizophrenia. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2009;34(6):1578–1589. doi: 10.1038/npp.2008.215. doi:10.1038/npp.2008.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ketheeswaranathan P, Turner NA, Spary EJ, Batten TF, McColl BW, Saha S. Changes in glutamate transporter expression in mouse forebrain areas following focal ischemia. Brain research. 2011;1418:93–103. doi: 10.1016/j.brainres.2011.08.029. doi:10.1016/j.brainres.2011.08.029. [DOI] [PubMed] [Google Scholar]
- 96.Kim K, Lee SG, Kegelman TP, Su ZZ, Das SK, Dash R, Dasgupta S, Barral PM, Hedvat M, Diaz P, Reed JC, Stebbins JL, Pellecchia M, Sarkar D, Fisher PB. Role of excitatory amino acid transporter-2 (EAAT2) and glutamate in neurodegeneration: opportunities for developing novel therapeutics. J Cell Physiol. 2011;226(10):2484–2493. doi: 10.1002/jcp.22609. doi:10.1002/jcp.22609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kim SY, Chao W, Choi SY, Volsky DJ. Cloning and characterization of the 3′-untranslated region of the human excitatory amino acid transporter 2 transcript. J Neurochem. 2003;86(6):1458–1467. doi: 10.1046/j.1471-4159.2003.01958.x. [DOI] [PubMed] [Google Scholar]
- 98.Kim SY, Choi SY, Chao W, Volsky DJ. Transcriptional regulation of human excitatory amino acid transporter 1 (EAAT1): cloning of the EAAT1 promoter and characterization of its basal and inducible activity in human astrocytes. J Neurochem. 2003;87(6):1485–1498. doi: 10.1046/j.1471-4159.2003.02128.x. [DOI] [PubMed] [Google Scholar]
- 99.Kirschner MA, Arriza JL, Copeland NG, Gilbert DJ, Jenkins NA, Magenis E, Amara SG. The mouse and human excitatory amino-acid transporter gene (eaat1) maps to mouse chromosome-15 and a region of syntenic homology on human-chromosome-5. Genomics. 1994;22:631–633. doi: 10.1006/geno.1994.1437. [DOI] [PubMed] [Google Scholar]
- 100.Koch HP, Hubbard JM, Larsson HP. Voltage-independent sodium-binding events reported by the 4B-4C loop in the human glutamate transporter excitatory amino acid transporter 3. J Biol Chem. 2007;282(34):24547–24553. doi: 10.1074/jbc.M704087200. doi:M704087200 [pii] 10.1074/jbc.M704087200. [DOI] [PubMed] [Google Scholar]
- 101.Koch HP, Lane Brown R, Larsson HP. The Glutamate-Activated Anion Conductance in Excitatory Amino Acid Transporters Is Gated Independently by the Individual Subunits. J Neurosci. 2007;27(11):2943–2947. doi: 10.1523/JNEUROSCI.0118-07.2007. doi:10.1523/jneurosci.0118-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Krisanova N, Sivko R, Kasatkina L, Borisova T. Neuroprotection by lowering cholesterol: a decrease in membrane cholesterol content reduces transporter-mediated glutamate release from brain nerve terminals. Biochim Biophys Acta. 2012;1822(10):1553–1561. doi: 10.1016/j.bbadis.2012.06.005. doi:10.1016/j.bbadis.2012.06.005. [DOI] [PubMed] [Google Scholar]
- 103.Kriz J, Nguyen MD, Julien JP. Minocycline slows disease progression in a mouse model of amyotrophic lateral sclerosis. Neurobiol Dis. 2002;10(3):268–278. doi: 10.1006/nbdi.2002.0487. [DOI] [PubMed] [Google Scholar]
- 104.Krnjevic K. Electrophysiology of cerebral ischemia. Neuropharmacology. 2008;55(3):319–333. doi: 10.1016/j.neuropharm.2008.01.002. doi:S0028-3908(08)00008-7 [pii] 10.1016/j.neuropharm.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 105.Kuersten S, Goodwin EB. The power of the 3′ UTR: translational control and development. Nat Rev Genet. 2003;4(8):626–637. doi: 10.1038/nrg1125. doi:10.1038/nrg1125. [DOI] [PubMed] [Google Scholar]
- 106.Kugler P. Expression of glutamate transporters in rat cardiomyocytes and their localization in the T-tubular system. J Histochem Cytochem. 2004;52(10):1385–1392. doi: 10.1177/002215540405201015. doi:10.1369/jhc.3A6233.2004 52/10/1385 [pii] [DOI] [PubMed] [Google Scholar]
- 107.Lagos-Quintana M, Rauhut R, Yalcin A, Meyer J, Lendeckel W, Tuschl T. Identification of tissue-specific microRNAs from mouse. Curr Biol. 2002;12(9):735–739. doi: 10.1016/s0960-9822(02)00809-6. [DOI] [PubMed] [Google Scholar]
- 108.Lang UE, Borgwardt S. Molecular Mechanisms of Depression: Perspectives on New Treatment Strategies. Cellular physiology and biochemistry: international journal of experimental cellular physiology, biochemistry, and pharmacology. 2013;31(6):761–777. doi: 10.1159/000350094. doi:10.1159/000350094. [DOI] [PubMed] [Google Scholar]
- 109.Larsson HP, Picaud SA, Werblin FS, Lecar H. Noise analysis of the glutamate-activated current in photoreceptors. Biophysl J. 1996;70(2):733–742. doi: 10.1016/S0006-3495(96)79613-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Larsson HP, Wang X, Lev B, Baconguis I, Caplan DA, Vyleta NP, Koch HP, Diez-Sampedro A, Noskov SY. Evidence for a third sodium-binding site in glutamate transporters suggests an ion/substrate coupling model. Proc Natl Acad Sci U S A. 2010;107(31):13912–13917. doi: 10.1073/pnas.1006289107. doi:1006289107 [pii] 10.1073/pnas.1006289107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Leary GP, Stone EF, Holley DC, Kavanaugh MP. The glutamate and chloride permeation pathways are colocalized in individual neuronal glutamate transporter subunits. J Neurosci. 2007;27(11):2938–2942. doi: 10.1523/JNEUROSCI.4851-06.2007. doi:27/11/2938 [pii] 10.1523/JNEUROSCI.4851-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ledesma MD, Dotti CG. The conflicting role of brain cholesterol in Alzheimer’s disease: lessons from the brain plasminogen system. Biochem Soc Symp. 2005;(72):129–138. doi: 10.1042/bss0720129. [DOI] [PubMed] [Google Scholar]
- 113.Lee ES, Sidoryk M, Jiang H, Yin Z, Aschner M. Estrogen and tamoxifen reverse manganese-induced glutamate transporter impairment in astrocytes. Journal of neurochemistry. 2009;110(2):530–544. doi: 10.1111/j.1471-4159.2009.06105.x. doi:10.1111/j.1471-4159.2009.06105.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lee SG, Su ZZ, Emdad L, Gupta P, Sarkar D, Borjabad A, Volsky DJ, Fisher PB. Mechanism of ceftriaxone induction of excitatory amino acid transporter-2 expression and glutamate uptake in primary human astrocytes. J Biol Chem. 2008;283(19):13116–13123. doi: 10.1074/jbc.M707697200. doi:10.1074/jbc.M707697200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lehmann C, Eisner F, Engele J. Role of endothelins as mediators of injury-induced alterations of glial glutamate turnover. J Neurosci Res. 2008;86(3):660–667. doi: 10.1002/jnr.21512. doi:10.1002/jnr.21512. [DOI] [PubMed] [Google Scholar]
- 116.Li L, Zuo Z. Glutamate transporter type 3 knockout reduces brain tolerance to focal brain ischemia in mice. Journal of cerebral blood flow and metabolism: official journal of the International Society of Cerebral Blood Flow and Metabolism. 2011;31(5):1283–1292. doi: 10.1038/jcbfm.2010.222. doi:10.1038/jcbfm.2010.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Loo DD, Hazama A, Supplisson S, Turk E, Wright EM. Relaxation kinetics of the Na+/glucose cotransporter. Proc Natl Acad Sci U S A. 1993;90(12):5767–5771. doi: 10.1073/pnas.90.12.5767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lu CC, Hilgemann DW. GAT1 (GABA:Na+:Cl-) cotransport function. Kinetic studies in giant Xenopus oocyte membrane patches. J Gen Physiol. 1999;114(3):445–457. doi: 10.1085/jgp.114.3.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ma K, Zheng S, Zuo Z. The transcription factor regulatory factor X1 increases the expression of neuronal glutamate transporter type 3. J Biol Chem. 2006;281(30):21250–21255. doi: 10.1074/jbc.M600521200. doi:10.1074/jbc.M600521200. [DOI] [PubMed] [Google Scholar]
- 120.Maeda S, Kawamoto A, Yatani Y, Shirakawa H, Nakagawa T, Kaneko S. Gene transfer of GLT-1, a glial glutamate transporter, into the spinal cord by recombinant adenovirus attenuates inflammatory and neuropathic pain in rats. Molecular pain. 2008;4:65. doi: 10.1186/1744-8069-4-65. doi:10.1186/1744-8069-4-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Martinez-Villarreal J, Garcia Tardon N, Ibanez I, Gimenez C, Zafra F. Cell surface turnover of the glutamate transporter GLT-1 is mediated by ubiquitination/deubiquitination. Glia. 2012;60(9):1356–1365. doi: 10.1002/glia.22354. doi:10.1002/glia.22354. [DOI] [PubMed] [Google Scholar]
- 122.Masliah E, Alford M, DeTeresa R, Mallory M, Hansen L. Deficient glutamate transport is associated with neurodegeneration in Alzheimer’s disease. Ann Neurol. 1996;40(5):759–766. doi: 10.1002/ana.410400512. doi:10.1002/ana.410400512. [DOI] [PubMed] [Google Scholar]
- 123.Massie A, Goursaud S, Schallier A, Vermoesen K, Meshul CK, Hermans E, Michotte Y. Time-dependent changes in GLT-1 functioning in striatum of hemi-Parkinson rats. Neurochemistry international. 2010;57(5):572–578. doi: 10.1016/j.neuint.2010.07.004. doi:10.1016/j.neuint.2010.07.004. [DOI] [PubMed] [Google Scholar]
- 124.Matoulkova E, Michalova E, Vojtesek B, Hrstka R. The role of the 3′ untranslated region in post-transcriptional regulation of protein expression in mammalian cells. RNA Biol. 2012;9(5):563–576. doi: 10.4161/rna.20231. doi:10.4161/rna.20231. [DOI] [PubMed] [Google Scholar]
- 125.Menaker D, Bendahan A, Kanner BI. The substrate specificity of a neuronal glutamate transporter is determined by the nature of the coupling ion. J Neurochem. 2006;99(1):20–28. doi: 10.1111/j.1471-4159.2006.04003.x. doi:JNC4003 [pii] 10.1111/j.1471-4159.2006.04003.x. [DOI] [PubMed] [Google Scholar]
- 126.Meyer T, Fromm A, Munch C, Schwalenstocker B, Fray AE, Ince PG, Stamm S, Gron G, Ludolph AC, Shaw PJ. The RNA of the glutamate transporter EAAT2 is variably spliced in amyotrophic lateral sclerosis and normal individuals. J Neurol Sci. 1999;170(1):45–50. doi: 10.1016/s0022-510x(99)00196-3. [DOI] [PubMed] [Google Scholar]
- 127.Meyer T, Ludolph AC, Morkel M, Hagemeier C, Speer A. Genomic organization of the human excitatory amino acid transporter gene GLT-1. Neuroreport. 1997;8(3):775–777. doi: 10.1097/00001756-199702100-00039. [DOI] [PubMed] [Google Scholar]
- 128.Mim C, Tao Z, Grewer C. Two conformational changes are associated with glutamate translocation by the glutamate transporter EAAC1. Biochemistry. 2007;46(31):9007–9018. doi: 10.1021/bi7005465. doi:10.1021/bi7005465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Morel L, Regan M, Higashimori H, Ng SK, Esau C, Vidensky S, Rothstein J, Yang Y. Neuronal exosomal miRNA-dependent translational regulation of astroglial glutamate transporter GLT1. J Biol Chem. 2013;288(10):7105–7116. doi: 10.1074/jbc.M112.410944. doi:10.1074/jbc.M112.410944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Muller F, Demeny MA, Tora L. New problems in RNA polymerase II transcription initiation: matching the diversity of core promoters with a variety of promoter recognition factors. J Biol Chem. 2007;282(20):14685–14689. doi: 10.1074/jbc.R700012200. doi:10.1074/jbc.R700012200. [DOI] [PubMed] [Google Scholar]
- 131.Munch C, Ebstein M, Seefried U, Zhu B, Stamm S, Landwehrmeyer GB, Ludolph AC, Schwalenstocker B, Meyer T. Alternative splicing of the 5′-sequences of the mouse EAAT2 glutamate transporter and expression in a transgenic model for amyotrophic lateral sclerosis. J Neurochem. 2002;82(3):594–603. doi: 10.1046/j.1471-4159.2002.01012.x. [DOI] [PubMed] [Google Scholar]
- 132.Munch C, Schwalenstocker B, Hermann C, Cirovic S, Stamm S, Ludolph A, Meyer T. Differential RNA cleavage and polyadenylation of the glutamate transporter EAAT2 in the human brain. Brain Res Mol Brain Res. 2000;80(2):244–251. doi: 10.1016/s0169-328x(00)00139-x. [DOI] [PubMed] [Google Scholar]
- 133.Mwaura J, Tao Z, James H, Albers T, Schwartz A, Grewer C. Protonation state of a conserved acidic amino acid involved in Na(+) binding to the glutamate transporter EAAC1. ACS Chemical Neuroscience. 2012;12:1073–1083. doi: 10.1021/cn300163p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Nothmann D, Leinenweber A, Torres-Salazar D, Kovermann P, Hotzy J, Gameiro A, Grewer C, Fahlke C. Hetero-oligomerization of neuronal glutamate transporters. J Biol Chem. 2011;286(5):3935–3943. doi: 10.1074/jbc.M110.187492. doi:M110.187492 [pii] 10.1074/jbc.M110.187492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Otis TS, Jahr CE. Anion currents and predicted glutamate flux through a neuronal glutamate transporter. J Neurosci. 1998;18(18):7099–7110. doi: 10.1523/JNEUROSCI.18-18-07099.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Otis TS, Kavanaugh MP. Isolation of current components and partial reaction cycles in the glial glutamate transporter EAAT2. J Neurosci. 2000;20(8):2749–2757. doi: 10.1523/JNEUROSCI.20-08-02749.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Owe SG, Marcaggi P, Attwell D. The ionic stoichiometry of the GLAST glutamate transporter in salamander retinal glia. J Physiol. 2006;577(Pt 2):591–599. doi: 10.1113/jphysiol.2006.116830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Palmada M, Kinne-Saffran E, Centelles JJ, Kinne RK. Benzodiazepines differently modulate EAAT1/GLAST and EAAT2/GLT1 glutamate transporters expressed in CHO cells. Neurochem Int. 2002;40(4):321–326. doi: 10.1016/s0197-0186(01)00087-0. [DOI] [PubMed] [Google Scholar]
- 139.Peacey E, Miller CC, Dunlop J, Rattray M. The four major N- and C-terminal splice variants of the excitatory amino acid transporter GLT-1 form cell surface homomeric and heteromeric assemblies. Mol Pharmacol. 2009;75(5):1062–1073. doi: 10.1124/mol.108.052829. doi:mol.108.052829 [pii] 10.1124/mol.108.052829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Peghini P, Janzen J, Stoffel W. Glutamate transporter EAAC-1-deficient mice develop dicarboxylic aminoaciduria and behavioral abnormalities but no neurodegeneration. The EMBO journal. 1997;16(13):3822–3832. doi: 10.1093/emboj/16.13.3822. doi:10.1093/emboj/16.13.3822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Perisic T, Holsboer F, Rein T, Zschocke J. The CpG island shore of the GLT-1 gene acts as a methylation-sensitive enhancer. Glia. 2012;60(9):1345–1355. doi: 10.1002/glia.22353. doi:10.1002/glia.22353. [DOI] [PubMed] [Google Scholar]
- 142.Perisic T, Zimmermann N, Kirmeier T, Asmus M, Tuorto F, Uhr M, Holsboer F, Rein T, Zschocke J. Valproate and amitriptyline exert common and divergent influences on global and gene promoter-specific chromatin modifications in rat primary astrocytes. Neuropsychopharmacology. 2010;35(3):792–805. doi: 10.1038/npp.2009.188. doi:10.1038/npp.2009.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Picaud SA, Larsson HP, Grant GB, Lecar H, Werblin FS. Glutamate-gated chloride channel with glutamate-transporter-like properties in cone photoreceptors of the tiger salamander. J Neurophys. 1995;74(4):1760–1771. doi: 10.1152/jn.1995.74.4.1760. [DOI] [PubMed] [Google Scholar]
- 144.Pines G, Danbolt NC, Bjoras M, Zhang Y, Bendahan A, Eide L, Koepsell H, Storm-Mathisen J, Seeberg E, Kanner BI. Cloning and expression of a rat brain L-glutamate transporter. Nature. 1992;360(6403):464–467. doi: 10.1038/360464a0. doi:10.1038/360464a0. [DOI] [PubMed] [Google Scholar]
- 145.Pines G, Danbolt NC, Bjoras M, Zhang Y, Bendahan A, Eide L, Koepsell H, Storm Mathisen J, Seeberg E, Kanner BI. Cloning and Expression of a Rat Brain L Glutamate Transporter. Nature. 1992;360(6403):464–467. doi: 10.1038/360464a0. [DOI] [PubMed] [Google Scholar]
- 146.Plaitakis A, Shashidharan P. Glutamate transport and metabolism in dopaminergic neurons of substantia nigra: implications for the pathogenesis of Parkinson’s disease. Journal of neurology. 2000;247(Suppl 2):II25–35. doi: 10.1007/pl00007757. [DOI] [PubMed] [Google Scholar]
- 147.Poitry-Yamate CL, Vutskits L, Rauen T. Neuronal-induced and glutamate-dependent activation of glial glutamate transporter function. J Neurochem. 2002;82(4):987–997. doi: 10.1046/j.1471-4159.2002.01075.x. doi:1075 [pii] [DOI] [PubMed] [Google Scholar]
- 148.Porcheray F, Leone C, Samah B, Rimaniol AC, Dereuddre-Bosquet N, Gras G. Glutamate metabolism in HIV-infected macrophages: implications for the CNS. American journal of physiology Cell physiology. 2006;291(4):C618–626. doi: 10.1152/ajpcell.00021.2006. doi:10.1152/ajpcell.00021.2006. [DOI] [PubMed] [Google Scholar]
- 149.Rao PS, Sari Y. Glutamate transporter 1: target for the treatment of alcohol dependence. Current medicinal chemistry. 2012;19(30):5148–5156. doi: 10.2174/092986712803530511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Rao VL, Dogan A, Todd KG, Bowen KK, Kim BT, Rothstein JD, Dempsey RJ. Antisense knockdown of the glial glutamate transporter GLT-1, but not the neuronal glutamate transporter EAAC1, exacerbates transient focal cerebral ischemia-induced neuronal damage in rat brain. The Journal of neuroscience. 2001;21(6):1876–1883. doi: 10.1523/JNEUROSCI.21-06-01876.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Rauen T. Diversity of glutamate transporter expression and function in the mammalian retina. Amino Acids. 2000;19(1):53–62. doi: 10.1007/s007260070033. [DOI] [PubMed] [Google Scholar]
- 152.Rauen T, Jeserich G, Danbolt NC, Kanner BI. Comparative analysis of sodium-dependent L-glutamate transport of synaptosomal and astroglial membrane vesicles from mouse cortex. FEBS Lett. 1992;312(1):15–20. doi: 10.1016/0014-5793(92)81401-7. doi:0014-5793(92)81401-7 [pii] [DOI] [PubMed] [Google Scholar]
- 153.Rauen T, Kanner BI. Localization of the glutamate transporter GLT-1 in rat and macaque monkey retinae. Neurosci Lett. 1994;169(1-2):137–140. doi: 10.1016/0304-3940(94)90375-1. [DOI] [PubMed] [Google Scholar]
- 154.Rauen T, Taylor WR, Kuhlbrodt K, Wiessner M. High-affinity glutamate transporters in the rat retina: a major role of the glial glutamate transporter GLAST-1 in transmitter clearance. Cell Tissue Res. 1998;291(1):19–31. doi: 10.1007/s004410050976. [DOI] [PubMed] [Google Scholar]
- 155.Rauen T, Wiessner M. Fine tuning of glutamate uptake and degradation in glial cells: common transcriptional regulation of GLAST1 and GS. Neurochem Int. 2000;37(2-3):179–189. doi: 10.1016/s0197-0186(00)00021-8. doi:S0197-0186(00)00021-8 [pii] [DOI] [PubMed] [Google Scholar]
- 156.Rauen T, Wiessner M, Sullivan R, Lee A, Pow DV. A new GLT1 splice variant: cloning and immunolocalization of GLT1c in the mammalian retina and brain. Neurochem Int. 2004;45(7):1095–1106. doi: 10.1016/j.neuint.2004.04.006. doi:10.1016/j.neuint.2004.04.006 S0197018604000919 [pii] [DOI] [PubMed] [Google Scholar]
- 157.Revett TJ, Baker GB, Jhamandas J, Kar S. Glutamate system, amyloid ss peptides and tau protein: functional interrelationships and relevance to Alzheimer disease pathology. Journal of psychiatry & neuroscience: JPN. 2013;38(1):6–23. doi: 10.1503/jpn.110190. doi:10.1503/jpn.110190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Reyes N, Ginter C, Boudker O. Transport mechanism of a bacterial homologue of glutamate transporters. Nature. 2009;462(7275):880–885. doi: 10.1038/nature08616. doi:nature08616 [pii] 10.1038/nature08616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Roberts PJ, Watkins JC. Structural requirements for the inhibition for L-glutamate uptake by glia and nerve endings. Brain Res. 1975;85(1):120–125. doi: 10.1016/0006-8993(75)91016-1. doi:0006-8993(75)91016-1 [pii] [DOI] [PubMed] [Google Scholar]
- 160.Robinson MB. The family of sodium-dependent glutamate transporters: a focus on the GLT-1/EAAT2 subtype. Neurochem Int. 1998;33(6):479–491. doi: 10.1016/s0197-0186(98)00055-2. [DOI] [PubMed] [Google Scholar]
- 161.Robinson MB. Regulated trafficking of neurotransmitter transporters: common notes but different melodies. J Neurochem. 2002;80(1):1–11. doi: 10.1046/j.0022-3042.2001.00698.x. [DOI] [PubMed] [Google Scholar]
- 162.Rosental N, Gameiro A, Grewer C, Kanner BI. A conserved aspartate residue located at the extracellular end of the binding pocket controls cation interactions in brain glutamate transporters. J Biol Chem. 2011;286(48):41381–41390. doi: 10.1074/jbc.M111.291021. doi:M111.291021 [pii] 10.1074/jbc.M111.291021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Rossi DJ, Oshima T, Attwell D. Glutamate release in severe brain ischaemia is mainly by reversed uptake. Nature. 2000;403(6767):316–321. doi: 10.1038/35002090. doi:10.1038/35002090. [DOI] [PubMed] [Google Scholar]
- 164.Rothstein JD. Current hypotheses for the underlying biology of amyotrophic lateral sclerosis. Annals of neurology. 2009;65(Suppl 1):S3–9. doi: 10.1002/ana.21543. doi:10.1002/ana.21543. [DOI] [PubMed] [Google Scholar]
- 165.Rothstein JD, Martin LJ, Kuncl RW. Decreased glutamate transport by the brain and spinal cord in amyotrophic lateral sclerosis. N Engl J Med. 1992;326(22):1464–1468. doi: 10.1056/NEJM199205283262204. doi:10.1056/NEJM199205283262204. [DOI] [PubMed] [Google Scholar]
- 166.Rothstein JD, Patel S, Regan MR, Haenggeli C, Huang YH, Bergles DE, Jin L, Dykes Hoberg M, Vidensky S, Chung DS, Toan SV, Bruijn LI, Su ZZ, Gupta P, Fisher PB. Beta-lactam antibiotics offer neuroprotection by increasing glutamate transporter expression. Nature. 2005;433(7021):73–77. doi: 10.1038/nature03180. doi:nature03180 [pii] 10.1038/nature03180. [DOI] [PubMed] [Google Scholar]
- 167.Rozyczka J, Engele J. Multiple 5′-splice variants of the rat glutamate transporter-1. Brain Res Mol Brain Res. 2005;133(1):157–161. doi: 10.1016/j.molbrainres.2004.09.021. [DOI] [PubMed] [Google Scholar]
- 168.Rozyczka J, Figiel M, Engele J. Endothelins negatively regulate glial glutamate transporter expression. Brain Pathol. 2004;14(4):406–414. doi: 10.1111/j.1750-3639.2004.tb00084.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Ryan RM, Mindell JA. The uncoupled chloride conductance of a bacterial glutamate transporter homolog. Nat Struct Mol Biol. 2007;14(5):365–371. doi: 10.1038/nsmb1230. doi:nsmb1230 [pii] 10.1038/nsmb1230. [DOI] [PubMed] [Google Scholar]
- 170.Ryan RM, Mitrovic AD, Vandenberg RJ. The chloride permeation pathway of a glutamate transporter and its proximity to the glutamate translocation pathway. J Biol Chem. 2004;279(20):20742–20751. doi: 10.1074/jbc.M304433200. doi:10.1074/jbc.M304433200 M304433200 [pii] [DOI] [PubMed] [Google Scholar]
- 171.Santos SD, Carvalho AL, Caldeira MV, Duarte CB. Regulation of AMPA receptors and synaptic plasticity. Neuroscience. 2009;158(1):105–125. doi: 10.1016/j.neuroscience.2008.02.037. doi:S0306-4522(08)00225-X [pii] 10.1016/j.neuroscience.2008.02.037. [DOI] [PubMed] [Google Scholar]
- 172.Sarthy VP, Pignataro L, Pannicke T, Weick M, Reichenbach A, Harada T, Tanaka K, Marc R. Glutamate transport by retinal Muller cells in glutamate/aspartate transporter-knockout mice. Glia. 2005;49(2):184–196. doi: 10.1002/glia.20097. doi:10.1002/glia.20097. [DOI] [PubMed] [Google Scholar]
- 173.Sato F, Tsuchiya S, Meltzer SJ, Shimizu K. MicroRNAs and epigenetics. FEBS J. 2011;278(10):1598–1609. doi: 10.1111/j.1742-4658.2011.08089.x. doi:10.1111/j.1742-4658.2011.08089.x. [DOI] [PubMed] [Google Scholar]
- 174.Saxonov S, Berg P, Brutlag DL. A genome-wide analysis of CpG dinucleotides in the human genome distinguishes two distinct classes of promoters. Proc Natl Acad Sci U S A. 2006;103(5):1412–1417. doi: 10.1073/pnas.0510310103. doi:10.1073/pnas.0510310103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Schlag BD, Vondrasek JR, Munir M, Kalandadze A, Zelenaia OA, Rothstein JD, Robinson MB. Regulation of the glial Na+-dependent glutamate transporters by cyclic AMP analogs and neurons. Mol Pharmacol. 1998;53(3):355–369. doi: 10.1124/mol.53.3.355. [DOI] [PubMed] [Google Scholar]
- 176.Schmitt A, Asan E, Lesch KP, Kugler P. A splice variant of glutamate transporter GLT1/EAAT2 expressed in neurons: cloning and localization in rat nervous system. Neuroscience. 2002;109(1):45–61. doi: 10.1016/s0306-4522(01)00451-1. [DOI] [PubMed] [Google Scholar]
- 177.Schousboe A, Hertz L. Role of astroglial cells in glutamate homeostasis. Adv Biochem Psychopharmacol. 1981;27:103–113. [PubMed] [Google Scholar]
- 178.Seki Y, Feustel PJ, Keller RW, Jr., Tranmer BI, Kimelberg HK. Inhibition of ischemia-induced glutamate release in rat striatum by dihydrokinate and an anion channel blocker. Stroke. 1999;30(2):433–440. doi: 10.1161/01.str.30.2.433. [DOI] [PubMed] [Google Scholar]
- 179.Sheldon AL, Gonzalez MI, Krizman-Genda EN, Susarla BT, Robinson MB. Ubiquitination-mediated internalization and degradation of the astroglial glutamate transporter, GLT-1. Neurochem Int. 2008;53(6-8):296–308. doi: 10.1016/j.neuint.2008.07.010. doi:10.1016/j.neuint.2008.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Sheldon AL, Gonzalez MI, Robinson MB. A carboxyl-terminal determinant of the neuronal glutamate transporter, EAAC1, is required for platelet-derived growth factor-dependent trafficking. J Biol Chem. 2006;281(8):4876–4886. doi: 10.1074/jbc.M504983200. doi:10.1074/jbc.M504983200. [DOI] [PubMed] [Google Scholar]
- 181.Sheldon AL, Robinson MB. The role of glutamate transporters in neurodegenerative diseases and potential opportunities for intervention. Neurochem Int. 2007;51(6-7):333–355. doi: 10.1016/j.neuint.2007.03.012. doi:10.1016/j.neuint.2007.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Shimamoto K, Lebrun B, Yasuda-Kamatani Y, Sakaitani M, Shigeri Y, Yumoto N, Nakajima T. DL-threo-beta-benzyloxyaspartate, a potent blocker of excitatory amino acid transporters. Mol Pharmacol. 1998;53(2):195–201. doi: 10.1124/mol.53.2.195. [DOI] [PubMed] [Google Scholar]
- 183.Shimamoto K, Sakai R, Takaoka K, Yumoto N, Nakajima T, Amara SG, Shigeri Y. Characterization of novel L-threo-beta-benzyloxyaspartate derivatives, potent blockers of the glutamate transporters. Mol Pharmacol. 2004;65(4):1008–1015. doi: 10.1124/mol.65.4.1008. doi:10.1124/mol.65.4.1008 65/4/1008 [pii] [DOI] [PubMed] [Google Scholar]
- 184.Shouffani A, Kanner BI. Cholesterol is required for the reconstruction of the sodium- and chloride-coupled, gamma-aminobutyric acid transporter from rat brain. J Biol Chem. 1990;265(11):6002–6008. [PubMed] [Google Scholar]
- 185.Shrivastava IH, Jiang J, Amara SG, Bahar I. Time-resolved mechanism of extracellular gate opening and substrate binding in a glutamate transporter. J Biol Chem. 2008;283(42):28680–28690. doi: 10.1074/jbc.M800889200. doi:M800889200 [pii] 10.1074/jbc.M800889200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Silverstein N, Crisman TJ, Forrest LR, Kanner BI. Cysteine scanning mutagenesis of transmembrane helix 3 of a brain glutamate transporter reveals two conformationally sensitive positions. J Biol Chem. 2013;288(2):964–973. doi: 10.1074/jbc.M112.403576. doi:M112.403576 [pii] 10.1074/jbc.M112.403576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Simons K, Gerl MJ. Revitalizing membrane rafts: new tools and insights. Nat Rev Mol Cell Biol. 2010;11(10):688–699. doi: 10.1038/nrm2977. doi:nrm2977 [pii] 10.1038/nrm2977. [DOI] [PubMed] [Google Scholar]
- 188.Sitcheran R, Gupta P, Fisher PB, Baldwin AS. Positive and negative regulation of EAAT2 by NF-kappaB: a role for N-myc in TNFalpha-controlled repression. Embo J. 2005;24(3):510–520. doi: 10.1038/sj.emboj.7600555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Sonders MS, Amara SG. Channels in transporters. Curr Opin Neurobiol. 1996;6(3):294–302. doi: 10.1016/s0959-4388(96)80111-5. [DOI] [PubMed] [Google Scholar]
- 190.Stephenson FA, Cousins SL, Kenny AV. Assembly and forward trafficking of NMDA receptors (Review) Mol Membr Biol. 2008;25(4):311–320. doi: 10.1080/09687680801971367. doi:791508052 [pii] 10.1080/09687680801971367. [DOI] [PubMed] [Google Scholar]
- 191.Stolzenberg S, Khelashvili G, Weinstein H. Structural intermediates in a model of the substrate translocation path of the bacterial glutamate transporter homologue GltPh. J Phys Chem B. 2012;116(18):5372–5383. doi: 10.1021/jp301726s. doi:10.1021/jp301726s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Storck T, Schulte S, Hofmann K, Stoffel W. Structure, expression, and functional analysis of a Na(+)-dependent glutamate/aspartate transporter from rat brain. Proc Natl Acad Sci U S A. 1992;89(22):10955–10959. doi: 10.1073/pnas.89.22.10955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Su ZZ, Leszczyniecka M, Kang DC, Sarkar D, Chao W, Volsky DJ, Fisher PB. Insights into glutamate transport regulation in human astrocytes: cloning of the promoter for excitatory amino acid transporter 2 (EAAT2) Proc Natl Acad Sci U S A. 2003;100(4):1955–1960. doi: 10.1073/pnas.0136555100. doi:10.1073/pnas.0136555100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Sullivan R, Rauen T, Fischer F, Wiessner M, Grewer C, Bicho A, Pow DV. Cloning, transport properties, and differential localization of two splice variants of GLT-1 in the rat CNS: implications for CNS glutamate homeostasis. Glia. 2004;45(2):155–169. doi: 10.1002/glia.10317. doi:10.1002/glia.10317. [DOI] [PubMed] [Google Scholar]
- 195.Suzuki Y, Tsunoda T, Sese J, Taira H, Mizushima-Sugano J, Hata H, Ota T, Isogai T, Tanaka T, Nakamura Y, Suyama A, Sakaki Y, Morishita S, Okubo K, Sugano S. Identification and characterization of the potential promoter regions of 1031 kinds of human genes. Genome Res. 2001;11(5):677–684. doi: 10.1101/gr.164001. doi:10.1101/gr.164001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Swanson RA, Liu J, Miller JW, Rothstein JD, Farrell K, Stein BA, Longuemare MC. Neuronal regulation of glutamate transporter subtype expression in astrocytes. J Neurosci. 1997;17(3):932–940. doi: 10.1523/JNEUROSCI.17-03-00932.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Szostak E, Gebauer F. Translational control by 3′-UTR-binding proteins. Brief Funct Genomics. 2013;12(1):58–65. doi: 10.1093/bfgp/els056. doi:10.1093/bfgp/els056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Tanaka K, Watase K, Manabe T, Yamada K, Watanabe M, Takahashi K, Iwama H, Nishikawa T, Ichihara N, Kikuchi T, Okuyama S, Kawashima N, Hori S, Takimoto M, Wada K. Epilepsy and exacerbation of brain injury in mice lacking the glutamate transporter GLT-1. Science. 1997;276:1699–1702. doi: 10.1126/science.276.5319.1699. [DOI] [PubMed] [Google Scholar]
- 199.Tao Z, Grewer C. Cooperation of the conserved aspartate 439 and bound amino acid substrate is important for high-affinity Na+ binding to the glutamate transporter EAAC1. J Gen Physiol. 2007;129(4):331–344. doi: 10.1085/jgp.200609678. doi:jgp.200609678 [pii] 10.1085/jgp.200609678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Tao Z, Rosental N, Kanner BI, Gameiro A, Mwaura J, Grewer C. Mechanism of cation binding to the glutamate transporter EAAC1 probed with mutation of the conserved amino acid residue Thr101. J Biol Chem. 2010;285(23):17725–17733. doi: 10.1074/jbc.M110.121798. doi:M110.121798 [pii] 10.1074/jbc.M110.121798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Tao Z, Zhang Z, Grewer C. Neutralization of the aspartic acid residue Asp-367, but not Asp-454, inhibits binding of Na+ to the glutamate-free form and cycling of the glutamate transporter EAAC1. J Biol Chem. 2006;281(15):10263–10272. doi: 10.1074/jbc.M510739200. doi:M510739200 [pii] 10.1074/jbc.M510739200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Tavoulari S, Rizwan AN, Forrest LR, Rudnick G. Reconstructing a chloride-binding site in a bacterial neurotransmitter transporter homologue. J Biol Chem. 2011;286(4):2834–2842. doi: 10.1074/jbc.M110.186064. doi:M110.186064 [pii] 10.1074/jbc.M110.186064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Tokusumi Y, Ma Y, Song X, Jacobson RH, Takada S. The new core promoter element XCPE1 (X Core Promoter Element 1) directs activator-, mediator-, and TATA-binding protein-dependent but TFIID-independent RNA polymerase II transcription from TATA-less promoters. Mol Cell Biol. 2007;27(5):1844–1858. doi: 10.1128/MCB.01363-06. doi:10.1128/MCB.01363-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Torres-Salazar D, Fahlke C. Intersubunit interactions in EAAT4 glutamate transporters. J Neurosci. 2006;26(28):7513, 7522. doi: 10.1523/JNEUROSCI.4545-05.2006. doi:26/28/7513 [pii] 10.1523/JNEUROSCI.4545-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Tsuru N, Ueda Y, Doi T. Amygdaloid kindling in glutamate transporter (GLAST) knockout mice. Epilepsia. 2002;43(8):805–811. doi: 10.1046/j.1528-1157.2002.36601.x. [DOI] [PubMed] [Google Scholar]
- 206.Unger T, Lakowa N, Bette S, Engele J. Transcriptional regulation of the GLAST/EAAT-1 gene in rat and man. Cell Mol Neurobiol. 2012;32(4):539–547. doi: 10.1007/s10571-011-9790-2. doi:10.1007/s10571-011-9790-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Utsunomiya-Tate N, Endou H, Kanai Y. Cloning and Functional Characterization of a System ASC-like Na+-dependent Neutral Amino Acid Transporter. J Biol Chem. 1996;271(25):14883–14890. doi: 10.1074/jbc.271.25.14883. [DOI] [PubMed] [Google Scholar]
- 208.Utsunomiya-Tate N, Endou H, Kanai Y. Tissue specific variants of glutamate transporter GLT-1. FEBS Lett. 1997;416(3):312–316. doi: 10.1016/s0014-5793(97)01232-5. [DOI] [PubMed] [Google Scholar]
- 209.Valladolid-Acebes I, Merino B, Principato A, Fole A, Barbas C, Lorenzo MP, Garcia A, Del Olmo N, Ruiz-Gayo M, Cano V. High-fat diets induce changes in hippocampal glutamate metabolism and neurotransmission. American journal of physiology Endocrinology and metabolism. 2012;302(4):E396–402. doi: 10.1152/ajpendo.00343.2011. doi:10.1152/ajpendo.00343.2011. [DOI] [PubMed] [Google Scholar]
- 210.Vandenberg RJ, Mitrovic AD, Johnston GA. Serine-O-sulphate transport by the human glutamate transporter, EAAT2. Br J Pharmacol. 1998;123(8):1593–1600. doi: 10.1038/sj.bjp.0701776. doi:10.1038/sj.bjp.0701776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Verdon G, Boudker O. Crystal structure of an asymmetric trimer of a bacterial glutamate transporter homolog. Nat Struct Mol Biol. 2012;19(3):355–357. doi: 10.1038/nsmb.2233. doi:nsmb.2233 [pii] 10.1038/nsmb.2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Veruki ML, Mørkve SH, Hartveit E. Activation of a presynaptic glutamate transporter regulates synaptic transmission through electrical signaling. Nat Neurosci. 2006;9(11):1388–1396. doi: 10.1038/nn1793. [DOI] [PubMed] [Google Scholar]
- 213.Wadiche JI, Amara SG, Kavanaugh MP. Ion fluxes associated with excitatory amino acid transport. Neuron. 1995;15:721–728. doi: 10.1016/0896-6273(95)90159-0. [DOI] [PubMed] [Google Scholar]
- 214.Wadiche JI, Arriza JL, Amara SG, Kavanaugh MP. Kinetics of a human glutamate transporter. Neuron. 1995;14(5):1019–1027. doi: 10.1016/0896-6273(95)90340-2. doi:0896-6273(95)90340-2 [pii] [DOI] [PubMed] [Google Scholar]
- 215.Wadiche JI, Kavanaugh MP. Macroscopic and microscopic properties of a cloned glutamate transporter/chloride channel. J Neurosci. 1998;18(19):7650–7661. doi: 10.1523/JNEUROSCI.18-19-07650.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Waly MI, Hornig M, Trivedi M, Hodgson N, Kini R, Ohta A, Deth R. Prenatal and Postnatal Epigenetic Programming: Implications for GI, Immune, and Neuronal Function in Autism. Autism research and treatment. 2012;2012:190930. doi: 10.1155/2012/190930. doi:10.1155/2012/190930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Watanabe T, Morimoto K, Hirao T, Suwaki H, Watase K, Tanaka K. Amygdala-kindled and pentylenetetrazole-induced seizures in glutamate transporter GLAST-deficient mice. Brain research. 1999;845(1):92–96. doi: 10.1016/s0006-8993(99)01945-9. [DOI] [PubMed] [Google Scholar]
- 218.Watase K, Hashimoto K, Kano M, Yamada K, Watanabe M, Inoue Y, Okuyama S, Sakagawa T, Ogawa S, Kawashima N, Hori S, Takimoto M, Wada K, Tanaka K. Motor discoordination and increased susceptibility to cerebellar injury in GLAST mutant mice. The European journal of neuroscience. 1998;10(3):976–988. doi: 10.1046/j.1460-9568.1998.00108.x. [DOI] [PubMed] [Google Scholar]
- 219.Watzke N, Bamberg E, Grewer C. Early intermediates in the transport cycle of the neuronal excitatory amino acid carrier EAAC1. J Gen Physiol. 2001;117(6):547–562. doi: 10.1085/jgp.117.6.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Watzke N, Grewer C. The anion conductance of the glutamate transporter EAAC1 depends on the direction of glutamate transport. FEBS Lett. 2001;503(2-3):121–125. doi: 10.1016/s0014-5793(01)02715-6. doi:S0014-5793(01)02715-6 [pii] [DOI] [PubMed] [Google Scholar]
- 221.Watzke N, Rauen T, Bamberg E, Grewer C. On the mechanism of proton transport by the neuronal excitatory amino acid carrier 1. J Gen Physiol. 2000;116(5):609–622. doi: 10.1085/jgp.116.5.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Wersinger E, Schwab Y, Sahel J-A, Rendon A, Pow DV, Picaud S, Roux MJ. The glutamate transporter EAAT5 works as a presynaptic receptor in mouse rod bipolar cells. J Physiol. 2006;577(1):221–234. doi: 10.1113/jphysiol.2006.118281. doi:10.1113/jphysiol.2006.118281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Wilson DF, Pastuszko A. Transport of cysteate by synaptosomes isolated from rat brain: evidence that it utilizes the same transporter as aspartate, glutamate, and cysteine sulfinate. J Neurochem. 1986;47(4):1091–1097. doi: 10.1111/j.1471-4159.1986.tb00725.x. [DOI] [PubMed] [Google Scholar]
- 224.Winter N, Kovermann P, Fahlke C. A point mutation associated with episodic ataxia 6 increases glutamate transporter anion currents. Brain: a journal of neurology. 2012;135(Pt 11):3416–3425. doi: 10.1093/brain/aws255. doi:10.1093/brain/aws255. [DOI] [PubMed] [Google Scholar]
- 225.Yang Y, Gozen O, Vidensky S, Robinson MB, Rothstein JD. Epigenetic regulation of neuron-dependent induction of astroglial synaptic protein GLT1. Glia. 2010;58(3):277–286. doi: 10.1002/glia.20922. doi:10.1002/glia.20922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Yernool D, Boudker O, Jin Y, Gouaux E. Structure of a glutamate transporter homologue from Pyrococcus horikoshii. Nature. 2004;431(7010):811–818. doi: 10.1038/nature03018. doi:nature03018 [pii] 10.1038/nature03018. [DOI] [PubMed] [Google Scholar]
- 227.Zelenaia O, Schlag BD, Gochenauer GE, Ganel R, Song W, Beesley JS, Grinspan JB, Rothstein JD, Robinson MB. Epidermal growth factor receptor agonists increase expression of glutamate transporter GLT-1 in astrocytes through pathways dependent on phosphatidylinositol 3-kinase and transcription factor NF-kappaB. Mol Pharmacol. 2000;57(4):667–678. doi: 10.1124/mol.57.4.667. [DOI] [PubMed] [Google Scholar]
- 228.Zerangue N, Kavanaugh MP. Flux coupling in a neuronal glutamate transporter. Nature. 1996;383(6601):634–637. doi: 10.1038/383634a0. doi:10.1038/383634a0. [DOI] [PubMed] [Google Scholar]
- 229.Zerangue N, Kavanaugh MP. Interaction of L-cysteine with a human excitatory amino acid transporter. Journal of Physiology. 1996;493(Pt 2):419–423. doi: 10.1113/jphysiol.1996.sp021393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Zhang R, Su B. Small but influential: the role of microRNAs on gene regulatory network and 3′UTR evolution. J Genet Genomics. 2009;36(1):1–6. doi: 10.1016/S1673-8527(09)60001-1. doi:10.1016/S1673-8527(09)60001-1. [DOI] [PubMed] [Google Scholar]
- 231.Zhang Z, Tao Z, Gameiro A, Barcelona S, Braams S, Rauen T, Grewer C. Transport direction determines the kinetics of substrate transport by the glutamate transporter EAAC1. Proc Natl Acad Sci U S A. 2007;104(46):18025–18030. doi: 10.1073/pnas.0704570104. doi:0704570104 [pii] 10.1073/pnas.0704570104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Zschocke J, Allritz C, Engele J, Rein T. DNA methylation dependent silencing of the human glutamate transporter EAAT2 gene in glial cells. Glia. 2007;55(7):663–674. doi: 10.1002/glia.20497. doi:10.1002/glia.20497. [DOI] [PubMed] [Google Scholar]
- 233.Zschocke J, Bayatti N, Behl C. Caveolin and GLT-1 gene expression is reciprocally regulated in primary astrocytes: association of GLT-1 with non-caveolar lipid rafts. Glia. 2005;49(2):275–287. doi: 10.1002/glia.20116. doi:10.1002/glia.20116. [DOI] [PubMed] [Google Scholar]