Abstract
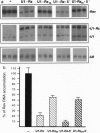
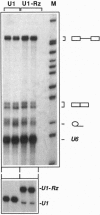
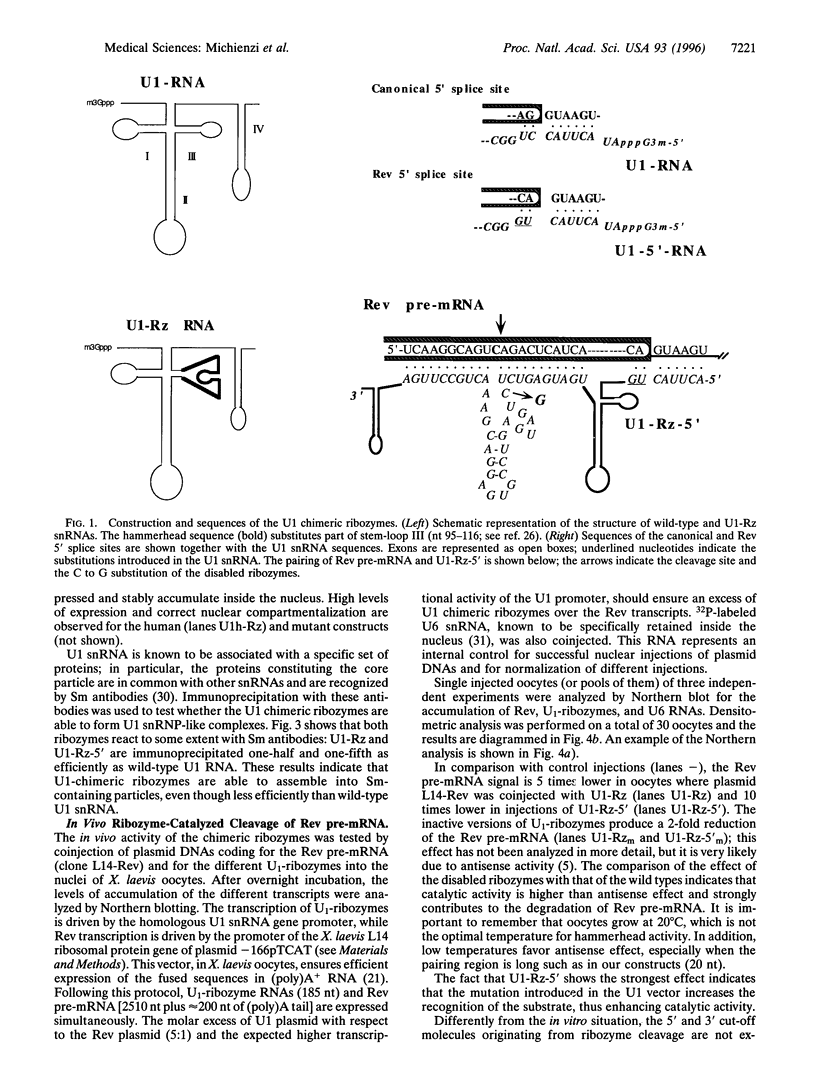
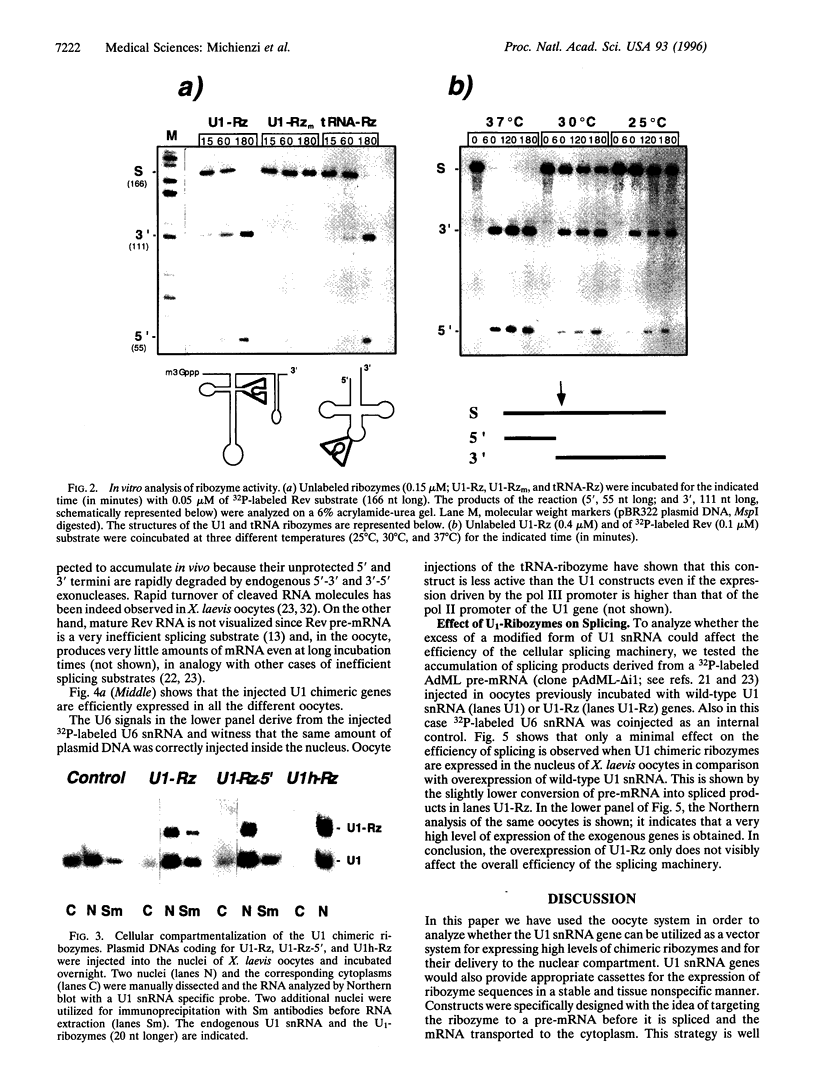
The in vivo effectiveness of ribozymes strongly depends on the correct choice of the vector molecule. High levels of expression, stability, active conformation, and correct cellular localization are the most important features for a ribozyme vector. We have exploited the utilization of the U1 small nuclear RNA (snRNA) as a vector for specifically targeting a ribozyme into the nucleus. The Rev pre-mRNA of human immunodeficiency virus type 1 was chosen as target for testing the activity of the Ul-ribozyme. The catalytic core of the hammerhead motif, plus the recognition sequences, substituted the stem-loop III of the U1 snRNA. The resulting construct displays efficient cleavage activity in vitro. In addition, in the in vivo system of Xenopus laevis oocytes, the Ul-chimeric ribozyme accumulates in large amounts in the nucleus and produces a considerable reduction of Rev pre-mRNA levels. The Rev-specific ribozyme was also inserted in a derivative of the Ul snRNA mutated in the region of pairing with the 5' splice site, such as to match it with the suboptimal splice junction of the Rev precursor. This construct shows more efficient reduction of Rev pre-mRNA in vivo than the wild-type U1 vector.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Caffarelli E., Fragapane P., Gehring C., Bozzoni I. The accumulation of mature RNA for the Xenopus laevis ribosomal protein L1 is controlled at the level of splicing and turnover of the precursor RNA. EMBO J. 1987 Nov;6(11):3493–3498. doi: 10.1002/j.1460-2075.1987.tb02674.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantor G. H., McElwain T. F., Birkebak T. A., Palmer G. H. Ribozyme cleaves rex/tax mRNA and inhibits bovine leukemia virus expression. Proc Natl Acad Sci U S A. 1993 Dec 1;90(23):10932–10936. doi: 10.1073/pnas.90.23.10932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carnevali F., La Porta C., Ilardi V., Beccari E. Nuclear factors specifically bind to upstream sequences of a Xenopus laevis ribosomal protein gene promoter. Nucleic Acids Res. 1989 Oct 25;17(20):8171–8184. doi: 10.1093/nar/17.20.8171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang D. D., Sharp P. A. Regulation by HIV Rev depends upon recognition of splice sites. Cell. 1989 Dec 1;59(5):789–795. doi: 10.1016/0092-8674(89)90602-8. [DOI] [PubMed] [Google Scholar]
- Dropulic B., Elkins D. A., Rossi J. J., Sarver N. Ribozymes: use as anti-HIV therapeutic molecules. Antisense Res Dev. 1993 Spring;3(1):87–94. doi: 10.1089/ard.1993.3.87. [DOI] [PubMed] [Google Scholar]
- Fischer U., Meyer S., Teufel M., Heckel C., Lührmann R., Rautmann G. Evidence that HIV-1 Rev directly promotes the nuclear export of unspliced RNA. EMBO J. 1994 Sep 1;13(17):4105–4112. doi: 10.1002/j.1460-2075.1994.tb06728.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fragapane P., Caffarelli E., Lener M., Prislei S., Santoro B., Bozzoni I. Identification of the sequences responsible for the splicing phenotype of the regulatory intron of the L1 ribosomal protein gene of Xenopus laevis. Mol Cell Biol. 1992 Mar;12(3):1117–1125. doi: 10.1128/mcb.12.3.1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fragapane P., Prislei S., Michienzi A., Caffarelli E., Bozzoni I. A novel small nucleolar RNA (U16) is encoded inside a ribosomal protein intron and originates by processing of the pre-mRNA. EMBO J. 1993 Jul;12(7):2921–2928. doi: 10.1002/j.1460-2075.1993.tb05954.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamm J., Kazmaier M., Mattaj I. W. In vitro assembly of U1 snRNPs. EMBO J. 1987 Nov;6(11):3479–3485. doi: 10.1002/j.1460-2075.1987.tb02672.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haseloff J., Gerlach W. L. Simple RNA enzymes with new and highly specific endoribonuclease activities. Nature. 1988 Aug 18;334(6183):585–591. doi: 10.1038/334585a0. [DOI] [PubMed] [Google Scholar]
- Krämer A., Keller W. Purification of a protein required for the splicing of pre-mRNA and its separation from the lariat debranching enzyme. EMBO J. 1985 Dec 16;4(13A):3571–3581. doi: 10.1002/j.1460-2075.1985.tb04119.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leavitt M. C., Yu M., Yamada O., Kraus G., Looney D., Poeschla E., Wong-Staal F. Transfer of an anti-HIV-1 ribozyme gene into primary human lymphocytes. Hum Gene Ther. 1994 Sep;5(9):1115–1120. doi: 10.1089/hum.1994.5.9-1115. [DOI] [PubMed] [Google Scholar]
- Lerner M. R., Steitz J. A. Antibodies to small nuclear RNAs complexed with proteins are produced by patients with systemic lupus erythematosus. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5495–5499. doi: 10.1073/pnas.76.11.5495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojwang J. O., Hampel A., Looney D. J., Wong-Staal F., Rappaport J. Inhibition of human immunodeficiency virus type 1 expression by a hairpin ribozyme. Proc Natl Acad Sci U S A. 1992 Nov 15;89(22):10802–10806. doi: 10.1073/pnas.89.22.10802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton J. R., Habets W., van Venrooij W. J., Pederson T. U1 small nuclear ribonucleoprotein particle-specific proteins interact with the first and second stem-loops of U1 RNA, with the A protein binding directly to the RNA independently of the 70K and Sm proteins. Mol Cell Biol. 1989 Aug;9(8):3360–3368. doi: 10.1128/mcb.9.8.3360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratner L., Haseltine W., Patarca R., Livak K. J., Starcich B., Josephs S. F., Doran E. R., Rafalski J. A., Whitehorn E. A., Baumeister K. Complete nucleotide sequence of the AIDS virus, HTLV-III. Nature. 1985 Jan 24;313(6000):277–284. doi: 10.1038/313277a0. [DOI] [PubMed] [Google Scholar]
- Ruffner D. E., Stormo G. D., Uhlenbeck O. C. Sequence requirements of the hammerhead RNA self-cleavage reaction. Biochemistry. 1990 Nov 27;29(47):10695–10702. doi: 10.1021/bi00499a018. [DOI] [PubMed] [Google Scholar]
- Staffa A., Cochrane A. The tat/rev intron of human immunodeficiency virus type 1 is inefficiently spliced because of suboptimal signals in the 3' splice site. J Virol. 1994 May;68(5):3071–3079. doi: 10.1128/jvi.68.5.3071-3079.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stutz F., Rosbash M. A functional interaction between Rev and yeast pre-mRNA is related to splicing complex formation. EMBO J. 1994 Sep 1;13(17):4096–4104. doi: 10.1002/j.1460-2075.1994.tb06727.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullenger B. A., Cech T. R. Tethering ribozymes to a retroviral packaging signal for destruction of viral RNA. Science. 1993 Dec 3;262(5139):1566–1569. doi: 10.1126/science.8248806. [DOI] [PubMed] [Google Scholar]
- Symons R. H. Small catalytic RNAs. Annu Rev Biochem. 1992;61:641–671. doi: 10.1146/annurev.bi.61.070192.003233. [DOI] [PubMed] [Google Scholar]
- Terns M. P., Dahlberg J. E., Lund E. Multiple cis-acting signals for export of pre-U1 snRNA from the nucleus. Genes Dev. 1993 Oct;7(10):1898–1908. doi: 10.1101/gad.7.10.1898. [DOI] [PubMed] [Google Scholar]
- Terns M. P., Dahlberg J. E. Retention and 5' cap trimethylation of U3 snRNA in the nucleus. Science. 1994 May 13;264(5161):959–961. doi: 10.1126/science.8178154. [DOI] [PubMed] [Google Scholar]
- Traboni C., Cortese R., Ciliberto G., Cesareni G. A general method to select for M13 clones carrying base pair substitution mutants constructed in vitro. Nucleic Acids Res. 1983 Jun 25;11(12):4229–4239. doi: 10.1093/nar/11.12.4229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlenbeck O. C. A small catalytic oligoribonucleotide. Nature. 1987 Aug 13;328(6131):596–600. doi: 10.1038/328596a0. [DOI] [PubMed] [Google Scholar]
- Yamada O., Yu M., Yee J. K., Kraus G., Looney D., Wong-Staal F. Intracellular immunization of human T cells with a hairpin ribozyme against human immunodeficiency virus type 1. Gene Ther. 1994 Jan;1(1):38–45. [PubMed] [Google Scholar]
- Yu M., Leavitt M. C., Maruyama M., Yamada O., Young D., Ho A. D., Wong-Staal F. Intracellular immunization of human fetal cord blood stem/progenitor cells with a ribozyme against human immunodeficiency virus type 1. Proc Natl Acad Sci U S A. 1995 Jan 31;92(3):699–703. doi: 10.1073/pnas.92.3.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaug A. J., Been M. D., Cech T. R. The Tetrahymena ribozyme acts like an RNA restriction endonuclease. Nature. 1986 Dec 4;324(6096):429–433. doi: 10.1038/324429a0. [DOI] [PubMed] [Google Scholar]
- Zeller R., Carri M. T., Mattaj I. W., De Robertis E. M. Xenopus laevis U1 snRNA genes: characterisation of transcriptionally active genes reveals major and minor repeated gene families. EMBO J. 1984 May;3(5):1075–1081. doi: 10.1002/j.1460-2075.1984.tb01931.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou C., Bahner I. C., Larson G. P., Zaia J. A., Rossi J. J., Kohn E. B. Inhibition of HIV-1 in human T-lymphocytes by retrovirally transduced anti-tat and rev hammerhead ribozymes. Gene. 1994 Nov 4;149(1):33–39. doi: 10.1016/0378-1119(94)90409-x. [DOI] [PubMed] [Google Scholar]