Introduction
Despite prolonged antiretroviral therapy (ART), HIV-1 persists as transcriptionally inactive proviruses in resting memory CD4+ T cells (Chun et al., 1997; Finzi et al., 1997; Wong et al., 1997). This latent reservoir (LR) has a long half-life, preventing cure by ART alone (Finzi et al., 1997; Siliciano et al., 2003; Strain et al., 2003). In resting CD4+ T cells, the lack of active forms of key cellular transcription factors (Bohnlein et al., 1988; Duh et al., 1989; Ganesh et al., 2003; Kinoshita et al., 1997; Nabel and Baltimore, 1987; West et al., 2001) and of HIV-1 Tat and its cellular cofactors (Cujec et al., 1997; Herrmann and Rice, 1995; Jones and Peterlin, 1994; Kao et al., 1987; Selby and Peterlin, 1990; Tyagi et al., 2010) limits the initiation and elongation, respectively, of viral transcription (Lassen et al., 2004; Williams and Greene, 2007). The latent reservoir may thus be established when activated CD4+ T cells become infected as they revert back to a resting memory state. In addition, DNA methylation and repressive histone modifications may silence proviruses (Blazkova et al., 2009; Coull et al., 2000; He and Margolis, 2002; Kauder et al., 2009; Van Lint et al., 1996; Verdin et al., 1993; Williams et al., 2006).
A major approach to eradicating HIV-1 involves reversing latency in patients on ART (Richman et al., 2009; Deeks, 2012). Cells harboring induced proviruses could then be lysed by HIV-1-specific cytolytic T lymphocytes (CTL) (Shan et al., 2012), while new rounds of infection are blocked by ART. Clinical trials exploring this strategy have used the histone deacetylase inhibitors (Lehrman et al., 2005; Archin et al., 2009; Contreras et al., 2009; Archin et al., 2012).
Accurate measurement of the LR is essential for evaluating eradication strategies. If the LR is eradicated, ART can be discontinued without rebound viremia. Interruption before complete eradication will likely result in rebound (Davey et al., 1999) and repopulation of the LR.
The standard assay for LR size is a viral outgrowth assay (VOA) (Finzi et al., 1997; Siliciano and Siliciano, 2005) measuring the frequency of resting CD4+ T cells that produce infectious virus after a single round of maximum in vitro T cell activation. Limiting dilutions of resting CD4+ T cells are stimulated with the mitogen phytohemagglutinin (PHA), which reverses latency by inducing T cell activation. Released viruses are expanded by addition of CD4+ T lymphoblasts from HIV-1-negative donors. Culture supernatants are examined for exponential viral growth by ELISA for HIV-1 p24. With this assay, the mean frequency of latently infected cells in patients on ART is ∼1/106 resting CD4+ T cells (Eriksson et al., 2013).
It has been assumed that LR size can be assessed with agents like PHA that induce uniform T cell activation (Patel et al., 1988; Hermankova et al., 2003). However, the frequency of latently infected cells detected in the VOA is 300 fold lower than the frequency of resting CD4+ T cells that harbor proviruses detectable by PCR (Eriksson et al., 2013). Thus at limiting dilution in the VOA, negative wells contain many proviruses, which we designate non-induced proviruses. The non-induced proviruses are generally considered defective, but have not been molecularly characterized. The magnitude of the challenge presented by the LR depends on whether non-induced proviruses can be induced in vivo. We present here the first molecular characterization of non-induced proviruses.
Results
Transwell VOA achieves maximum in vitro activation and outgrowth
To analyze proviruses that did not give rise to infectious virus in the VOA (non-induced proviruses), we first established that the conditions were sufficient to activate 100% of resting CD4+ T cells. Resting CD4+ T cells from patients on suppressive ART for >6 months were labeled with carboxyfluorescein succinimidyl ester (CFSE) and stimulated with PHA and irradiated allogeneic peripheral blood mononuclear cells (PBMC) under conditions used in the VOA. By day 7, >99.8% of patient cells had divided at least once (Figure S1A), confirming that PHA causes uniform T cell activation.
In the VOA, viruses released after reversal of latency replicate in healthy donor CD4+ lymphoblasts added to the cultures. To facilitate cloning of non-induced proviruses, we tested whether comparable levels of activation and viral outgrowth could be achieved in transwell cultures in which patient cells were separated from donor lymphoblasts by a cell-impermeable membrane (Figure S1B). In side-by-side comparison with standard VOA cultures from 10 patients, transwell cultures showed comparable cellular activation in both p24+ and p24- wells, as >95% of patient cells expressed HLA-DR and/or CD25 on day 21 (Figure S1C). Transwell cultures also showed viral outgrowth comparable to standard VOA cultures (Figure S1D). Non-induced proviruses were thus cloned from p24-wells of limiting dilution transwell and standard cultures.
Clonal amplification and sequencing of non-induced proviruses
We obtained near full-length clonal sequences of non-induced proviruses from 8 patients on suppressive ART. Patient characteristics are in Table S1. Non-induced proviruses were obtained from wells seeded with 4×104 or 2×105 resting CD4+ T cells that were p24- on day 21. In clonal VOA cultures, wells with replicating virus are p24+ by day 10–14 (Laird et al., 2013). Even with a more sensitive RT-PCR assay for HIV-1 mRNA (Laird et al., 2013), none of the p24- wells showed exponential growth. Thus the non-induced proviruses were obtained from wells with no replicating virus despite maximal T cell activation.
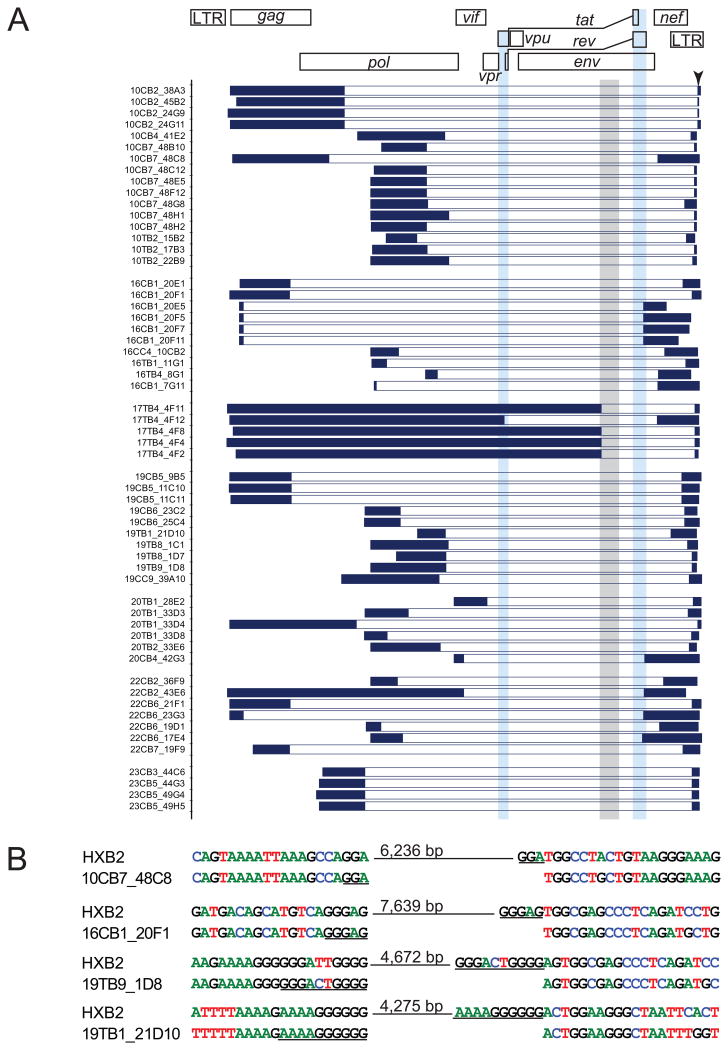
Non-induced proviruses were amplified in limiting dilution PCRs to avoid in vitro recombination. A near full-length 9.1 kb outer PCR (Li et al., 2007) spanning U5 to U5 (positions 623–9,686, HXB2 coordinates) was followed by nested inner PCRs. Aliquots from outer PCRs were first amplified in a nested inner gag PCR (Figure 1A). Cell dilutions for which <20% of the inner reactions were positive were selected because positive wells at these dilutions have >90% probability of being clonal. Aliquots from the gag+, clonal outer PCRs were amplified using 4 sets of inner PCR primers to obtain fragments overlapping by 150–3,173 bp (Figure 1A). Importantly, instead of cloning PCR products, we directly sequenced them. This dramatically reduces PCR errors since errors occurring after the 1st or 2nd cycle are present in too small a fraction of the final products to be observed. Sequences with double peaks or non-identical overlap regions were discarded. We identified 213 non-induced proviruses from 8 patients.
Figure 1. Characterization of non-induced proviruses.
(A) Strategy and results of analysis of non-induced proviruses. Limiting dilution VOA cultures were established from 8 patients. A total of 51 p24- wells containing a total of 8.9×106 resting CD4+ T cells were analyzed. 213 non-induced proviruses were identified by near-full length limiting dilution PCR followed by a nested gag PCR. The gag PCR products were directly sequenced, and proviruses with APOBEC3G-mediated G→A hypermutation were identified using the Los Alamos Hypermut algorithm (Rose and Korber, 2000). Non-hypermutated proviruses were analyzed by nested amplification of 4 overlapping subgenomic fragments (labeled A-D). Amplicons with patient HIV-1 sequence but discernibly smaller than expected size in 1% agarose gel electrophoresis were considered to contain large internal deletions which, where possible, were mapped by direct sequencing. Other lethal defects including single nucleotide insertions and deletions (INDELs), packaging signal (Ψ) deletions, and major splice donor (MSD) site mutations were identified by direct sequencing.
(B) Results of 1% agarose gel electrophoresis of 4 subgenomic amplicons (A–D) from representative non-induced proviruses with different defects. Left to right: intact genome, 16 bp Ψ deletion, deletion with defined junction, and deletion with undefined junction. Clone name is give below each gel. Triangles, amplicons with correct size. Asterisks, amplicons with smaller than expected size.
(C) Summary of the analysis of 213 non-induced proviruses.
See also Figure S1 and Table S1.

Hypermutation and large internal deletions render most non-induced proviruses defective
Most (88.3%) non-induced proviruses had obvious defects precluding replication (Figure 1C). Direct sequencing of the nested gag PCR product revealed that ∼1/3 (32.4%) of non-induced proviruses had APOBEC3G-mediated G→A hypermutation occurring in the expected sequence context (GG or GGG)(Yu et al., 2004). Hypermutated proviruses are replication-defective due to start codon mutations and numerous tryptophan→stop codon mutations (Figure S2). Although the gag gene was analyzed here, other regions of the genome show even greater hypermutation (Yu et al., 2004). Importantly, it is unlikely that hypermutated proviruses could produce functional viral proteins due to stop codons in most open reading frames (ORFs).
Non-induced proviruses that were not hypermutated were further analyzed by nested amplification of 4 overlapping subgenomic fragments (Figure 1A). Of the 144 clonal non-induced proviruses without hypermutation, 97 had large internal deletions identified by smaller amplicon size in electrophoresis (Figure 1B). We mapped the deletion junctions in 58 of these clones (Figure 2A). For example, clone 10CB7_48H1 (Figure 1B) gave a smaller amplicon for the nested C reaction, and amplification of fragments A, B, and D failed due to deletion of nucleotides 4,869–9,533. All 58 mapped deletions would affect expression of the essential regulatory proteins Tat and Rev (Figure 2A) because the deletions encompass the tat and rev exons, the splice sites, and the Rev-responsive element (RRE).
Figure 2. Mapping of large internal deletions in non-induced proviruses.
(A) Locations of deletions. Dark blue horizontal bars: continuous sequencing reads interrupted by deletions. White horizontal bars: deletions. Light blue vertical bars, tat and rev ORFs. Gray vertical bar: RRE element.
(B) Representative sequences at deletion junctions. Short repeats (underlined) at the deletion junctions suggest a single reverse transcriptase jump due to copy choice recombination (Sanchez et al., 1997; Temin, 1993). Hypermutated sequences (see Figure S2) were not analyzed for deletions.
Deletions are not unexpected. HIV-1 is prone to recombination due to pseudodiploidity (2 RNA copies/virion with physical proximity for recombination). Frequent template switching events occur during reverse transcription (Simon-Loriere and Holmes, 2011). Switching between short repeats in a single genome results in deletion of the intervening sequence and one repeat (Temin, 1993). Large deletions have been observed in unfractionated PBMC from viremic patients (Sanchez et al., 1997). Several lines of evidencesuggest that these deletions occur in vivo rather than during in vitro analysis. First, deletions were observed following direct sequencing of uncloned PCR products. Second, for a given provirus, the same deletion junctions were observed in different nested PCRs using different primers. Third, short amplicons were not seen in control experiments with plasmids carrying the reference proviral genomes NL4-3 and BaL. Plasmids were mixed, diluted to 8 copies/105 human genome equivalents, and amplified under the same conditions. No deletion or recombination was observed. Fourth, short sequence repeats were identified at some deletion junctions (Figure 2B), consistent with a single polymerase jump due to copy choice recombination during reverse transcription of the minus strand (Sanchez et al., 1997). Taken together, these results demonstrate that a large fraction of non-induced proviruses are non-functional due to large internal deletions, likely introduced during reverse transcription.
The precise fraction of proviruses with deletions could be underestimated by this analysis because deletions could affect PCR primer binding sites. For 39 clones, the exact deletion junction could not be identified, probably because the deletions encompassed binding sites for primers used in the nested PCRs. For these clones, we obtained at least 2 sequences to ensure that the amplicons contained non-hypermutated, patient-specific sequences. For 12 mapped deletions, the deletion included the reverse gag primer binding site, but mapping was possible because other nested reactions were successful.
Mutations in cis elements render some non-induced proviruses defective
Proviruses with correct amplicon size were directly sequenced. A small fraction (8/213) had non-sense mutations and/or frame-shifting insertions or deletions in one or more ORFs. Small deletions (8–98 bp) were found in the packaging signal (Ψ) in 12 sequences (Figure S3A). The deletions encompassed the major splice donor (MSD) site. Point mutations in the MSD site were found in 2 proviruses. Full genome sequencing showed that 7 of the non-induced proviruses with Ψ or MSD mutations were otherwise intact. To determine whether these mutations rendered non-induced proviruses defective, we reconstructed 3 clones by genome synthesis as described below. The reconstructed proviruses included 2 clones with short (8 bp and 16 bp) Ψ deletions in packaging stem loop 2 and one with a MSD site mutation (TG|GT→TG|GG). Although these clones had intact ORFs, they did not replicate in healthy donor CD4+ lymphoblasts (Figure S3B) under conditions in which other reconstructed proviruses replicated well (see below). Thus mutations in cis elements render otherwise intact proviruses defective.
A significant proportion of non-induced proviruses are replication-competent
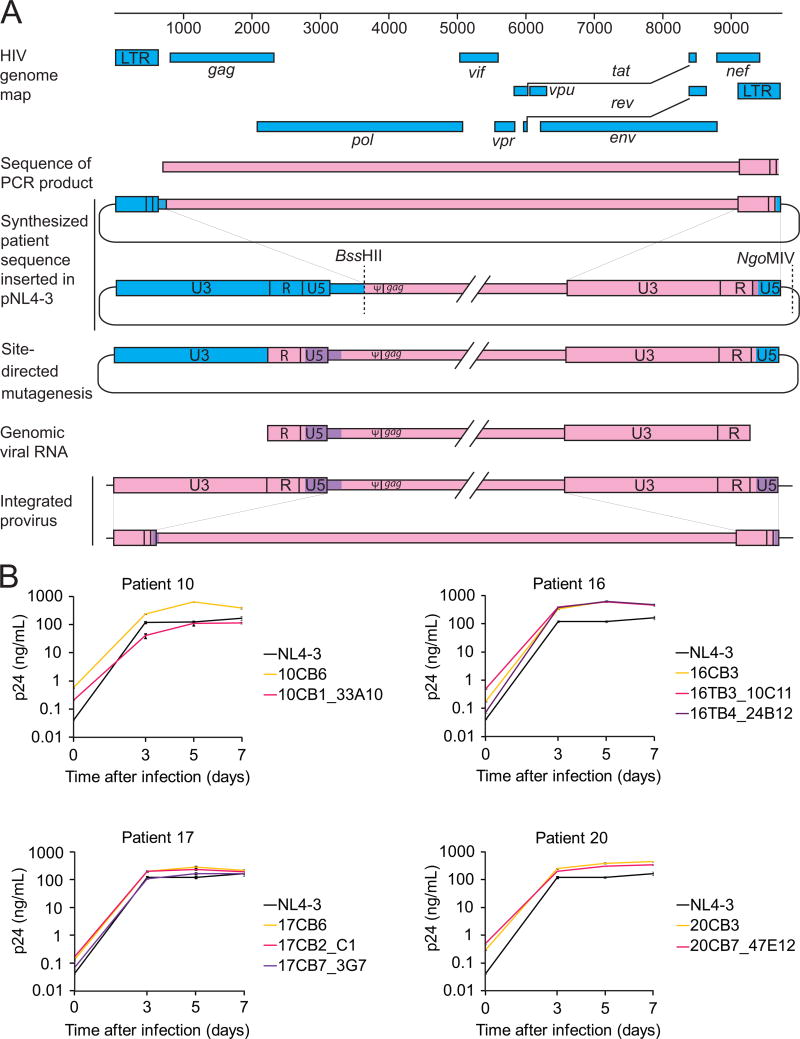
Of 213 non-induced proviruses, 25 (11.7%) had intact ORFs and cis elements. When compared with induced proviruses from the same patient, no known lethal mutations were seen. To determine whether these intact non-induced proviruses are replication-competent, we used the direct sequencing results to reconstruct full-length non-induced proviral clones by de novo genome synthesis (Figure 3A). This strategy avoids PCR and cloning-induced errors. We reconstructed 6 non-induced proviruses from 4 patients by inserting the synthesized sequence into a plasmid carrying the reference isolate NL4-3. Not captured in our PCR strategy is a 108 bp segment of the provirus, representing nucleotides (nt) 565–672 (HXB2 coordinates). This segment in the 5′ untranslated region includes part of U5 and the primer binding site (pbs) (Figure 3A). Although U5 deletions may not affect replication-competence (Vicenzi et al., 1994), we took additional steps to make the reconstructed clones fully patient-derived, without any NL4-3 sequence. We used limiting dilution PCR to amplify the LTR-gag region from cells in p24-wells. Using a 424 bp segment from the 5′ U3-R-U5 region (HXB2 nt 140–564), we constructed phylogenetic trees (Figure S4). Then, using site-directed mutagenesis, we corrected the 108 bp segment from NL4-3 to the phylogenetically closest sequence from the same patient (Figure 3A). This process results in proviruses that are 100% patient-derived and 98.2 % equivalent to specific proviruses present in vivo with the remaining 1.8% equivalent to a very closely related provirus from the same patient. Given the high sequence conservation in the 108 bp segment (Figure S4), we estimate that the reconstructed clones could differ from the parent clones by at most 3 nt, or 0.03% of the genome. For each patient, we also reconstructed an induced viral clone from a p24+ well.
Figure 3. Growth kinetics of reconstructed non-induced viruses.
(A) Reconstruction of full-length non-induced proviruses. Pink, clonal patient-derived sequence. Blue, pNL4-3. Purple, sequence from the most closely related patient-specific virus.
(B) Growth kinetics of reconstructed non-induced viruses from p24- wells (pink and purple), reconstructed induced viruses from p24+ wells (yellow), and NL4-3 (black). Data are presented as mean +/- SEM.
See also Figure S4.
Strikingly, all 6 reconstructed non-induced proviruses from 4 different patients showed replication fitness comparable to that of the NL4-3 and reconstructed induced proviruses from the same patients (Figure 3B). It is unlikely that all of these intact non-induced proviruses could have actually been defective with inactivating mutations in the 108 base pair segment that was not directly sequenced, as we showed that an additional round of PHA stimulation causes some non-induced proviruses to produce replication-competent virus (see below). Taken together, these results indicate that a substantial fraction of non-induced proviruses are intact and capable of generating infectious virus if induced in vivo.
Non-induced proviruses have intact promoter function unless hypermutated
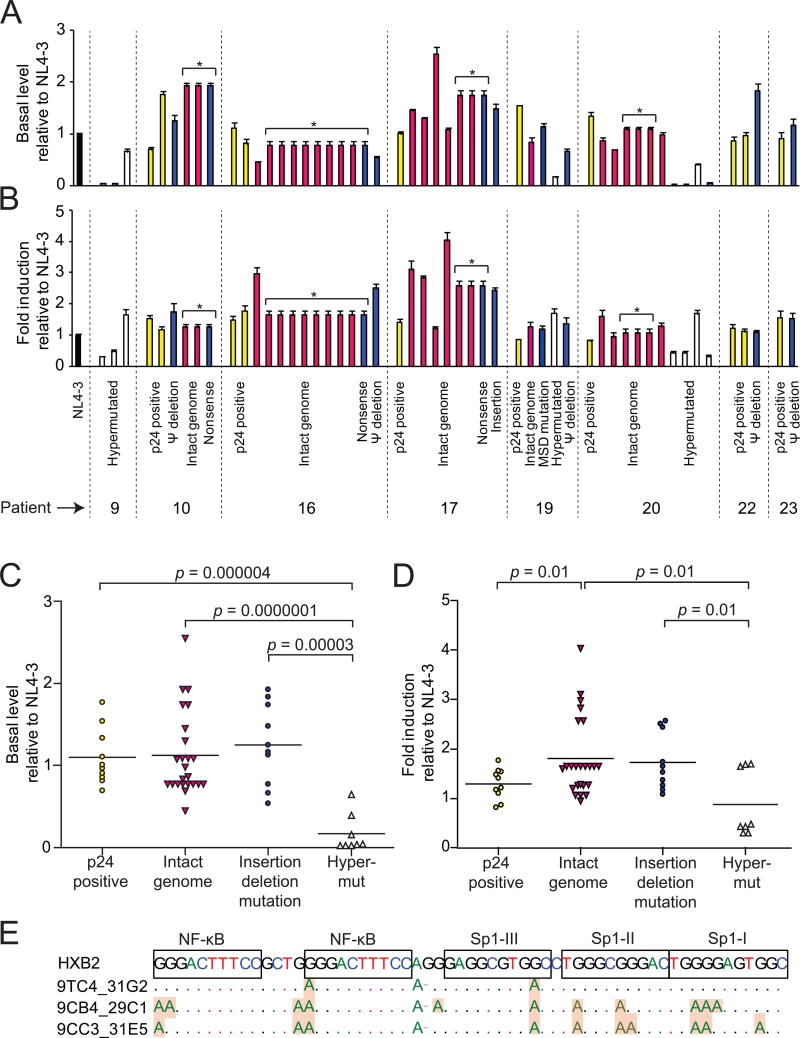
The ability of intact non-induced proviruses to produce infectious virus suggests that, at the primary sequence level, their promoters are functional. To confirm this, we cloned LTRs from representative non-induced proviruses into a luciferase reporter construct (Yang et al., 2009). We measured luciferase activity in transfected resting CD4+ T cells before and 4 hours after stimulation with phorbol myristate acetate (PMA) and ionomycin. We also analyzed LTRs from induced proviruses from the same patients and NL4-3. In general, LTRs from non-induced proviruses showed basal (Figure 4A, C) and stimulated (Figure 4B, D) activity comparable to LTRs from induced proviruses and NL4-3. Decreased LTR function was only observed for hypermutated clones. This likely reflects G→A hypermutation in binding sites for the transcription factors NF-κB and Sp1 (Figure 4E). Thus, most non-induced proviruses have LTRs that are intact at the primary sequence level.
Figure 4. LTR activity of non-induced proviruses.
LTR activity measured before (A, C) and 4 hrs after PMA/ionomycin stimulation (B, D). Resting CD4+ T cells were transfected with LTR-firefly luciferase reporter constructs containing LTRs from non-induced proviruses or induced proviruses from p24+ wells (yellow bars). Firefly luciferase activity was normalized to an internal control, Renilla luciferase driven by a constitutive thymidine kinase promoter. The resulting values were then expressed relative to the NL4-3 control. Non-induced proviruses included those with intact genomes (red bars), insertions or deletions (blue bars), and hypermutation (white bars). Asterisks indicate different clones with the same LTR sequence. Data are presented as mean +/- SEM.
(E) Impact of G→A hypermutation on transcription factor binding sites. Hypermutated sites in representative clones are shaded.
Most non-induced proviruses are integrated into active transcription units
We next determined the locations of non-induced proviruses in the host genome to understand whether they were integrated into sites unfavorable for transcription. Bushman et al. showed that HIV-1 typically integrates into transcription units (Schroder et al., 2002). However, in some model systems, integration into regions of heterochromatin is associated with latency (Jordan et al., 2003). Using inverse PCR at limiting dilution, we found that 92.9% of 70 non-induced proviruses resided in transcription units (Figure 5A), consistent with previous observations in patient resting CD4+ T cells (91%)(Han et al., 2004). Based on transcript levels measured in a serial analysis of gene expression (SAGE) library from a primary cell model of latency (Shan et al., 2011), most non-induced proviruses were integrated into genes transcribed at moderate levels in both resting and activated CD4+ T cells (Figure 5B). Non-induced proviruses were found in both orientations with respect to the host genes (Figure 5C). Overall these results indicate that non-induced proviruses are not integrated into chromosomal regions that are repressive for transcription, and thus other factors must have prevented expression.
Figure 5. Integration sites of non-induced proviruses.
(A) Distribution of integration sites of non-induced proviruses. Intron and exon boundaries are determined as previously described (Shan et al., 2011).
(B) Transcription level of host genes in which non-induced proviruses were integrated. Transcription levels were determined by SAGE analysis of Bcl-2 transduced activated and resting primary CD4+ T cells as previously described (Shan et al., 2011). Blue circles, transcript levels in resting and activated CD4+ T cells. Red squares, transcript levels for the subset of genes in which integration sites of non-induced proviruses were mapped.
(C) Transcriptional orientation of non-induced proviruses relative to the host gene.

Lack of CpG methylation in the LTR of non-induced proviruses
We next examined whether non-induced proviruses were silenced by CpG methylation. CpG islands are present in the HIV-1 genome (Chavez et al., 2011), including one in the U3 region of the LTR which contains critical transcription factor binding sites (Figure 6). Therefore, DNA from freshly isolated resting CD4+ T cells and from cells in p24- wells was treated with bisulfite, and then the LTR region was amplified under limiting dilution conditions (Blazkova et al., 2012). Direct sequencing was used to determine the extent of CpG methylation. Only 3.1% of the LTR CpGs were methylated in resting CD4+ T cells from study patients. Even fewer (0.9%) of the CpGs in the LTRs of non-induced proviruses were methylated (Figure 6). In contrast, we readily detected methylation at a CpG island in the env region (75.5%), indicating that this method did not selectively amplify non-methylated sites. Although CpG methylation at the LTR clearly is well documented in some models of HIV-1 latency (Kauder et al., 2009), our results indicate that non-induced proviruses are not silenced through CpG methylation at the 5′ LTR.
Figure 6. CpG methylation of non-induced proviruses.
(A) Positions of CpGs analyzed.
(B) CpG methylation in the 5′ LTR of proviruses in resting CD4+ T cells from the indicated patients. Each row represents a single provirus amplified by limiting dilution PCR after bisulfite treatment. Open circles, nonmethylated CpGs. Closed circles, methylated CpGs. Missing circles, no CpG present due to sequence polymorphism.
(C) CpG methylation in the env gene of proviruses from resting CD4+ T cells.
(D) CpG methylation in the 5′ LTR of non-induced proviruses from p24- wells.
(E) CpG methylation in the env gene of non-induced proviruses from p24- wells.
Intact non-induced proviruses may increase LR size by ∼60 fold
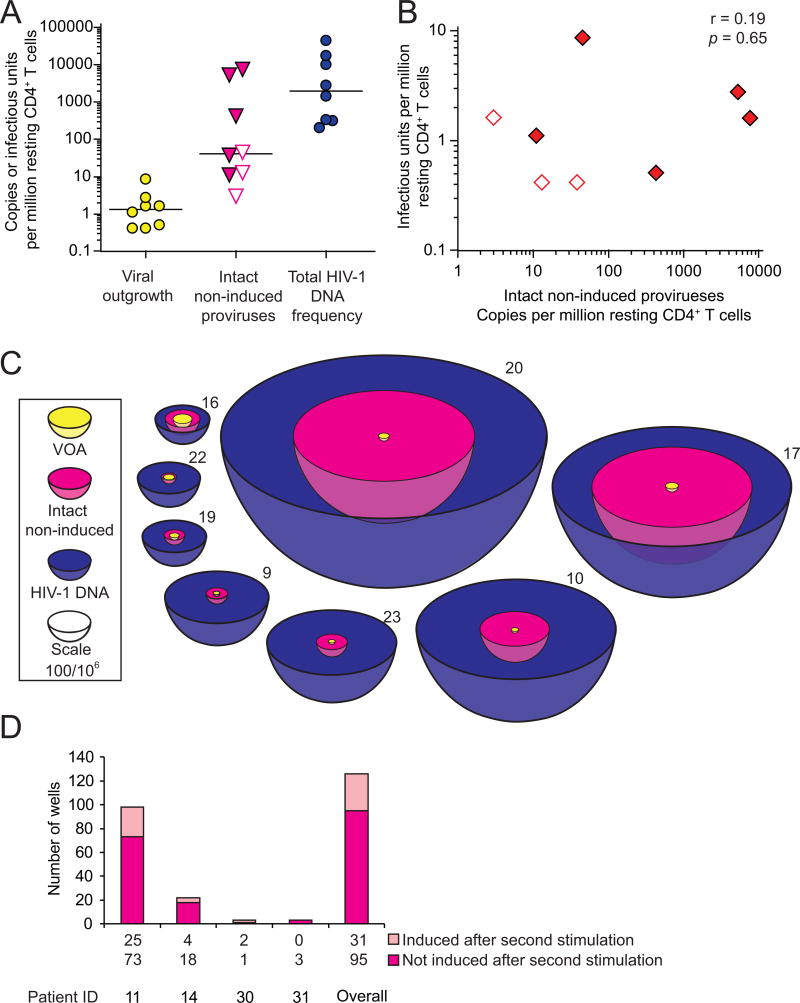
The above results indicate that although most non-induced proviruses have identifiable lethal defects, a substantial fraction are intact and replication-competent at the primary sequence level. Analysis of LTR function, integration sites, and methylation status suggests that these intact non-induced proviruses could be induced in vivo, thereby increasing LR size. We compared the frequency of induced proviruses (defined using the VOA) and intact non-induced proviruses (quantitated as the product of total proviral DNA frequency and the fraction of non-induced proviruses that are intact) among the total pool of proviruses (measured by quantitative real-time PCR). Bayesian analysis was chosen instead of maximum likelihood estimation because the former provides nonzero point estimates for patients from whom no clones with intact genomes were identified. The positive VOA results in every patient and the successful detection of intact non-induced proviruses in patients for whom >20 clones were analyzed suggest that intact non-induced proviruses could be detected in every patient if enough clones are examined. The fraction of intact non-induced proviruses was calculated as the median of an empirical Bayesian posterior, the most conservative of 5 models tested (Table S2), with a prior distribution chosen to reflect the observed data. Both the fraction of intact non-induced proviruses and the total number of proviruses/106 resting CD4+ T cells varied dramatically from patient to patient (Figure 7A). There was no correlation between the VOA and the frequency of intact non-induced proviruses (Figure 7B). All statistical models (Table S2) indicated that the median frequency of intact non-induced proviruses was at least ∼60 fold higher than the frequency of induced proviruses detected in the VOA. If the intact non-induced proviruses described here can be induced in vivo, then the size of the LR is much greater than previously thought (Figure 7C).
Figure 7. Quantification of intact non-induced proviruses.
(A) Comparison of the frequency of cells detected in the VOA, cells carrying intact non-induced proviruses, and cells carrying HIV-1 DNA. The frequency of cells with HIV-1 DNA was measured by quantitative PCR on freshly isolated resting CD4+ T cells. The frequency of cells with intact, non-induced proviruses was calculated as frequency of cells with HIV-1 DNA times the proportion of intact non-induced proviruses estimated for each patient using an empirical Bayesian model (Table S2). Open symbols, no intact proviruses detected, empirical Bayesian estimate plotted. Bars, median value.
(B) Correlation between the frequency of cells detected in the VOA and the frequency of cells with intact non-induced proviruses. Open symbols, see (A).
(C) Scale representation of the frequencies of the infected resting CD4+ T cell populations. Volume reflects population size. Yellow circles, minimum size of the LR as measured by VOA. Magenta, frequency of cells with intact proviruses, calculated as the frequency of cells detected in the VOA plus the frequency of cells with intact non-induced proviruses. This is the potential size of the LR if intact non-induced proviruses can be induced in vivo. Blue, cells with HIV-1 DNA.
(D) Repeated stimulation induces additional proviruses. Bars indicated p24 ELISA results from pairs of split wells cultured with or without a second round of stimulation with PHA and irradiated allogeneic PBMC.
See also Figure S5 and Table S2.
To determine whether intact non-induced proviruses are permanently silenced or potentially inducible under certain conditions, we tested whether repeated PHA stimulation could induce additional non-induced proviruses (Figure S5). We stimulated multiple replicate cultures of 2×105 patient resting CD4+ T cells with PHA in VOA conditions. We then split each culture well equally into two wells on day 7. As all patient cells have divided by day 7 (Figure S1), each split well contained daughter cells derived from cells activated in the original well. One set of the split cultures wells was activated again with PHA while the other set was cultured without additional stimulation. We then compared supernatant p24 levels after another 14 days of culture. Among 126 p24- wells from 4 patients, 31 (24.6%) became p24+ after the additional round of PHA stimulation, while their paired culture well that did not receive an additional round of stimulation remained p24- (Figure 7D). It is not yet clear what fraction of the intact non-induced proviruses are inducible in vivo. Nevertheless, these results demonstrate that at least some intact non-induced proviruses can be induced under repeated stimulation.
Discussion
The LR in resting CD4+ T cells is the major barrier to HIV-1 eradication, and as efforts to cure the infection proceed, accurate measurement of LR size will be essential. This study provides a molecular basis for understanding measures of the LR. Through an analysis of proviruses that did not give rise to infectious virus following a single round of T cell activation (non-induced proviruses), we have provided a definitive explanation for the large discrepancy between results of PCR and culture assays of LR size. In addition, the discovery of intact non-induced proviruses indicates that the size of the LR may be much greater than previously thought. We reconstructed full-length, intact non-induced proviruses from multiple patients, and all showed growth kinetics comparable to induced proviruses from the same patient and a reference isolate. These intact non-induced proviruses are not detected in standard culture assays but may nevertheless prevent cure. Thus the present study provides new insights into the extent of the challenge posed by the LR and may lead to novel strategies that target intact non-induced proviruses.
We show here that most non-induced proviruses were rendered defective during reverse transcription by APOBEC3G-induced hypermutation (Yu et al., 2004), by internal deletions caused by copy choice recombination during reverse transcription (Sanchez et al., 1997), or by frame-shift or nonsense mutations caused by the error-prone reverse transcriptase (Bebenek et al., 1989). The resulting defective viral genomes can still integrate because only defects at the ends of the genome affect integration. The defective genomes will be detected in most PCR-based assays of proviral DNA provided that the primer binding sites are intact. Many of the defective proviruses have large internal deletions encompassing the Tat and Rev ORFs and the RRE. Tat-mediated transactivation is required for effective transcriptional elongation (Kao et al., 1987) and the production of virus particles requires that singly spliced and unspliced HIV-1 mRNAs be exported from the nucleus in a Rev-dependent fashion (Malim et al., 1989). Thus these deleted proviruses may not produce viral proteins even after successful induction of transcription. The same is true for hypermutated proviruses, which have stop codons in every ORF. Importantly, eradication strategies depend on the production of viral proteins which allows recognition of the infected cells by HIV-1 specific CTL (Shan et al., 2012). Defective proviruses with large internal deletions and/or APOBEC3G-induced hypermutation may not be eliminated even by strategies that effectively eliminate cells carrying replication-competent virus. These considerations highlight the difficulty of assessing eradication strategies with PCR-based assays.
Although difficult and time-consuming, the VOA (Eriksson et al., 2013; Finzi et al., 1997), which has recently been simplified (Laird et al., 2013), does allow detection of cells harboring replication-competent virus. However, the discovery of intact, non-induced proviruses raises the possibility that this assay may dramatically underestimate LR size. Several lines of evidence suggest that this is not simply an issue of assay sensitivity. We showed that the PHA stimulation activates all resting CD4+ T cells as assessed by proliferation and activation marker expression. Using prolonged culture and sensitive RT-PCR assays, we also verified that wells from which non-induced proviruses were obtained were truly negative for viral outgrowth. It is also unlikely that these p24- wells remained negative because of reduced viral fitness, as we showed that intact non-induced proviruses had growth kinetics comparable to induced viruses from p24+ wells. Taken together, these results confirm that we are examining a population of intact proviruses that were not induced to produce infectious virus after a single round of maximum in vitro activation.
To prove replication-competence, we reconstructed 6 intact, non-induced proviruses from 6 different p24- wells from 4 patients. Surprisingly, all reconstructed viruses replicated as well as the standard reference isolate and control viruses reconstructed from p24+ wells. A sterilizing cure requires elimination of all replication-competent HIV-1, and therefore the discovery that intact non-induced proviruses are replication-competent means that the number of proviruses that must be eliminated is much higher than previously thought. We conservatively estimate that the number may be ∼60 fold higher than estimates based on the VOA. Some statistical models suggest an even higher number (medians of 97–273 fold). Importantly, there is large inter-patient variation in this and other measures of LR size. Overall, our results indicate that the “shock and kill” strategy (Archin et al., 2012; Deeks, 2012) is challenged with a large but unmeasured hidden population of replication-competent proviruses. Interestingly, despite the intense search for novel latency reversing agents, none of the drugs tested to date reaches the robust level of in vitro HIV-1 induction achieved by PHA. Thus the finding that the true size of the LR may be ∼60 fold greater than that estimated using PHA activation is particularly disturbing. However, it is also important to point out that even a low level of virus gene expression may be sufficient to allow the elimination of infected cells by an appropriately primed CTL response (Shan et al., 2012), and that the critical variable may the fraction of latently infected cells induced to express HIV-1 genes.
Understanding why intact non-induced proviruses did not produce infectious virus after maximum in vitro T cell activation is critical for determining their clinical significance. Possible explanations include silencing by repressive chromatin modifications or transcriptional interference. We analyzed CpG methylation of the LTRs of non-induced proviruses at the clonal level. In contrast to some in vitro models of HIV-1 latency (Kauder et al., 2009), we found that in patient CD4+ T cells, there was little CpG methylation at the LTR, consistent with another recent study (Blazkova et al., 2012). We also examined whether non-induced proviruses are silenced by integration into heterochromatin. We found that most of the non-induced proviruses were integrated into active transcription units, consistent with previous studies showing that most HIV-1 proviruses are integrated into introns of actively transcribed genes in cell lines (Schroder et al., 2002) and patient resting CD4+ T cells (Han et al., 2004). Another potential explanation for the non-induced proviruses is transcriptional interference (Han et al., 2008; Lenasi et al., 2008). Since T cell activation may overcome transcriptional interference due to the high affinity of NF-κB for its binding sites in the LTR (Lenasi et al., 2008), transcriptional interference may not be a major cause of silencing of the non-induced proviruses.
We propose that despite maximum T cell activation, the induction of latent proviruses is stochastic. Cellular gene expression levels may follow a digital or analogue distribution after T cell receptor activation, as a result of stochastic and dynamic processes (Chakraborty and Das, 2010). Elegant experimental and theoretical studies have shown that HIV-1 proviruses may show stochastic fluctuations in expression depending on levels of Tat (Burnett et al., 2009; Singh et al., 2010; Weinberger et al., 2005; Weinberger et al., 2008). We propose that intact proviruses undergo stochastic induction even after maximum cellular activation. Some will be induced by one round of activation, while others will remain silent but retain the potential to be activated subsequently. These results indicate an increased barrier to cure, as all intact non-induced proviruses need to be eradicated. Underestimation of intact proviruses by VOAs could be reflected in delayed viral rebound after an apparent “cure”, and overestimation of LR size resulting from detection of defective proviruses by PCR assays could result in prolonged, excessive exposure to toxic latency reversing agents. Thus, the molecular analysis of non-induced proviruses contributes in an important way to HIV-1 eradication efforts.
Experimental Procedures
Study subjects
Peripheral blood was obtained from healthy volunteers and infected donors who had suppression of viremia to <50 copies HIV-1 RNA/ml for >6 months on ART. This study was approved by the Johns Hopkins Institutional Review Board. Written informed consent was obtained from all participants.
VOA
VOAs were performed as described previously (Finzi et al., 1997; Siliciano and Siliciano, 2005) except that patient cells and donor lymphoblasts were placed in separate chambers in transwell plates. CD8-depleted donor lymphoblasts were added on days 1, 7 and 14. Culture supernatants were examined for p24 by ELISA (PerkinElmer) after 21 days.
Characterization of full-length non-induced proviruses
Genomic DNA isolated from p24- wells seeded with 4×104 or 2×105 patient resting CD4+ T cells was subjected to limiting dilution prior to amplification with an initial near full genome length outer PCR (U5 to U5) followed nested amplification of a segment of the gag gene. Aliquots from clonal (P>0.9) positive outer PCR wells were subjected to 4 inner PCRs to obtain near full-length genome sequence. Procedures are described in Extended Experimental Procedures. PCR conditions are listed in Table S3. PCR products were directly sequenced. Sequences of 213 non-induced clones and 5 induced clones were submitted to GenBank (accession numbers KF626120-KF526339).
Reconstruction of non-induced proviruses
The reconstruction of full length non-induced proviruses is described in Extended Experimental Procedures. Reconstructed plasmids were checked by restriction digestion and sequencing and then transfected into HEK 293T cells for virus production. The virus-containing supernatants were adjusted to 200 ng/mL of p24 and used to infect healthy donor CD4+ T lymphoblasts for analysis of growth kinetics.
Measurement of LTR function
Patient-derived LTR sequences were cloned into wt-LTR-Luc reporter and transfected into resting CD4+ T cells from healthy donors using nucleofection (Amaxa)(Yang et al., 2009). TKRLuc was used as internal control. After 48 hours, basal luciferase activity was measured. We also measured luciferase activity 4 hours after stimulation with PMA (10 ng/mL) and ionomycin (1 μM).
Integration site, CpG methylation, and quantitative PCR
Integration site analysis was carried out by inverse PCR as previously described (Han et al., 2004). Transcription levels of the host genes containing integration sites were determined using a previously described SAGE analysis of Bcl-2-transduced primary resting and activated CD4+ T cells (Shan et al., 2011). CpG methylation analysis was carried out as described by Blazkova et al. (Blazkova et al., 2012). Total proviral DNA in resting CD4+ T cells was quantified using a real-time PCR assay (Durand et al., 2012). Copy numbers of the human RNaseP gene copies were measured in separate reactions to quantify cell number (TaqMan Copy Number Reference Assay, RNaseP, Invitrogen)(Spivak et al., 2011).
Statistical analysis
Activation marker expression, viral outgrowth, and LTR function were compared by 2-tailed Student's t test for independent samples. The correlation between viral outgrowth and intact non-induced provirus frequency was analyzed using MedCalc software. Bayesian inference was used to estimate the proportion of intact non-induced genomes in each patient. An empirical Bayesian prior, among 5 models (Table S3 and Supplemental Experimental Procedures), was chosen to reflect the distribution of the observed data. These proportions were multiplied by total HIV-1 DNA copies per 106 resting CD4+ T cells to yield an estimate of intact non-induced proviruses per 106 cells.
Supplementary Material
Highlights.
11.7% of non-induced HIV-1 proviruses have intact genomes and LTR function
Reconstructed intact non-induced proviruses are replication-competent
They are integrated into transcription units and have no CpG methylation in the LTR
The size of the latent reservoir for HIV-1 may be underestimated by ∼60 fold
Acknowledgments
We thank all study participants. We thank S. Alireza Rabi, Gregory Laird, and Drs. Stuart Ray and Joel Pomerantz for critical advice, Linda Alston for patient recruitment, David Walker for advice on CpG methylation, and Dr. Gautam Sahu for PCR suggestions. This work was supported by amfAR as part of the amfAR Research Collaboration on HIV Eradication (ARCHE), and also by the Martin Delaney CARE and DARE Collaboratories (NIH grants AI096113 and 1U19AI096109), by NIH grant AI043222, by the Johns Hopkins Center for AIDS Research, and by the Howard Hughes Medical Institute. Y.-H. H. is a Howard Hughes Medical Institute International Student Research Fellow.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Archin NM, Espeseth A, Parker D, Cheema M, Hazuda D, Margolis DM. Expression of latent HIV induced by the potent HDAC inhibitor suberoylanilide hydroxamic acid. AIDS Res Hum Retroviruses. 2009;25:207–212. doi: 10.1089/aid.2008.0191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archin NM, Liberty AL, Kashuba AD, Choudhary SK, Kuruc JD, Crooks AM, Parker DC, Anderson EM, Kearney MF, Strain MC, et al. Administration of vorinostat disrupts HIV-1 latency in patients on antiretroviral therapy. Nature. 2012;487:482–485. doi: 10.1038/nature11286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bebenek K, Abbotts J, Roberts JD, Wilson SH, Kunkel TA. Specificity and mechanism of error-prone replication by human immunodeficiency virus-1 reverse transcriptase. J Biol Chem. 1989;264:16948–16956. [PubMed] [Google Scholar]
- Blazkova J, Murray D, Justement JS, Funk EK, Nelson AK, Moir S, Chun TW, Fauci AS. Paucity of HIV DNA methylation in latently infected, resting CD4+ T cells from infected individuals receiving antiretroviral therapy. J Virol. 2012;86:5390–5392. doi: 10.1128/JVI.00040-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blazkova J, Trejbalova K, Gondois-Rey F, Halfon P, Philibert P, Guiguen A, Verdin E, Olive D, Van Lint C, Hejnar J, Hirsch I. CpG methylation controls reactivation of HIV from latency. PLoS Pathog. 2009;5:e1000554. doi: 10.1371/journal.ppat.1000554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohnlein E, Lowenthal JW, Siekevitz M, Ballard DW, Franza BR, Greene WC. The same inducible nuclear proteins regulates mitogen activation of both the interleukin-2 receptor-alpha gene and type 1 HIV. Cell. 1988;53:827–836. doi: 10.1016/0092-8674(88)90099-2. [DOI] [PubMed] [Google Scholar]
- Burnett JC, Miller-Jensen K, Shah PS, Arkin AP, Schaffer DV. Control of stochastic gene expression by host factors at the HIV promoter. PLoS Pathog. 2009;5:e1000260. doi: 10.1371/journal.ppat.1000260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakraborty AK, Das J. Pairing computation with experimentation: a powerful coupling for understanding T cell signalling. Nat Rev Immunol. 2010;10:59–71. doi: 10.1038/nri2688. [DOI] [PubMed] [Google Scholar]
- Chavez L, Kauder S, Verdin E. In vivo, in vitro, and in silico analysis of methylation of the HIV-1 provirus. Methods. 2011;53:47–53. doi: 10.1016/j.ymeth.2010.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun TW, Stuyver L, Mizell SB, Ehler LA, Mican JA, Baseler M, Lloyd AL, Nowak MA, Fauci AS. Presence of an inducible HIV-1 latent reservoir during highly active antiretroviral therapy. Proc Natl Acad Sci U S A. 1997;94:13193–13197. doi: 10.1073/pnas.94.24.13193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contreras X, Schweneker M, Chen CS, McCune JM, Deeks SG, Martin J, Peterlin BM. Suberoylanilide hydroxamic acid reactivates HIV from latently infected cells. J Biol Chem. 2009;284:6782–6789. doi: 10.1074/jbc.M807898200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coull JJ, Romerio F, Sun JM, Volker JL, Galvin KM, Davie JR, Shi Y, Hansen U, Margolis DM. The human factors YY1 and LSF repress the human immunodeficiency virus type 1 long terminal repeat via recruitment of histone deacetylase 1. J Virol. 2000;74:6790–6799. doi: 10.1128/jvi.74.15.6790-6799.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cujec TP, Okamoto H, Fujinaga K, Meyer J, Chamberlin H, Morgan DO, Peterlin BM. The HIV transactivator TAT binds to the CDK-activating kinase and activates the phosphorylation of the carboxy-terminal domain of RNA polymerase II. Genes Dev. 1997;11:2645–2657. doi: 10.1101/gad.11.20.2645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davey RT, Jr, Bhat N, Yoder C, Chun TW, Metcalf JA, Dewar R, Natarajan V, Lempicki RA, Adelsberger JW, Miller KD, et al. HIV-1 and T cell dynamics after interruption of highly active antiretroviral therapy (HAART) in patients with a history of sustained viral suppression. Proc Natl Acad Sci U S A. 1999;96:15109–15114. doi: 10.1073/pnas.96.26.15109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deeks SG. HIV: Shock and kill. Nature. 2012;487:439–440. doi: 10.1038/487439a. [DOI] [PubMed] [Google Scholar]
- Duh EJ, Maury WJ, Folks TM, Fauci AS, Rabson AB. Tumor necrosis factor alpha activates human immunodeficiency virus type 1 through induction of nuclear factor binding to the NF-kappa B sites in the long terminal repeat. Proc Natl Acad Sci U S A. 1989;86:5974–5978. doi: 10.1073/pnas.86.15.5974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durand CM, Ghiaur G, Siliciano JD, Rabi SA, Eisele EE, Salgado M, Shan L, Lai JF, Zhang H, Margolick J, et al. HIV-1 DNA is detected in bone marrow populations containing CD4+ T cells but is not found in purified CD34+ hematopoietic progenitor cells in most patients on antiretroviral therapy. J Infect Dis. 2012;205:1014–1018. doi: 10.1093/infdis/jir884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson S, Graf EH, Dahl V, Strain MC, Yukl SA, Lysenko ES, Bosch RJ, Lai J, Chioma S, Emad F, et al. Comparative analysis of measures of viral reservoirs in HIV-1 eradication studies. PLoS Pathog. 2013;9:e1003174. doi: 10.1371/journal.ppat.1003174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finzi D, Hermankova M, Pierson T, Carruth LM, Buck C, Chaisson RE, Quinn TC, Chadwick K, Margolick J, Brookmeyer R, et al. Identification of a reservoir for HIV-1 in patients on highly active antiretroviral therapy. Science. 1997;278:1295–1300. doi: 10.1126/science.278.5341.1295. [DOI] [PubMed] [Google Scholar]
- Ganesh L, Burstein E, Guha-Niyogi A, Louder MK, Mascola JR, Klomp LW, Wijmenga C, Duckett CS, Nabel GJ. The gene product Murr1 restricts HIV-1 replication in resting CD4+ lymphocytes. Nature. 2003;426:853–857. doi: 10.1038/nature02171. [DOI] [PubMed] [Google Scholar]
- Han Y, Lassen K, Monie D, Sedaghat AR, Shimoji S, Liu X, Pierson TC, Margolick JB, Siliciano RF, Siliciano JD. Resting CD4+ T cells from human immunodeficiency virus type 1 (HIV-1)-infected individuals carry integrated HIV-1 genomes within actively transcribed host genes. J Virol. 2004;78:6122–6133. doi: 10.1128/JVI.78.12.6122-6133.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Y, Lin YB, An W, Xu J, Yang HC, O'Connell K, Dordai D, Boeke JD, Siliciano JD, Siliciano RF. Orientation-dependent regulation of integrated HIV-1 expression by host gene transcriptional readthrough. Cell Host Microbe. 2008;4:134–146. doi: 10.1016/j.chom.2008.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He G, Margolis DM. Counterregulation of chromatin deacetylation and histone deacetylase occupancy at the integrated promoter of human immunodeficiency virus type 1 (HIV-1) by the HIV-1 repressor YY1 and HIV-1 activator Tat. Mol Cell Biol. 2002;22:2965–2973. doi: 10.1128/MCB.22.9.2965-2973.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermankova M, Siliciano JD, Zhou Y, Monie D, Chadwick K, Margolick JB, Quinn TC, Siliciano RF. Analysis of human immunodeficiency virus type 1 gene expression in latently infected resting CD4+ T lymphocytes in vivo. J Virol. 2003;77:7383–7392. doi: 10.1128/JVI.77.13.7383-7392.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann CH, Rice AP. Lentivirus Tat proteins specifically associate with a cellular protein kinase, TAK, that hyperphosphorylates the carboxyl-terminal domain of the large subunit of RNA polymerase II: candidate for a Tat cofactor. J Virol. 1995;69:1612–1620. doi: 10.1128/jvi.69.3.1612-1620.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones KA, Peterlin BM. Control of RNA initiation and elongation at the HIV-1 promoter. Annu Rev Biochem. 1994;63:717–743. doi: 10.1146/annurev.bi.63.070194.003441. [DOI] [PubMed] [Google Scholar]
- Jordan A, Bisgrove D, Verdin E. HIV reproducibly establishes a latent infection after acute infection of T cells in vitro. EMBO J. 2003;22:1868–1877. doi: 10.1093/emboj/cdg188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao SY, Calman AF, Luciw PA, Peterlin BM. Anti-termination of transcription within the long terminal repeat of HIV-1 by tat gene product. Nature. 1987;330:489–493. doi: 10.1038/330489a0. [DOI] [PubMed] [Google Scholar]
- Kauder SE, Bosque A, Lindqvist A, Planelles V, Verdin E. Epigenetic regulation of HIV-1 latency by cytosine methylation. PLoS Pathog. 2009;5:e1000495. doi: 10.1371/journal.ppat.1000495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita S, Su L, Amano M, Timmerman LA, Kaneshima H, Nolan GP. The T cell activation factor NF-ATc positively regulates HIV-1 replication and gene expression in T cells. Immunity. 1997;6:235–244. doi: 10.1016/s1074-7613(00)80326-x. [DOI] [PubMed] [Google Scholar]
- Laird GM, Eisele EE, Rabi SA, Lai J, Chioma S, Blankson JN, Siliciano JD, Siliciano RF. Rapid Quantification of the Latent Reservoir for HIV-1 Using a Viral Outgrowth Assay. PLoS Pathog. 2013;9:e1003398. doi: 10.1371/journal.ppat.1003398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lassen K, Han Y, Zhou Y, Siliciano J, Siliciano RF. The multifactorial nature of HIV-1 latency. Trends Mol Med. 2004;10:525–531. doi: 10.1016/j.molmed.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Lehrman G, Hogue IB, Palmer S, Jennings C, Spina CA, Wiegand A, Landay AL, Coombs RW, Richman DD, Mellors JW, et al. Depletion of latent HIV-1 infection in vivo: a proof-of-concept study. Lancet. 2005;366:549–555. doi: 10.1016/S0140-6736(05)67098-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenasi T, Contreras X, Peterlin BM. Transcriptional interference antagonizes proviral gene expression to promote HIV latency. Cell Host Microbe. 2008;4:123–133. doi: 10.1016/j.chom.2008.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B, Gladden AD, Altfeld M, Kaldor JM, Cooper DA, Kelleher AD, Allen TM. Rapid reversion of sequence polymorphisms dominates early human immunodeficiency virus type 1 evolution. J Virol. 2007;81:193–201. doi: 10.1128/JVI.01231-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malim MH, Hauber J, Le SY, Maizel JV, Cullen BR. The HIV-1 rev trans-activator acts through a structured target sequence to activate nuclear export of unspliced viral mRNA. Nature. 1989;338:254–257. doi: 10.1038/338254a0. [DOI] [PubMed] [Google Scholar]
- Nabel G, Baltimore D. An inducible transcription factor activates expression of human immunodeficiency virus in T cells. Nature. 1987;326:711–713. doi: 10.1038/326711a0. [DOI] [PubMed] [Google Scholar]
- Patel SS, Duby AD, Thiele DL, Lipsky PE. Phenotypic and functional characterization of human T cell clones. J Immunol. 1988;141:3726–3736. [PubMed] [Google Scholar]
- Richman DD, Margolis DM, Delaney M, Greene WC, Hazuda D, Pomerantz RJ. The challenge of finding a cure for HIV infection. Science. 2009;323:1304–1307. doi: 10.1126/science.1165706. [DOI] [PubMed] [Google Scholar]
- Rose PP, Korber BT. Detecting hypermutations in viral sequences with an emphasis on G → A hypermutation. Bioinformatics. 2000;16:400–401. doi: 10.1093/bioinformatics/16.4.400. [DOI] [PubMed] [Google Scholar]
- Sanchez G, Xu X, Chermann JC, Hirsch I. Accumulation of defective viral genomes in peripheral blood mononuclear cells of human immunodeficiency virus type 1-infected individuals. J Virol. 1997;71:2233–2240. doi: 10.1128/jvi.71.3.2233-2240.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroder AR, Shinn P, Chen H, Berry C, Ecker JR, Bushman F. HIV-1 integration in the human genome favors active genes and local hotspots. Cell. 2002;110:521–529. doi: 10.1016/s0092-8674(02)00864-4. [DOI] [PubMed] [Google Scholar]
- Selby MJ, Peterlin BM. Trans-activation by HIV-1 Tat via a heterologous RNA binding protein. Cell. 1990;62:769–776. doi: 10.1016/0092-8674(90)90121-t. [DOI] [PubMed] [Google Scholar]
- Shan L, Deng K, Shroff NS, Durand CM, Rabi SA, Yang HC, Zhang H, Margolick JB, Blankson JN, Siliciano RF. Stimulation of HIV-1-specific cytolytic T lymphocytes facilitates elimination of latent viral reservoir after virus reactivation. Immunity. 2012;36:491–501. doi: 10.1016/j.immuni.2012.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shan L, Yang HC, Rabi SA, Bravo HC, Shroff NS, Irizarry RA, Zhang H, Margolick JB, Siliciano JD, Siliciano RF. Influence of host gene transcription level and orientation on HIV-1 latency in a primary-cell model. J Virol. 2011;85:5384–5393. doi: 10.1128/JVI.02536-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siliciano JD, Kajdas J, Finzi D, Quinn TC, Chadwick K, Margolick JB, Kovacs C, Gange SJ, Siliciano RF. Long-term follow-up studies confirm the stability of the latent reservoir for HIV-1 in resting CD4+ T cells. Nat Med. 2003;9:727–728. doi: 10.1038/nm880. [DOI] [PubMed] [Google Scholar]
- Siliciano JD, Siliciano RF. Enhanced culture assay for detection and quantitation of latently infected, resting CD4+ T-cells carrying replication-competent virus in HIV-1-infected individuals. Methods Mol Biol. 2005;304:3–15. doi: 10.1385/1-59259-907-9:003. [DOI] [PubMed] [Google Scholar]
- Simon-Loriere E, Holmes EC. Why do RNA viruses recombine? Nat Rev Microbiol. 2011;9:617–626. doi: 10.1038/nrmicro2614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh A, Razooky B, Cox CD, Simpson ML, Weinberger LS. Transcriptional bursging from the HIV-1 promoter is a significant source of stochastic noise in HIV-1 gene expression. Biophys J. 2010;98:L32–34. doi: 10.1016/j.bpj.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spivak AM, Salgado M, Rabi SA, O'Connell KA, Blankson JN. Circulating monocytes are not a major reservoir of HIV-1 in elite suppressors. J Virol. 2011;85:10399–10403. doi: 10.1128/JVI.05409-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strain MC, Gunthard HF, Havlir DV, Ignacio CC, Smith DM, Leigh-Brown AJ, Macaranas TR, Lam RY, Daly OA, Fischer M, et al. Heterogeneous clearance rates of long-lived lymphocytes infected with HIV: intrinsic stability predicts lifelong persistence. Proc Natl Acad Sci U S A. 2003;100:4819–4824. doi: 10.1073/pnas.0736332100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temin HM. Retrovirus variation and reverse transcription: abnormal strand transfers result in retrovirus genetic variation. Proc Natl Acad Sci U S A. 1993;90:6900–6903. doi: 10.1073/pnas.90.15.6900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyagi M, Pearson RJ, Karn J. Establishment of HIV latency in primary CD4+ cells is due to epigenetic transcriptional silencing and P-TEFb restriction. J Virol. 2010;84:6425–6437. doi: 10.1128/JVI.01519-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Lint C, Emiliani S, Ott M, Verdin E. Transcriptional activation and chromatin remodeling of the HIV-1 promoter in response to histone acetylation. EMBO J. 1996;15:1112–1120. [PMC free article] [PubMed] [Google Scholar]
- Verdin E, Paras P, Jr, Van Lint C. Chromatin disruption in the promoter of human immunodeficiency virus type 1 during transcriptional activation. EMBO J. 1993;12:3249–3259. doi: 10.1002/j.1460-2075.1993.tb05994.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicenzi E, Dimitrov DS, Engelman A, Migone TS, Purcell DF, Leonard J, Englund G, Martin MA. An integration-defective U5 deletion mutant of human immunodeficiency virus type 1 reverts by eliminating additional long terminal repeat sequences. J Virol. 1994;68:7879–7890. doi: 10.1128/jvi.68.12.7879-7890.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberger LS, Burnett JC, Toettcher JE, Arkin AP, Schaffer DV. Stochastic gene expression in a lentiviral positive-feedback loop: HIV-1 Tat fluctuations drive phenotypic diversity. Cell. 2005;122:169–182. doi: 10.1016/j.cell.2005.06.006. [DOI] [PubMed] [Google Scholar]
- Weinberger LS, Dar RD, Simpson ML. Transient-mediated fate determination in a transcriptional circuit of HIV. Nat Genet. 2008;40:466–470. doi: 10.1038/ng.116. [DOI] [PubMed] [Google Scholar]
- West MJ, Lowe AD, Karn J. Activation of human immunodeficiency virus transcription in T cells revisited: NF-kappaB p65 stimulates transcriptional elongation. J Virol. 2001;75:8524–8537. doi: 10.1128/JVI.75.18.8524-8537.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams SA, Chen LF, Kwon H, Ruiz-Jarabo CM, Verdin E, Greene WC. NF-kappaB p50 promotes HIV latency through HDAC recruitment and repression of transcriptional initiation. EMBO J. 2006;25:139–149. doi: 10.1038/sj.emboj.7600900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams SA, Greene WC. Regulation of HIV-1 latency by T-cell activation. Cytokine. 2007;39:63–74. doi: 10.1016/j.cyto.2007.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong JK, Hezareh M, Gunthard HF, Havlir DV, Ignacio CC, Spina CA, Richman DD. Recovery of replication-competent HIV despite prolonged suppression of plasma viremia. Science. 1997;278:1291–1295. doi: 10.1126/science.278.5341.1291. [DOI] [PubMed] [Google Scholar]
- Yang HC, Shen L, Siliciano RF, Pomerantz JL. Isolation of a cellular factor that can reactivate latent HIV-1 without T cell activation. Proc Natl Acad Sci U S A. 2009;106:6321–6326. doi: 10.1073/pnas.0809536106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Q, Konig R, Pillai S, Chiles K, Kearney M, Palmer S, Richman D, Coffin JM, Landau NR. Single-strand specificity of APOBEC3G accounts for minus-strand deamination of the HIV genome. Nat Struct Mol Biol. 2004;11:435–442. doi: 10.1038/nsmb758. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.