Abstract
Intestinal disorders such as inflammatory bowel disease (IBD) result in chronic illness requiring lifelong therapy. Our aim was to evaluate the efficacy of recombinant adeno-associated virus (AAV) vector-mediated gene delivery to intestinal epithelial cells in vitro and in vivo. Human colon epithelial cell lines and colon biopsies were transduced using AAV pseudotypes 2/1, 2/2, and 2/5 encoding green fluorescence protein (GFP). Mice were administered the same vectors through oral, enema, intraperitoneal (IP) injection and superior mesenteric artery (SMA) injection routes. Tropism and efficiency were determined by microscopy, flow cytometry, immunohistochemistry and PCR. Caco2 cells were more permissive to AAV transduction. Human colon epithelial cells in organ culture were more effectively transduced by AAV2/2. SMA injection provided the most effective means of vector gene transfer to small intestine and colonic epithelial cells in vivo. Transgene detection 80 days post AAV treatment suggests transduction of crypt progenitor cells. This study shows the feasibility of AAV-mediated intestinal gene delivery, applicable for the investigation of IBD pathogenesis and novel therapeutic options, but also revealed the need for further studies to identify more efficient pseudotypes.
Keywords: Adeno-associated virus, Colon, Small intestine, Gene delivery, Epithelial, Green fluorescence protein
Introduction
Gene transfer, widely studied for the potential treatment of monogenetic diseases, has also shown progress in the therapy of auto-immunity, malignancy and chronic inflammatory disease. Although in its infancy, transgene products such as proteins and antisense RNA that intently down-regulate pathogenic responses or induce protective immune responses are becoming a reality [1]. The difficulty in treating chronic inflammatory intestinal disorders such inflammatory bowel disease (IBD) warrant the investigation of biological therapy within the gastrointestinal tract via localized gene delivery and endogenous protein expression [2–4]. Additionally, recent research has identified genetic mutations in IBD patients [5], which further supports the need to pursue gene transfer research in the intestine.
Transduction of the intestinal tract via liposomal [6], retroviral [7], lentiviral [8], herpes simplex viral [9] and adenoviral [10, 11] vector systems has been previously demonstrated. Limited success has been achieved with these modalities either due to short duration of transgene expression or adverse host immune responses [12]. The discovery of single-stranded DNA adeno-associated virus (AAV) [13, 14] has led to the development of recombinant AAV vectors [15, 16]. The AAV vector is attractive for gene transfer as it lacks all viral genes, is nonreplicative, and is able to transduce both dividing and quiescent cells for sustained transgene expression [17, 18]. Furthermore, AAVs are associated with limited cell-mediated immune response, especially when compared to adenoviral vectors [19].
To date, only two publications have described successful AAV transduction of the intestinal epithelium. During and colleagues [20] demonstrated transgene expression in the stomach, duodenum and proximal jejunum following oral delivery. Shao and colleagues [21] described gastric and small bowel transduction with AAV2, but no protein expression, following oral delivery. However, no study has yet examined AAV-mediated colonic epithelium transduction or AAV type 1 and 5 intestinal tropism. Pseudotypes AAV1 and 5 are constructed using their respective capsids to encapsidate a recombinant AAV2-based vector genome and are referred to as AAV2/1 and 2/5, respectively. The purpose of the present study was to determine AAV2/1, 2/2, and 2/5 pseudotype tropism and transduction efficiency characteristics in human colon cancer cell lines, human colon epithelial cells from biopsies in organ culture, and mouse small intestine and colon in vivo. As vector administration route could affect transduction efficiency, we assessed different routes of delivery via oral, enema, intraperitoneal (IP) injection and superior mesenteric artery (SMA) injection for each AAV pseudotype.
Methods
Preparation of recombinant AAV-GFP vectors
Recombinant AAV vectors (AAV) were obtained from the Powell Gene Therapy Center at the University of Florida (Gainesville, FL). The AAV2/2-green fluorescence protein (GFP) vector genome contains a “humanized” enhanced GFP as the transgene and is driven by a chicken beta-actin promoter with a CMV enhancer [22]. Production of AAV2/2 and recombinant pseudotyped vectors AAV2/1 and AAV2/5 were performed according to methods previously described [23, 24]. Physical AAV particles were quantified by dot blot analysis.
Colon epithelial cell line transduction
Colon-derived human epithelial cell lines used: HT29 (ATCC HTB-38), T84 (ATCC CCL-248), and Caco2 (ATCC HTB-37). See supplemental materials and methods for detailed cell culture information. Samples of 1 × 105 non-polarized cells were treated with AAV-GFP at a multiplicity of infection (MOI) of 100 or 1,000 DNase-resistant physical particles, alone or in co-infection with wild type (wt) AdV5 co-infection (MOI 1). Control treatments were performed simultaneously using phosphate-buffered saline (PBS) or wt AdV5 (MOI 1). Transductions were carried out at cell growth times of 24, 48 and 96 h alongside controls and performed at least in four separate experiments. For long-term evaluation of transgene expression, 5 × 105 HT29 cells were transduced with AAV2/2 (particle MOI 10,000), without AdV co-infection, passaged in standard culture protocol and assessed for GFP expression via flow cytometry every 6–8 weeks as described below. GFP fluorescence in living cells was observed with a Leica DM ILT fluorescence microscope (Leica Microsystems; Wetzlar, Germany) or an MRC 1024 ES Confocal Laser Scanning Microscope (Bio Rad; Hercules, CA). Images were captured with a Spot RT color camera using Spot software version 3.4 (Diagnostic Instruments; Sterling Heights, MI) or Laser Sharp software (Bio Rad), respectively.
Flow cytometric analysis
Cells were fixed in sterile filtered 1% paraformaldehyde (pH 7.4) and analyzed within 2 h. GFP-positive cells were counted using a FACSort flow cytometer with an argon laser tuned to 488 nm (Becton Dickinson, Franklin Lakes, NJ) within the ICBR Flow Cytometry Core at the University of Florida (Gainesville, FL). See supplemental materials and methods for detailed flow cytometer settings. Three flow analysis parallels were performed for every experiment.
Human biopsy culture and transduction
Colon mucosal biopsies were obtained from adults undergoing routine investigative colonoscopy after obtaining informed consent. Tissue was obtained in accordance with a protocol approved by the University of Florida Institutional Review Board. Biopsies were taken from macroscopically normal appearing mucosa, from a localized area in the transverse colon in all subjects. Immediately, specimens were washed in warmed RPMI 1640 medium (Cellgro by Mediatech; Herndon, VA), 0.1 mM dithiothreitol (Invitrogen; Carlsbad, CA), and 1× PBS; then placed mucosal side up in individual wells of tissue culture plates (Becton Dickinson) containing 100 μl RPMI 1640 containing (10% FBS, 4.0 mM l-glutamine, 0.1 unit/ml bovine insulin, 1% ABAM).
Each specimen was treated with 5 × 103 particles of AAV2/1, 2/2 or 2/5 encoding GFP, co-infected with wt AdV5 (100 particles) and incubated up to 36 h at 37°C (5% CO2). A 100 μl aliquot of fresh medium was added to each specimen every 8–12 h. Formalin/alcohol fixation reduced sensitivity and intensity of GFP fluorescence, therefore transduction was evaluated on frozen sections, as described in detail in the online supplementary material. Slides were viewed on an Axioskop microscope (Zeiss, Germany) with a GFP filter (Chroma, 41028) and representative digital images taken with an AxioCam color camera. Auto-fluorescence was evaluated in the same field with a rhodamine filter (Zeiss Filter set 14, 510–560/590).
Mice and AAV delivery
Male BALB/c mice (5 weeks old) obtained from Jackson Laboratory (Bar Harbor, ME) were housed in standard conditions at the Animal Care Services Facility, University of Florida, fed normal rodent chow and provided water ad libitum. All procedures and handling of mice were performed in accordance with the Institutional Animal Care and Use Committees and Institutional Biosafety Committees of the University of Florida. Samples of 1 × 1011 particles of AAV2/1, 2/2 or 2/5 encoding GFP in 0.1 ml PBS were delivered by a single injection via oral gavage, enema, IP injection or SMA injection. In the oral gavage and enema groups, a sterile 24G × 1.5 in (38 mm) × 1.25 mm bulb tip needle (Roboz, Rockville, MD) was inserted into the stomach or transanally, respectively. In the IP injection group, mice were injected in the left lower midline with a 28 gauge needle and syringe. In the SMA injection group, mice were anesthetized with 2% inhaled isoflurane. A midline incision was made, the small intestine partially exteriorized, and the SMA isolated. Under a Wild M32 surgical microscope (Wild Heerbrugg, Heerbrugg, Switzerland) a microvascular clamp was placed on the proximal SMA and a 33 gauge needle fitted on a gas tight syringe (Hamilton, Reno, NV) was used to cannulate the SMA. After injection, residual bleeding was treated with direct pressure. This operative procedure was found to carry a mortality risk of 11% as one mouse died 20 days after the procedure.
Three animals were treated per AAV pseudotype per route of delivery. A single sham-treated animal per route of delivery was administered 0.1 ml sterile PBS. Necropsy was performed 80 days after treatment and the small intestine and colon were removed, using sterile scalpels between organs and animals to prevent contamination, fixed in 10% neutral buffered formalin and embedded in paraffin. Formalin fixation significantly reduced direct GFP fluorescence; therefore GFP expression was evaluated by immunohistochemistry (IHC).
GFP IHC
IHC was performed by the Molecular Pathology Core, Department of Pathology, Immunology and Laboratory Medicine, University of Florida as described in detail in the supplemental materials and methods. Serial sections were incubated with rabbit anti-GFP, 1:800, (Abcam, Cambridge, MA) or normal goat IgG (negative control), then with a biotinylated goat anti-rabbit secondary followed by streptavidin-biotin-peroxidase complex technique, Vectastain Elite (Vector Laboratories, Burlingame, CA) with diamino-benzidine (DAB) as the chromagen.
Routine and real-time PCR
Three 10 mm sections were cut from each block with a sterile microtome and de-paraffinized with Hemo-D (Fisher, Pittsburg, PA). DNA was isolated using a QIAamp DNA Micro kit (Qiagen, Valencia, CA). RNA was isolated using the Optimum FFPE RNA isolation kit (Ambion, Austin, TX). Samples of 10 μl of purified DNA were used per 25 μl standard PCR reaction with 1× buffer, 50 mM MgCl2, 0.2 mM dNTP and 1 U Platinum Taq (Invitrogen, Carlsbad, CA); 200 nM GFP primer or 200 nM mouse beta-actin primers (Invitrogen, Carlsbad, CA) were used (see supplemental materials for primer sequences). PCR of 30 cycles using a 62–57°C touchdown protocol was performed. Negative control DNA was from the corresponding sham-treated animal tissue. Positive control DNA was sham-treated DNA spiked with pTR-UF11 GFP plasmid (0.1 pg/ml). PCR products were analyzed on 2% agarose gels.
For quantitative real-time PCR, each reaction had a final concentration of 200 nM GFP or beta-actin primer and 1× SYBR Green (Bio-Rad, Richmond, CA); plus 5 μl DNA isolated from each triplicate treatment group. ABI SDS 7000 (Applied Biosystems, Foster City, CA) program: 10 min at 95°C, 40 cycles of 30 s at 95°C, 1 min at 58°C, and 1 min at 72°C. Each sample was run in triplicate simultaneously alongside beta-actin and the average CT (cycle threshold) was determined for each sample. Replicates of each animal group were performed on two separate days. Since DNA samples were compared by equal volumes and not by concentration, mouse beta-actin was used as the reference gene to normalize the GFP DNA and allow comparison between groups (see the supplemental material for the specific calculations). DNA copy numbers were extrapolated from a standard curve prepared as outlined in the ABI SDS 7000 bulletin, by serial dilution from 200 pg/μl or 2.5 × 107 copies to 0.012 pg/μl or 1,547 copies. See supplemental materials for the DNA copy number equation and the standard curve equation.
Statistical analysis
One way analysis of variances with Scheffe post hoc testing (Analyse-it v1.73, Leeds, UK) was used to analyze flow cytometric data. Animal treatment results did not have a normal distribution, therefore the Kruskal-Wallis test with Conover post hoc analysis (Analyse-it v1.73, Leeds, UK) was used to analyze quantitative PCR results. An α equal to a 0.05 level of significance was used for all tests.
Results
AAV tropism in human colon epithelial cells is cell line dependent
Three transformed, colon-derived human epithelial cell lines, HT29, T84 and Caco2, were successfully transduced with AAV2/1, 2/2 and 2/5 encoding GFP, although at lower efficiency as compared to AAV preferred host cell lines such as A293 and Cos7. Due to limitations of time in cell culture and lack of in vivo mechanisms, experiments were also performed using wt AdV5 coinfection, which has become a conventional method to hasten transduction in vitro [25]. Undifferentiated or non-polarized cells were used in all experiments. Transduction was verified by observing live cellular green fluorescence. The greatest number of GFP-positive cells was seen with wt AdV co-infection, 48–96 h after treatment (Fig. 1A). Transgene expression was quantified using flow cytometric analysis, which allowed for only 0.5 % background green fluorescence (auto-fluorescence).
Fig. 1a–c.
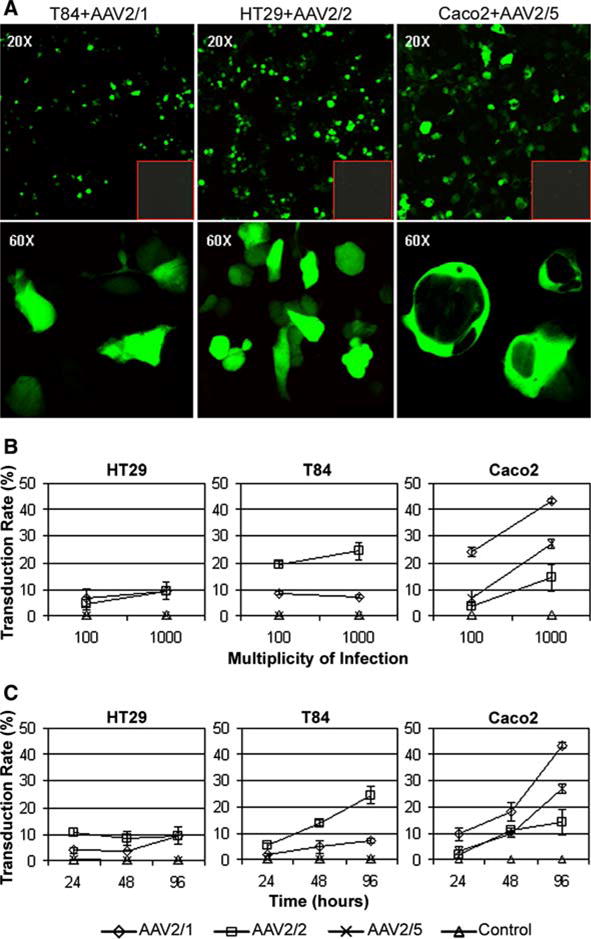
Adeno-associated virus (AAV)-GFP transduced human colonic epithelial cell lines. a Representative confocal laser scanning microscopy images of live cells in culture expressing cytoplasmic GFP, 96 h after transduction with AAV-GFP at a particle multiplicity of infection (MOI) of 1,000, shown at 20× and 60× magnification. Red outlined insets Simultaneous negative control transductions. b, c Variation of dose curve and time course between cell lines. Cells were transduced with a particle MOI of 100 or 1,000 of AAV2/1, 2/2 & 2/5 encoding GFP. The percentage of GFP-positive cells (transduction rate) was quantified 96 h after transduction using flow cytometry (b). The transduction rate of cells transduced with MOI 1,000 was determined at 24, 48 and 96 h with each pseudotype (c). Data are presented as mean +/– SD of three separate experiments
In general, both increased vector concentration and time in cell culture after transduction improved transduction efficiency (Fig. 1b,c). Only HT29 and T84 cells treated with AAV2/5 failed to exhibit better transduction efficiencies at an MOI of 1,000 (Fig. 1b). Longer post treatment culture time showed better transduction rates in HT29 cells treated with AAV2/1, T84 cells treated with AAV2/1 and AAV2/2, and Caco2 cells treated with all pseudotypes (Fig. 1c). HT29 and T84 cells were poorly transduced by AAV2/5. Pseudotype tropism was determined for each cell line with and without AdV5 coinfection using AAV MOI 1,000 and 96 h cell culture time. AdV5 coinfection improved transduction efficiency by nearly four fold in each cell line; however, the transduction rate pattern remained essentially identical when compared to that obtained with AAV alone (Fig. 2a,b). HT29 cells were best transduced by AAV2/2, but with low mean efficiency (3.3%), T84 cells were also best transduced with AAV2/2 (5.2%) and Caco2 cells best transduced with AAV2/1 (11.6%) (Fig. 2a). AAV2/5 could transduce only Caco2 cells, with a mean efficiency of 4.9% and 27.0% without and with AdV5 coinfection, respectively (Fig. 2a,b). Overall, undifferentiated Caco2 cells demonstrated the best transduction efficiencies.
Fig. 2a,b.
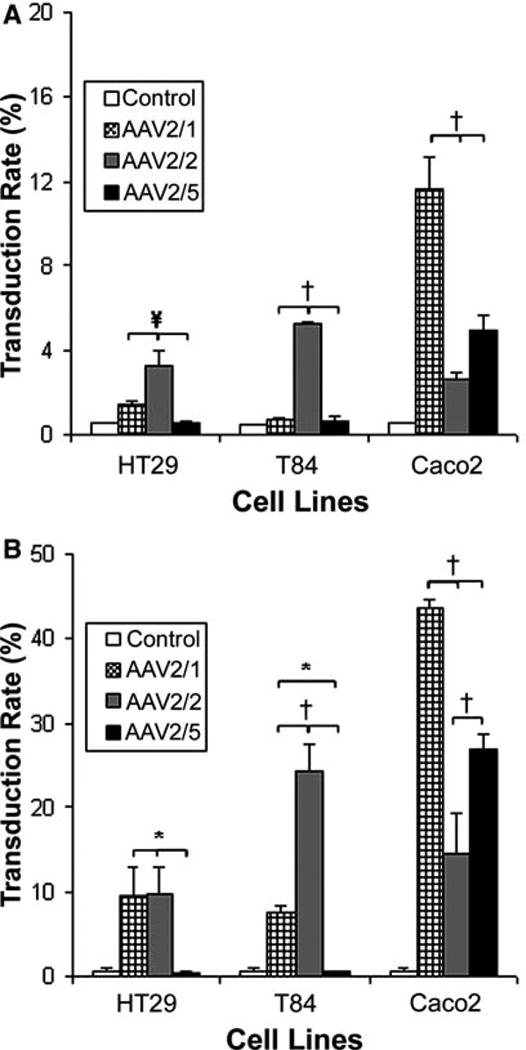
AAV pseudotype transduction efficiency in vitro.Cells were transduced with AAV2/1, 2/2 or 2/5 (MOI 1,000) encoding GFP, without wild type (wt) AdV coinfection (a), and compared to transduction efficiencies with wt AdV coinfection (b) initially depicted in Fig. 1c. The percentage of GFP-expressing cells was quantified by flow cytometry 96 h after transduction. Caco2 cells were most permissive to AAV2/1 and 2/5 transduction, while HT29 and T84 cells were most permissive to AAV2/2 transduction. Data are presented as mean +/– SD from four separate experiments. *P < 0.02, †P < 0.001, ¥P < 0.005
HT29 cells can be readily cultured for multiple passages and were transduced with AAV2/2 encoding GFP and passaged in culture for 52 weeks. Cellular GFP expression was followed by flow cytometric analysis every 6–8 weeks. The percentage of GFP-positive cells dropped by only 6% from week 3 to week 52. This degree of sustained transgene expression in a colonic epithelial cell line could translate to long-term transgene expression in vivo.
Human colon epithelial cells can be transduced in organ culture
Human colon biopsy specimens from normal patients were transduced in organ culture with AAV2/1, 2/2 and 2/5 encoding GFP. Due to time limitations in organ culture, wt AdV5 coinfection was used in each experiment. GFP-positive intestinal epithelial cells were identified on multiple sections on both the superficial and basal regions of the glands (Fig. 3). Transduction efficiency varied amongst four subjects, but on average three positive colonic intestinal epithelial cells were seen per cut of tissue using AAV2/2. In contrast, only rare GFP positive cells were seen after AAV2/1 and 2/5 treatments.
Fig. 3a–d.
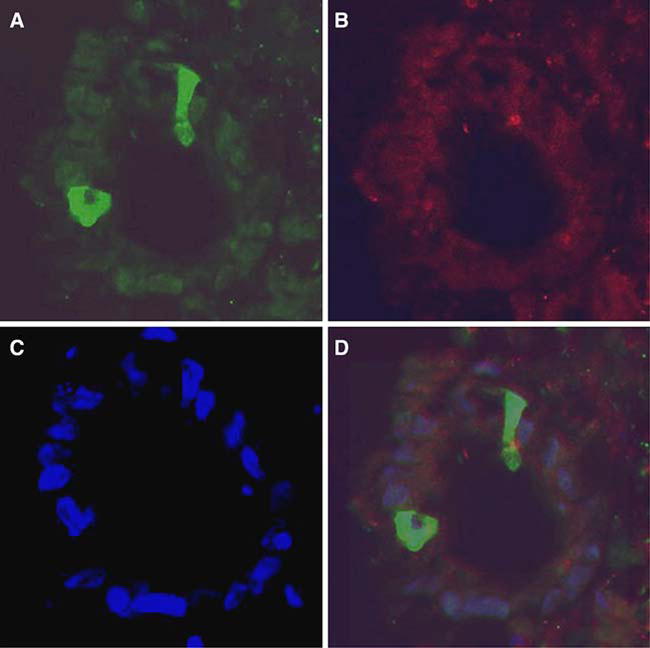
Transduction of human colon biopsy tissue in organ culture with AAV2/2 encoding GFP and wt AdV coinfection. After frozen sectioning, GFP-positive epithelial cells are seen on cross-section of a villous at 40× magnification (a). The same image is seen under a rhodamine filter (b) for auto-fluorescence and a DAPI filter (c) for nuclear staining. Nonspecific or yellow staining was not seen in the overlay image (d)
Murine small intestine and colon tropism
To determine intestinal tropism and efficiency in vivo, 5-week-old male BALB/c mice were treated with the three AAV pseudotypes encoding GFP. Vector was administered via oral gavage, enema, IP injection or SMA injection in three mice per pseudotype. IHC was used to demonstrate the presence and location of cells expressing GFP, with positive cells demonstrating diffuse cytoplasmic staining.
Staining for GFP was not seen at all in the orally treated mice with any of the pseudotypes used. A few rarely scattered epithelial cells were seen in only one AAV1/2 enema-treated mouse. Scattered areas of GFP staining were seen in the smooth muscle and serosa of IP-treated mice, but no epithelial staining was seen. A low level of GFP expression was seen in the intestine of the SMA injected mice, with more frequent staining seen in AAV2/2-treated animals. In the SMA group, GFP-positive intestinal epithelial cells were seen more clearly in the proximal colon including the crypt or gland cells (Fig. 4). Significant staining in the lamina propria was not observed. A low level of GFP expression was seen in the small intestine, but this required higher concentrations of anti-GFP antibody that resulted in higher background, subsequently reducing the confidence of the IHC. Overall, staining was infrequent and patchy in the colon and present at a low level in the small intestine of SMA-injected animals. Hematoxylin and eosin staining of the small intestine and colon did not reveal inflammatory infiltrates or architectural distortion in the areas surrounding the transduced epithelial cells.
Fig. 4a–d.
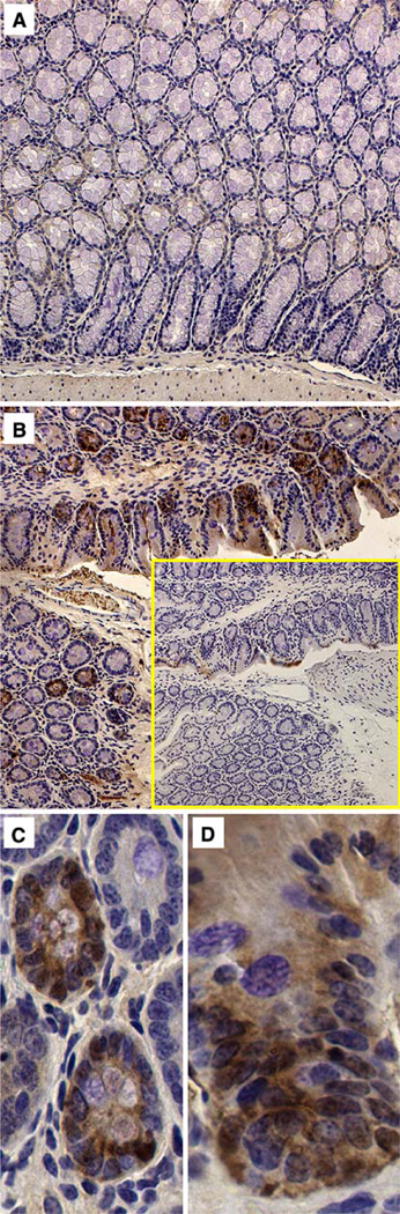
GFP immunohistochemistry (IHC) in representative sections of colon from vector-treated mice taken 80 days following superior mesenteric artery (SMA) injection of 1 × 1011 particles of AAV2/2 encoding GFP. Diffuse brown diamino-benzidine (DAB) cytoplasmic staining indicates the presence of GFP. Sham (a) is compared to vector-treated tissue (b) and staining negative control of adjacent tissue (b inset) at 20× magnification. Epithelial and crypt staining was seen in the colon at 40×of AAV2/2 treated mice (c, d)
Gene transfer efficiency of pseudotypes in murine small intestine and colon
The presence of GFP DNA in the intestines of mice from all treatment groups was initially screened by standard PCR. Pooled DNA isolated from the fixed small intestine and colon of each treated animal was screened by amplification of vector-derived GFP DNA (Fig. 5a). Multiple GFP bands or positive animals were noted in the SMA-treated group. Only one of three AAV2/1 mice had a clear amplified band in the enema-treated group. Five mice in the IP-injected group were positive. In agreement with the IHC, GFP DNA was not found in the orally treated animals. Therefore, further quantitative analysis was not performed in the oral treatment group.
Fig. 5a–c.
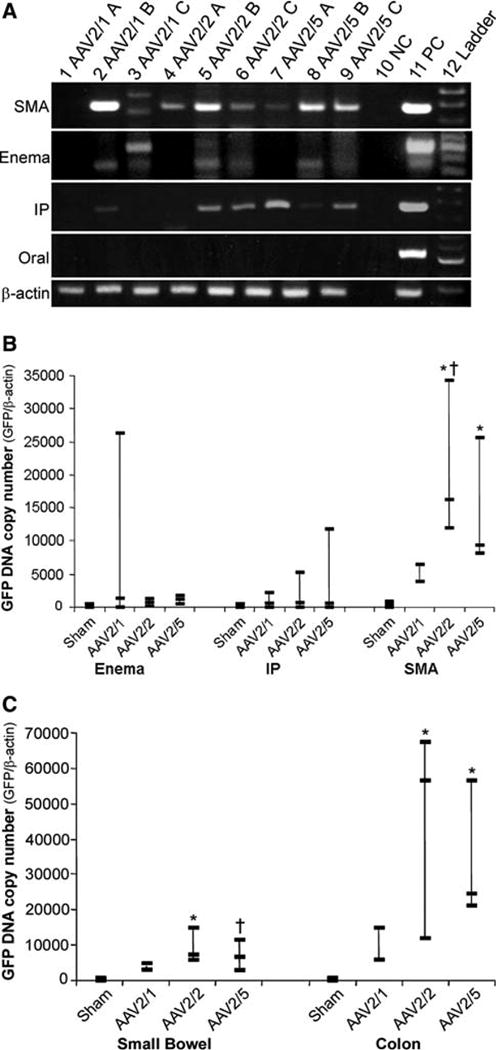
Detection and quantification of vector genomes. Tissue from animal treatment groups was initially screened for the presence of the GFP transgene by PCR. Pooled DNA extracted from random sections of both small intestine and colon was amplified for GFP by standard PCR. Products were examined on a 2% agarose gel where a GFP band (452 bp) could be visualized (a). Controls were run with each PCR experiment using DNA spiked with GFP DNA (PC) and DNA from sham-treated animals (NC). Quality of DNA used for PCR was checked by PCR amplification of mouse beta-actin (246 bp). Water was used in the beta-actin negative control PCR reaction. Gene transfer efficiency was compared between the three most positive treatment groups by determining GFP DNA copy number from pooled small intestine and colon DNA for each animal (b). SMA injection demonstrated better gene transfer efficiency as compared to other routes of administration. AAV2/2 showed higher copy numbers as compared to sham (*P < 0.005) and AAV2/1 (†P < 0.01) (b). AAV2/5 also revealed higher copy number compared to sham (*P = 0.005) (b). Further analysis of the SMA treatment group revealed a greater degree of gene transfer to the colon compared to the small intestine (c). AAV2/2 and 2/5 transduced the colon and the small bowel effectively as compared to sham (*P < 0.008, †P < 0.05) (c). Three animals were used per vector in each treatment group except the SMA AAV2/1 group which had only two animals
GFP DNA copy number was determined by quantitative real-time PCR using DNA isolated from the small intestine and colon of triplicate animals. Comparison of GFP DNA among treatment groups showed that the SMA injection route of vector delivery was more efficient than IP injection and enema treatment (Fig. 5b). In the enema treatment group, only one mouse treated with AAV2/1 had a demonstrable GFP copy number. There were measurable GFP copy numbers in the IP treatment group with each pseudotype, but none were statistically significant versus the control. AAV2/2 and AAV2/5 showed significant efficiency of gene transfer in the SMA treatment group compared to the control (Fig. 5b). Interestingly, when DNA from the small intestine and colon of the SMA injection group was analyzed separately the gene transfer efficiency was higher in the colon (Fig. 5c). AAV2/1 had higher copy numbers of DNA compared to sham, but the difference was not statistically significant. AAV2/2 and 2/5 were most efficient in the small intestine and colon as compared to control (cf. Fig. 5b and c).
Discussion
The favorable properties of AAV vectors have permitted its entry into human clinical studies for diseases such as cystic fibrosis [26, 27], Canavan disease [28] and hemophilia B [29]. The luminal gastrointestinal tract, as a target for gene transfer, could provide a therapeutic strategy and aid research to help decipher the pathogenesis of a variety of epithelium-derived diseases such as IBDs, hemochromatosis and intestinal cancers. The potential for the application of gene therapy in diseases related to the gastrointestinal tract—especially the colon—has been the subject of several recent reviews [1, 30, 31]. The results of this project demonstrate that colonic epithelial cells are susceptible to in vitro and ex vivo transduction with AAV pseudotypes 2/1, 2/1 and 2/5. Similar to previous studies [32], HT29 and T84 cells were poorly transduced by AAV. However, non-polarized Caco2 cells provide a good intestinal cell type for AAV2/1, 2/2 and 2/5 transduction. Additionally, AAV2/5 could transduce only Caco2 cells that express 2,3-linked sialic acid epitopes (S. Polyak, unpublished data), a cellular receptor for AAV5 [33], which may be reduced or missing on T84 and HT29 cells. The variability in pseudotype tropism observed among cancer cell lines may be due to their cell surface protein heterogeneity [34] or deficient transduction/translational machinery. To improve transduction efficiency in vitro without wt AdV coinfection we found that a higher concentration of vector is required; an expensive practice at this time. We acknowledge that use of wt AdV may not simulate in vivo transduction with AAV, but the transduction pattern was similar with and without coinfection in vitro (Fig. 2).
Organ culture allowed us to test gene delivery potential in non-transformed intestinal epithelial cells, but was limited by a reduced post transduction culture time. Time is limited to 36–48 h in culture before autolysis inhibits GFP analysis, which may not provide enough time to fully evaluate AAV2/1 and 2/5. In an attempt to overcome time limitations we used wt AdV coinfection, a potential weakness in pseudotype evaluations ex vivo. The use of newly developed self-complementary AAV vectors may provide more insight into vector tropism in future studies [35]. However, we can speculate that if AAV2/2 shows tropism for colonic epithelial cells in animal models in vivo, it could also be used for gene delivery to human colonic mucosa.
The sustained long-term transgene expression seen in HT29 cells after AAV2/2 transduction supported our in vivo findings. Presence of GFP in the intestine of mice sacrificed 80 days after transduction confirms the reputation of long-term AAV-delivered transgene expression [36, 37]. Without evaluation at an earlier time point, our data may underestimate the initial gene transfer efficiency in the intestine due to villous epithelial cell turnover. However, our goals are to identify pseudotypes that will provide sustained intestinal transgene expression. Only rare lamina propria cells were positive for GFP, but from this work it is unknown if more or other nonepithelial lamina propria cell types were transduced early after transduction. Considering the known turnover physiology of villous intestinal epithelial cells, the presence of GFP-positive cells 80 days after transduction suggests delivery to crypt stem cells.
The low level of GFP expression in the intestine did not allow for quantitative evaluation of transduction efficiency through IHC. A weakness of our study was the determination of efficiency using GFP DNA rather than RNA. Our attempts to isolate RNA from the fixed tissues resulted in poor quality RNA preparations and unreliable RT-PCR results. From our PCR data, we can make conclusions regarding the efficiency of DNA delivery, but not on GFP protein expression. Future work in the intestine will require fresh tissue isolation for RNA or protein work.
As anticipated, the route of delivery influenced transduction and gene transfer efficiencies [38]. Oral gavage and enema utilized an apical or luminal approach, and IP and SMA injection (two different vascular routes) utilized a basolateral approach. We compared the IP and SMA routes since IP injection offers access to the systemic circulation through absorption into the vascular peritoneal lining and is technically easier than SMA injection. Luminal delivery was ineffective in transducing the epithelium as demonstrated by IHC and PCR. The actual sensitivity of our PCR reaction was not determined and it is feasible that a very low level of gene transfer might not have been detected, but given that both IHC and PCR were negative it would likely not be physiologically relevant. Repeat PCR was performed separately at 40 cycles and still found to be negative (data not shown). Our findings are in agreement with those of Shao and colleagues [21], but are contradictory to the peroral results of During et al. [20]. Variations among our experiments that could have led to differing results included our use of mice rather than rats, and our use of GFP rather than beta-galactosidase as the delivered transgene. Additionally, During and colleagues selected older animals that developed lactose intolerance over time, not genetically lactase-deficient animals, thus adding the possibility of selecting animals with altered enterocytes or mucosal modifications that made them more susceptible to AAV infection. Previous gene transfer research has demonstrated the protective limitations of the luminal surface of the gastrointestinal tract. Extracellular barriers such as tight junctions, cell surface glycocalyces, mucus and enzymes may prevent vector entry [33, 39–41]. Additionally AAV capsid characteristics can also determine if apical or basolateral infection is more optimal [34, 42].
SMA injection proved to be the most promising route for intestinal transduction with these three pseudotypes. Sferra and colleagues also demonstrated intestinal transduction via the SMA, as a selective vascular route for vector delivery, using an adenoviral vector in a rat model [43]. Our work confirms that this method can also be applied to the murine model using AAV. The vascular or basolateral approach has previously been successful using AAV2/2 [37], and this also proved to be the optimal pseudotype in our experiments.
The discrepancy between gene transfer (PCR) and gene expression (IHC) for all pseudotypes may reflect the low sensitivity of our IHC for vector-delivered GFP, or rate-limiting steps in AAV-mediated transduction such as second strand synthesis [35]. In the IP injection group, the positive PCR results and the negative IHC staining could also have arisen from scattered vector transgene in fibrous tissues or muscle on the outer portions of the intestine and tissue contamination during resection. Even though AAV2/1 demonstrated a measurable GFP DNA copy number in the SMA treatment group, it failed to show statistically significant results over sham, likely due to the fact that one animal died in the AAV2/1 group, reducing the number of values. Further efforts were not made to treat and replace the animal as AAV2/2 and 2/5 were more efficient than AAV1/2.
Interestingly, the colon had a higher GFP copy number than the small intestine after SMA injection, which also correlated with the level of GFP expression seen. SMA injection can be considered a systemic approach with the possibility of variably transducing the vascular intestine. One might also speculate that the cellular concentration in the small intestine exceeds that of the colon, therefore diluting the vector gene transfer efficiency.
In conclusion, in vitro experimental techniques in Caco2 cells may provide the necessary pathways for testing AAV-delivered transgene effects in the intestine. AAV1/2, 2/2 and 2/5 tropism and efficiency in cell lines did not reflect tropism and efficiency in the intestine. This difference in pseudotype tropism between our in vitro results and our in vivo findings could reflect physiological and receptor variations between differentiated and undifferentiated cells. Overall, in vivo transduction of the epithelium was sporadic, as demonstrated by IHC, but represents the first step in transduction of an important target organ. A benefit of gastrointestinal tract transduction is the potential for crypt stem cell propagation of the transgene, which may prevent the need for repeat treatments. Future research is needed to identify new serotypes [44] that may provide more efficient intestinal transduction and to investigate the limiting factors in intestinal transduction through the use of self complementary AAV vectors [35].
Supplementary Material
Acknowledgments
We thank Melissa Chen for assistance with flow cytometry, and Samuel Wu and Linda Young for their statistical consultation. This work was supported by the NIH (T32 DK60443) and the Crohn's and Colitis Foundation of America Career Development Award to S.P.
Footnotes
Electronic supplementary material The online version of this article (doi: 10.1007/s10620-007-9991-1) contains supplementary material, which is available to authorized users.
Contributor Information
Steven Polyak, Email: steven.polyak@medicine.ufl.edu, Division of Gastroenterology, Department of Medicine, University of Florida, Gainesville, FL 32610, USA.
Cathryn Mah, Division of Cellular and Molecular Therapy, Department of Pediatrics, University of Florida, Gainesville, FL 32610, USA.
Stacy Porvasnik, Division of Pediatric Cardiology, Department of Pediatrics, Powell Gene Therapy Center, University of Florida, Gainesville, FL 32610, USA.
John-David Herlihy, Department of Neuroscience, University of Florida, Gainesville, FL 32610, USA.
Martha Campbell-Thompson, Department of Pathology, Immunology and Laboratory Medicine, University of Florida, Gainesville, FL 32610, USA.
Barry J. Byrne, Division of Pediatric Cardiology, Department of Pediatrics, Powell Gene Therapy Center, University of Florida, Gainesville, FL 32610, USA
John F. Valentine, Division of Gastroenterology, Department of Medicine, University of Florida, Gainesville, FL 32610, USA
References
- 1.Wirtz S, Neurath MF. Gene transfer approaches for the treatment of inflammatory bowel disease. Gene Ther. 2003;10:854–860. doi: 10.1038/sj.gt.3302013. [DOI] [PubMed] [Google Scholar]
- 2.Lindsay JO, Sandison A, Cohen P, Brennan FM, Hodgson HJ. IL-10 gene therapy is therapeutic for dextran sodium sulfate-induced murine colitis. Dig Dis Sci. 2003;52:363–369. doi: 10.1023/b:ddas.0000037830.22065.71. [DOI] [PubMed] [Google Scholar]
- 3.Lindsay J, van Montfrans C, Brennan F, van Deventer S, Drillenburg P, Hodgson H, te Velde A, Sol Rodriguez Pena M. IL-10 gene therapy prevents TNBS-induced colitis. Gene Ther. 2002;9:1715–1721. doi: 10.1038/sj.gt.3301841. [DOI] [PubMed] [Google Scholar]
- 4.Jobin C, Panja A, Hellerbrand C, Iimuro Y, Didonato J, Brenner DA, Sartor RB. Inhibition of proinflammatory molecule production by adenovirus-mediated expression of a nuclear factor kappa B super-repressor in human intestinal epithelial cells. J Immunol. 1998;160:410–418. [PubMed] [Google Scholar]
- 5.Vermeire S, Rutgeerts P. Current status of genetics research in inflammatory bowel disease. Genes Immun. 2005;6:637–645. doi: 10.1038/sj.gene.6364257. [DOI] [PubMed] [Google Scholar]
- 6.Westbrook CA, Chmura SJ, Arenas RB, Kim SY, Otto G. Human APC gene expression in rodent colonic epithelium in vivo using liposomal gene delivery. Hum Mol Genet. 1994;3:2005–2010. doi: 10.1093/hmg/3.11.2005. [DOI] [PubMed] [Google Scholar]
- 7.Li M, Lonial H, Citarella R, Lindh D, Colina L, Kramer R. Tumor inhibitory activity of anti-ras ribozymes delivered by retroviral gene transfer. Cancer Gene Ther. 1996;3:221–229. [PubMed] [Google Scholar]
- 8.Seppen J, Barry SC, Klinkspoor JH, Katen LJ, Lee SP, Garcia JV, Osborne WR. Apical gene transfer into quiescent human and canine polarized intestinal epithelial cells by lentivirus vectors. J Virol. 2000;74:7642–7645. doi: 10.1128/jvi.74.16.7642-7645.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Varghese S, Rabkin SD. Oncolytic herpes simplex virus vectors for cancer virotherapy. Cancer Gene Ther. 2002;9:967–978. doi: 10.1038/sj.cgt.7700537. [DOI] [PubMed] [Google Scholar]
- 10.Foreman PK, Wainwright MJ, Alicke B, Kovesdi I, Wickham TJ, Smith JG, Meier-Davis S, Fix JA, Daddona P, Gardner P, Huang MT. Adenovirus-mediated transduction of intestinal cells in vivo. Hum Gene Ther. 1998;9:1313–1321. doi: 10.1089/hum.1998.9.9-1313. [DOI] [PubMed] [Google Scholar]
- 11.Wirtz S, Becker C, Blumberg R, Galle PR, Neurath MF. Treatment of T cell-dependent experimental colitis in SCID mice by local administration of an adenovirus expressing IL-18 antisense mRNA. J Immunol. 2002;168:411–420. doi: 10.4049/jimmunol.168.1.411. [DOI] [PubMed] [Google Scholar]
- 12.Hackett NR, Kaminsky SM, Sondhi D, Crystal RG. Antivector and antitransgene host responses in gene therapy. Curr Opin Mol Ther. 2000;2:376–382. [PubMed] [Google Scholar]
- 13.Hoggan MD, Blacklow NR, Rowe WP. Studies of small DNA viruses found in various adenovirus preparations: physical, biological, and immunological characteristics. Proc Natl Acad Sci USA. 1966;55:1467–1474. doi: 10.1073/pnas.55.6.1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Berns KI, Hauswirth WW. Adeno-associated viruses. Adv Virus Res. 1979;25:407–449. doi: 10.1016/s0065-3527(08)60574-6. [DOI] [PubMed] [Google Scholar]
- 15.Samulski RJ, Berns KI, Tan M, Muzyczka N. Cloning of adeno-associated virus into pBR322: rescue of intact virus from the recombinant plasmid in human cells. Proc Natl Acad Sci USA. 1982;79:2077–2081. doi: 10.1073/pnas.79.6.2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhou X, Muzyczka N. In vitro packaging of adeno-associated virus DNA. J Virol. 1998;72:3241–3247. doi: 10.1128/jvi.72.4.3241-3247.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Monahan PE, Samulski RJ. Adeno-associated virus vectors for gene therapy: more pros than cons? Mol Med Today. 2000;6:433–440. doi: 10.1016/s1357-4310(00)01810-4. [DOI] [PubMed] [Google Scholar]
- 18.Stilwell JL, Samulski RJ. Adeno-associated virus vectors for therapeutic gene transfer. Biotechniques. 2003;34:148–159. doi: 10.2144/03341dd01. [DOI] [PubMed] [Google Scholar]
- 19.Zaiss AK, Liu Q, Bowen GP, Wong NC, Bartlett JS, Muruve DA. Differential activation of innate immune responses by adenovirus and adeno-associated virus vectors. J Virol. 2002;76:4580–4590. doi: 10.1128/JVI.76.9.4580-4590.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.During MJ, Xu R, Young D, Kaplitt MG, Sherwin RS, Leone P. Peroral gene therapy of lactose intolerance using an adeno-associated virus vector. Nat Med. 1998;4:1131–1135. doi: 10.1038/2625. [DOI] [PubMed] [Google Scholar]
- 21.Shao G, Greathouse K, Huang Q, Wang C, Sferra T. Gene transfer to the gastrointestinal tract after peroral administration of recombinant adeno-associated virus type 2 vectors. J Pediatr Gastroenterol Nutr. 2006;43:168–179. doi: 10.1097/01.mpg.0000228118.59853.ba. [DOI] [PubMed] [Google Scholar]
- 22.Zolotukhin S, Potter M, Hauswirth WW, Guy J, Muzyczka N. A “humanized” green fluorescent protein cDNA adapted for high-level expression in mammalian cells. J Virol. 1996;70:4646–4654. doi: 10.1128/jvi.70.7.4646-4654.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zolotukhin S, Byrne BJ, Mason E, Zolotukhin I, Potter M, Chesnut K, Summerford C, Samulsk RJ, Muzyczka N. Recombinant adeno-associated virus purification using novel methods improves infectious titer and yield. Gene Ther. 1999;6:973–985. doi: 10.1038/sj.gt.3300938. [DOI] [PubMed] [Google Scholar]
- 24.Zolotukhin S, Potter M, Zolotukhin I, Sakai Y, Loiler S, Fraites TJ, Jr, Chiodo VA, Phillipsberg T, Muzyczka N, Hauswirth WW, Flotte TR, Byrne BJ, Snyder RO. Production and purification of serotype 1, 2, and 5 recombinant adeno-associated viral vectors. Methods. 2002;28:158–167. doi: 10.1016/s1046-2023(02)00220-7. [DOI] [PubMed] [Google Scholar]
- 25.Ferrari FK, Samulski T, Shenk T, Samulski RJ. Secondstrand synthesis is a rate-limiting step for efficient transduction by recombinant adeno-associated virus vectors. J Virol. 1996;70:3227–3234. doi: 10.1128/jvi.70.5.3227-3234.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rochlitz CF. Gene therapy of cancer. Drugs Today. 2000;36:619–629. doi: 10.1358/dot.2000.36.9.593779. [DOI] [PubMed] [Google Scholar]
- 27.Wagner JA, Nepomuceno IB, Messner AH, Moran ML, Batson EP, Dimiceli S, Brown BW, Desch JK, Norbash AM, Conrad CK, Guggino WB, Flotte TR, Wine JJ, Carter BJ, Reynolds TC, Moss RB, Gardner P. A phase II, double-blind, randomized, placebo-controlled clinical trial of tgAAVCF using maxillary sinus delivery in patients with cystic fibrosis with antrostomies. Hum Gene Ther. 2002;3:1349–1359. doi: 10.1089/104303402760128577. [DOI] [PubMed] [Google Scholar]
- 28.Leone P, Janson CG, Bilaniuk L, Wang Z, Sorgi F, Huang L, Matalon R, Kaul R, Zeng Z, Freese A, McPhee SW, Mee E, During MJ. Aspartoacylase gene transfer to the mammalian central nervous system with therapeutic implications for Canavan disease. Ann Neurol. 2000;48:27–38. doi: 10.1002/1531-8249(200007)48:1<27::aid-ana6>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 29.Kay MA, Manno CS, Ragni MV, Larson PJ, Couto LB, McClelland A, Glader B, Chew AJ, Tai SJ, Herzog RW, Arruda V, Johnson F, Scallan C, Skarsgard E, Flake AW, High KA. Evidence for gene transfer and expression of factor IX in haemophilia B patients treated with an AAV vector. Nat Genet. 2000;24:257–261. doi: 10.1038/73464. [DOI] [PubMed] [Google Scholar]
- 30.Londei M, Quaratino S, Maiuri L. Celiac disease: a model autoimmune disease with gene therapy applications. Gene Ther. 2003;10:835–843. doi: 10.1038/sj.gt.3302041. [DOI] [PubMed] [Google Scholar]
- 31.Prieto J, Herraiz M, Sangro B, Qian C, Mazzolini G, Melero I, Ruiz J. The promise of gene therapy in gastrointestinal and liver diseases. Gut. 2003;52(S2):49–54. doi: 10.1136/gut.52.suppl_2.ii49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tang SC, Sambanis A, Sibley E. Proteosome modulating agents induce rAAV2-mediated transgene expression in human intestinal epithelial cells. Biochem Biophys Res Commun. 2005;31:1392–1400. doi: 10.1016/j.bbrc.2005.03.245. [DOI] [PubMed] [Google Scholar]
- 33.Walters RW, Pilewski JM, Chiorini JA, Zabner J. Secreted and transmembrane mucins inhibit gene transfer with AAV4 more efficiently than AAV5. J Biol Chem. 2002;277:23709–23713. doi: 10.1074/jbc.M200292200. [DOI] [PubMed] [Google Scholar]
- 34.Summerford C, Samulski RJ. Membrane-associated heparan sulfate proteoglycan is a receptor for adeno-associated virus type 2 virions. J Virol. 1998;72:1438–1445. doi: 10.1128/jvi.72.2.1438-1445.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McCarty DM, Fu H, Monahan PE, Toulson CE, Naik P, Samulski RJ. Adeno-associated virus terminal repeat (TR) mutant generates self-complementary vectors to overcome the rate-limiting step to transduction in vivo. Gene Ther. 2003;10:2112–2118. doi: 10.1038/sj.gt.3302134. [DOI] [PubMed] [Google Scholar]
- 36.Kessler PD, Podsakoff GM, Chen X, McQuiston SA, Colosi PC, Matelis LA, Kurtzman GJ, Byrne BJ. Gene delivery to skeletal muscle results in sustained expression and systemic delivery of a therapeutic protein. Proc Natl Acad Sci USA. 1996;93:14082–14087. doi: 10.1073/pnas.93.24.14082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Song S, Embury J, Laipis PJ, Berns KI, Crawford JM, Flotte TR. Stable therapeutic serum levels of human alpha-1 antitrypsin (AAT) after portal vein injection of recombinant adeno-associated virus (rAAV) vectors. Gene Ther. 2001;8:1299–1306. doi: 10.1038/sj.gt.3301422. [DOI] [PubMed] [Google Scholar]
- 38.Bals R, Xiao W, Sang N, Weiner D, Meegalla R, Wilson J. Transduction of well-differentiated airway epithelium by recombinant adeno-associated virus is limited by vector entry. J Virol. 1999;73:6085–6088. doi: 10.1128/jvi.73.7.6085-6088.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sandberg JW, Lau C, Jacomino M, Finegold M, Henning SJ. Improving access to intestinal stem cells as a step toward intestinal gene transfer. Hum Gene Ther. 1994;5:323–329. doi: 10.1089/hum.1994.5.3-323. [DOI] [PubMed] [Google Scholar]
- 40.Pickles RJ, Fahrner JA, Petrella JM, Boucher RC, Bergelson JM. Retargeting the coxsackievirus and adenovirus receptor to the apical surface of polarized epithelial cells reveals the glycocalyx as a barrier to adenovirus-mediated gene transfer. J Virol. 2000;74:6050–6057. doi: 10.1128/jvi.74.13.6050-6057.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xie X, Forsmark CE, Lau JY. Effect of bile and pancreatic juice on adenoviral-mediated gene delivery: implications on the feasibility of gene delivery through ERCP. Dig Dis Sci. 2000;45:230–236. doi: 10.1023/a:1005431703317. [DOI] [PubMed] [Google Scholar]
- 42.Seiler MP, Miller AD, Zabner J, Halbert CL. Adeno-associated virus types 5 and 6 use distinct receptors for cell entry. Hum Gene Ther. 2006;17:10–19. doi: 10.1089/hum.2006.17.10. [DOI] [PubMed] [Google Scholar]
- 43.Sferra TJ, McNeely D, Johnson PR. Gene transfer to the intestinal tract: a new approach using selective injection of the superior mesenteric artery. Hum Gene Ther. 1997;8:681–687. doi: 10.1089/hum.1997.8.6-681. [DOI] [PubMed] [Google Scholar]
- 44.Gao GP, Alvira MR, Wang L, Calcedo R, Johnston J, Wilson JM. Novel adeno-associated viruses from rhesus monkeys as vectors for human gene therapy. Proc Natl Acad Sci USA. 2002;99:11854–11859. doi: 10.1073/pnas.182412299. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


