Abstract
Age-related differences in sensitivity to the acute effects of alcohol may play an important role in the increased risk for the development of alcoholism seen in teens that begin drinking at an early age. The present study evaluated the acute and protracted (hangover) effects of ethanol in adolescent (P33–P40) and adult (P100–P107) Wistar rats, using the cortical electroencephalogram (EEG). Six minutes of EEG was recorded during waking, 15 min after administration of 0, 1.5, or 3.0 g/kg ethanol, and for 3 h at 20 h post ethanol, during the rats’ next sleep cycle. Significantly higher overall frontal and parietal cortical power was seen in a wide range of EEG frequencies in adolescent rats as compared to adult rats in their waking EEG. Acute administration of ethanol did not produce differences between adolescents and adults on behavioral measures of acute intoxication. However, it did produce a significantly less intense acute EEG response to ethanol in the theta frequencies in parietal cortex in the adolescents as compared to the adults. At 20 h following acute ethanol administration, during the rats’ next sleep cycle, a decrease in slow-wave frequencies (1–4 Hz) was seen and the adolescent rats were found to display more reduction in the slow-wave frequencies than the adults did. The present study found that adolescent rats, as compared to adults, demonstrate low sensitivity to acute ethanol administration in the theta frequencies and more susceptibility to disruption of slow-wave sleep during hangover. These studies may lend support to the idea that these traits may contribute to increased risk for alcohol use disorders seen in adults who begin drinking in their early teenage years.
Keywords: Adolescence, Alcohol, EEG, Alcoholism, Slow-wave sleep, Hangover
Introduction
Adolescence is a critical time when most individuals first experiment with alcohol intake. However, early age onset of alcohol use (e.g., 11–14 years old) is also associated with approximately a 2–3 fold increase in the risk for developing an alcohol use disorder (Andersen, Due, Holstein, & Iversen, 2003; DeWit, Adlaf, Offord, & Ogborne, 2000; Grant & Dawson, 1997; Hawkins et al., 1997; Rose, Dick, Viken, Pulkkinen, & Kaprio, 2001). It has been suggested that the reason that early alcohol use is associated with increased risk for the development of problem drinking may be that those with a genetic susceptibility to alcoholism also have other externalizing behaviors that lead them to begin drinking early (Iacono, Carlson, Malone, & McGue, 2002; Jessor & Jessor, 1977). Alternatively, it is also possible that responses to alcohol during adolescence differ from those of adults in some fundamental way that makes alcohol inherently more addicting to teens that begin drinking early. Studies in a variety of populations suggest that sensitivity or “level of response” to alcohol may be one of the most relevant biologically based risk factors for the development of alcohol use disorders (Schuckit, 1994; Schuckit & Smith, 1996). It has been demonstrated that individuals at higher risk for alcohol dependence, such as sons of alcoholics, are less “sensitive” to or have a less “intense” response to alcohol and therefore must drink larger amounts to experience the same effects as other drinkers. This characteristic then, at least theoretically, puts them at higher risk for heavy drinking and its consequences.
Interestingly, studies in adolescent rats have demonstrated that they are less sensitive than adult rats to acute administration of ethanol on a number of measures, including ethanol-induced motor incoordination, sedation, hypothermia, and electrophysiological effects (Ernst, Dempster, Yee, Dennis, & Nakano, 1976; Hollstedt, Olsson, & Rydberg, 1980; Little, Kuhn, Wilson, & Swartzwelder, 1996; Moy, Duncan, Knapp, & Breese, 1998; Pian, Criado, Walker, & Ehlers, 2008; Silveri & Spear, 1998, 2000; Varlinskaya & Spear, 2002). Adolescent rats have also been shown to have a higher tolerance to acute administration of high doses of ethanol, as shown by requiring significantly less time to regain the righting reflex at high blood alcohol levels, as compared to adult rats (Pian, Criado, Walker, & Ehlers, 2008; Silveri & Spear, 1998). Due to these age-related differences in the sensitivity to the sedative effects of alcohol, adolescents may have higher neurological and physiological limits of alcohol intake compared to adults. This may lead to higher consumption and tolerance to alcohol than adults. The higher consumption and tolerance of alcohol have been proposed to enhance risk for alcohol dependence in adolescents (Ehlers, Slutske, Gilder, Lau, & Wilhelmsen, 2006; Spear, 2000; Witt, 1994).
Adolescent rats appear to be not only more resistant and tolerant to the sedative effects of acute ethanol, but also differ with respect to the degree of withdrawal/hangover symptoms. For example, anxiety, hyperthermia, and social suppression are all less severe in adolescents compared to adults following ethanol withdrawal (Brasser & Spear, 2002; Doremus-Fitzwater & Spear, 2007; Doremus, Brunell, Varlinskaya, & Spear, 2003; Slawecki & Roth, 2004; Varlinskaya & Spear, 2004). However, other symptoms of alcohol withdrawal, such as the development of hypoactivity and increases in high frequency EEG activity, are more pronounced in adolescents (Slawecki & Roth, 2004; Slawecki, Roth, & Gilder, 2006). Since a hangover consists of several aversive symptoms that occur sometime after the end of a drinking period, it has been proposed that individuals who are especially prone to hangovers may be somewhat protected from developing alcohol use disorders (Piasecki, Robertson, & Epler, 2010; Piasecki, Sher, Slutske, & Jackson, 2005; Rohsenow et al., 2012). Alternatively, individuals with severe hangover may “self-medicate” symptoms of hangover by drinking more alcohol, thus leading to a vicious cycle of heavy drinking and more hangover symptoms. Recently, it has also been shown that individuals with lower sensitivity to alcohol may have more hangovers but also tend to be differentially resistant to hangovers at a given number of drinks (Piasecki et al., 2012). Thus, it has been postulated that hangover frequency and hangover resistance as well as heavy drinking may all be manifestations of a less intense response to alcohol (Piasecki et al., 2012). Because adolescent rats, as compared to adults, demonstrate both low sensitivity to ethanol and differential responses to some of the symptoms of hangover, these traits may form part of the substrate of the increased risk for alcohol use disorders.
One of the results of acute alcohol exposure, which can also influence hangover symptoms, is alcohol-induced disturbances of sleep (Penning, van Nuland, Fliervoet, Olivier, & Verster, 2010; Rohsenow, Howland, Minsky, & Arnedt, 2006; Rohsenow et al., 2010; Swift & Davidson, 1998). Alcohol use has marked effects on sleep (Brower, 2001; Hasler, Smith, Cousins, & Bootzin, 2012), including reductions in slow-wave sleep (SWS) in both humans and rodents (Ehlers & Slawecki, 2000; Irwin, Miller, Gillin, De Modena, & Ehlers, 2000). Sleep difficulties have been reported to be common in human adolescents and furthermore, poor or inadequate sleep has been shown to be associated with negative outcomes in adolescents, including emotional dysfunction, behavioral problems, and consequently increased risk for alcohol use disorders (Hasler & Clark, 2013). We have previously demonstrated that SWS is preferentially impacted by adolescent ethanol exposure in the rat vapor model. Significant reductions in the mean duration of SWS episodes and the total amount of time spent in SWS were found in adult rats treated with ethanol vapor during adolescence (Criado, Wills, Walker, & Ehlers, 2008). It is yet unknown whether withdrawal from acute ethanol exposure (hangover) impacts sleep differently in adolescents than in adults.
The primary goal of the present study was to further characterize the effects of acute ethanol exposure and hangover on cortical EEG measures of waking and SWS during hangover in adolescent and adult rats. The working hypothesis of this study is that adolescents are less sensitive to the acute effects of ethanol and that SWS may be differently affected by acute ethanol withdrawal in adolescents than in adults.
Materials and methods
Subjects
Nineteen male adolescent Wistar rats at ages postnatal days (P) 33–P40 and 19 male adult Wistar rats (P100–P107) were used during the study (Charles River, Wilmington, MA). Upon receipt, adolescent rats (P24) averaged 68.1 ± 5.6 g; the adult rats (P91) averaged 370.0 ± 9.34 g. All the animals were pair-housed in standard plastic cages on a 12-h light/dark cycle (lights on at 8:00 AM) and food and water were provided ad libitum. The experimental procedure described below adheres to the guidelines stipulated in the National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals (NIH publication No. 80-23, revised 1996) and was reviewed and approved by The Scripps Research Institute's Institutional Animal Care and Use Committee.
Surgical procedures
At least one week prior to the electrophysiological recordings, the rats were surgically prepared with screw electrodes placed in the skull overlying the frontal (adolescent: AP + 1.5, ML ± 2.0; adult: AP + 1.5, ML ± 2.0; adult: AP + 1.5, ML ± 3.0) and parietal lobes (adolescent: AP – 4.0, ML ± 3.5; adult: AP – 4.5, ML ± 4.5). An electrode placed over the cerebellum was used as ground. Two electromyography (EMG) wire electrodes were also inserted into the rats’ neck muscles on the right and left. The rats were anesthetized with isoflurane (1–3%), and atropine (0.4 mg/mL: 0.03 mL for adolescents and 0.06 mL for adults; subcutaneously) was co-administered to minimize respiratory suppression during the surgical procedures. EMG and EEG electrode connections either were made to an Amphenol 5-pin connector (adult rats) or were assembled into a custom 5-pin cap (for the adolescent rats). The assembly was anchored to the skull with dental acrylic and anchor screws.
Ethanol administration
The 38 ethanol-naive rats were injected intraperitoneally (I.P.) on a randomized schedule of 3 injections on 3 different days: a saline dose, a high (3.0 g/kg) and a low (1.5 g/kg) dose of 20% w/v ethanol. Adult rats were between P100 and P107 days old at the time of the injections; adolescent rats were between P33 and P40 days old. These moderate to high doses of ethanol were chosen because previous studies have demonstrated that EEG differences in ethanol response between adolescents and adults may be more significant at higher ethanol doses (Slawecki, 2002). The ethanol concentrations were prepared fresh for each day of testing for groups containing 3–4 rats each, and the saline doses were given in equal frequency and at equivalent volumes to the ethanol doses. The rats were placed in the electrophysiological recording chamber approximately 15 min post-injection. The injection volumes ranged from 0.7 to 6.1 mL based on the subjects’ body weight (92–424 g). All rats were habituated to the testing procedure before the first experimental recording, and the rats were given at least 72 h between doses. All rats were scored visually for intoxication by using a modified 5-point scale that was similar to that described by Freund (1969) for mice. Intoxication was rated just before the onset of the EEG recordings. On that scale, a score of 0 indicated no signs of intoxication, 1 indicated that the rat was calm with decreased muscle tone during handling, 2 indicated mild ataxia with a rapid gait, 3 indicated that the gait was impaired with the animal falling to one side, and a score of 4 was given if the rat was immobile with little muscle tone.
Electrophysiological recording procedures
Cortical electroencephalogram (EEG) recordings were conducted on the rats at 2 different times. The first was 15 min after the ethanol or saline dose administration (6-min EEG recordings) and the second was 20 h later (8:00 AM) during the rats’ next sleep cycle (3-h EEG recordings). EEGs were recorded from 2 monopolar leads referenced to the cerebellum ground (i.e. frontal and parietal cortex) and were recorded on a preamplifier/amplifier unit (Sensorium Inc., Shelburne, VT) with a band pass of 0.53–70 Hz. Data were digitized at a rate of 256 Hz, and consecutive 4-s epochs of EEG were Fourier-transformed over a spectrum of 1–64 Hz. Artifact epochs were excluded only after being verified by visual analysis of the raw EEG and spectral distributions; only non-artifact epochs were included in the analyses. Non rapid eye movement (NREM) sleep was also identified by visual inspection using EEG and EMG amplitude and frequency characteristics previously described (Criado et al., 2008; Ehlers & Slawecki, 2000). Individual spectra from each 4-s epoch were then averaged and compressed into 4 frequency bands: 1–4, 4–8, 8–16, and 16–32 Hz. Mean spectral power and peak frequency were calculated for each of the frequency bands as described previously (Ehlers & Havstad, 1982). Peak frequency over the entire spectral range (1–50 Hz) was also calculated.
Sleep analysis
Slow-wave sleep (SWS) was visually identified as synchronized slow-wave activity (1–4 Hz) during the 3-h EEG recording session, and at 20 h following saline/ethanol administration, during the rats’ next sleep cycle. Increases in EEG power of at least twice the amplitude of waking baseline EEG power lasting longer than 8 s were counted as episodes of SWS. Sleep patterns were analyzed by 1) determining the onset latency of the first episode of SWS, 2) calculating the mean duration of all episodes of SWS, and 3) counting the total number of instances of SWS. The onset latency of the first SWS episode lasting at least 8 s was determined from the raw EEG. The onset of the first SWS episode in the EEG was identified as the first transition from low-amplitude high-frequency EEG to SWS (high-amplitude low-frequency EEG).
Statistical analysis
Repeated-measures analysis of variance (ANOVA) was used to assess the effects of developmental age and the effects of ethanol on intoxication scores and EEG power and peak frequency means in the 4 frequency bands at the 2 different time points (15 min following ethanol, 20 h following ethanol). The adolescent and adult rats were compared using a 2 × 3 ANOVA where age was a between-group factor (adolescent vs. adult) and ethanol dose was a within-group factor (saline, 1.5 g/kg, 3.0 g/kg). Two different sets of repeated-measures analyses were conducted in this manner for the 2 recording periods (6-min awake EEG, 15 min after administration and the 3-h sleep EEG, 20 h later) for the 2 brain regions (frontal and parietal lobes). For significant main effects and interactions for group and dose, a repeated-measures post hoc analysis was used. For analyses of latency to sleep onset, nonparametric statistics were also employed. Degrees of freedom are presented in whole integers. All analyses were conducted using the statistical program SPSS (v15.0, Chicago, SPSS Inc.).
Results
Behavioral state assessment
Subjective visual inspection of adolescent and adult rats preceding ethanol administration showed normal exploratory and grooming behaviors in both groups. Acute administration of ethanol produced dose-dependent effects on the rats’ behavior. Consistent with previous studies (e.g., Slawecki, 2002), some mild ataxia with a rapid gait was apparent 15 min post injection of 1.5 g/kg of ethanol (mean intoxication score: adolescents 2.17 ± 0.20; adults 2.71 ± 0.21), whereas the 3.0 g/kg ethanol dose impaired gait with rats often falling to one side as well as producing immobility in some animals (mean intoxication score: adolescents 3.78 ± 0.12; adults 3.71 ± 0.12). A main effect of ethanol administration was seen on intoxication score (F = 69.56, df = 2,66; p < 0.0001). No significant main effects of age or ethanol × age interactions were observed.
EEG assessment
Frontal and parietal cortical EEG power following acute alcohol challenge
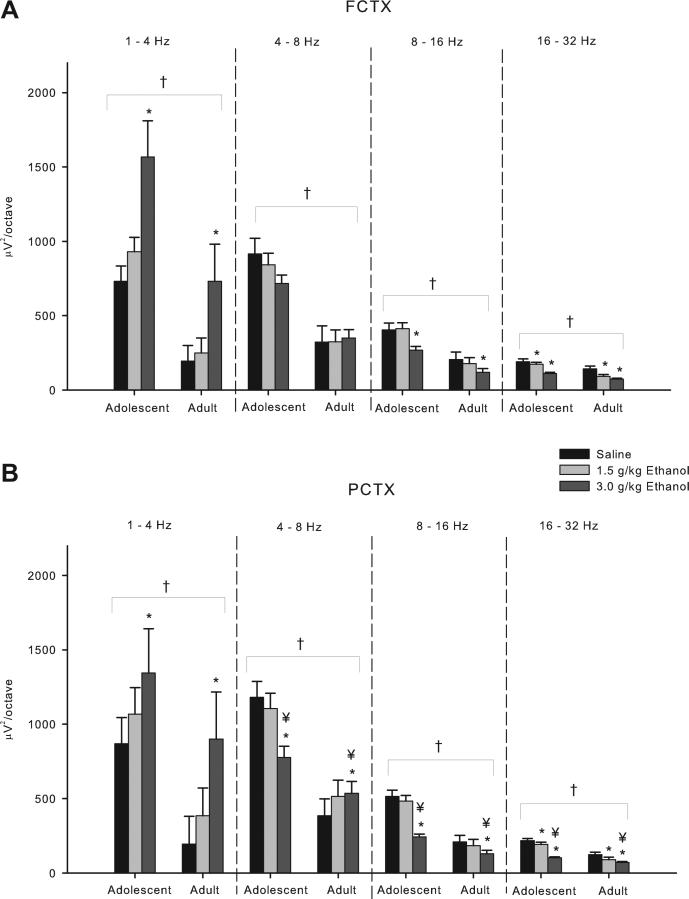
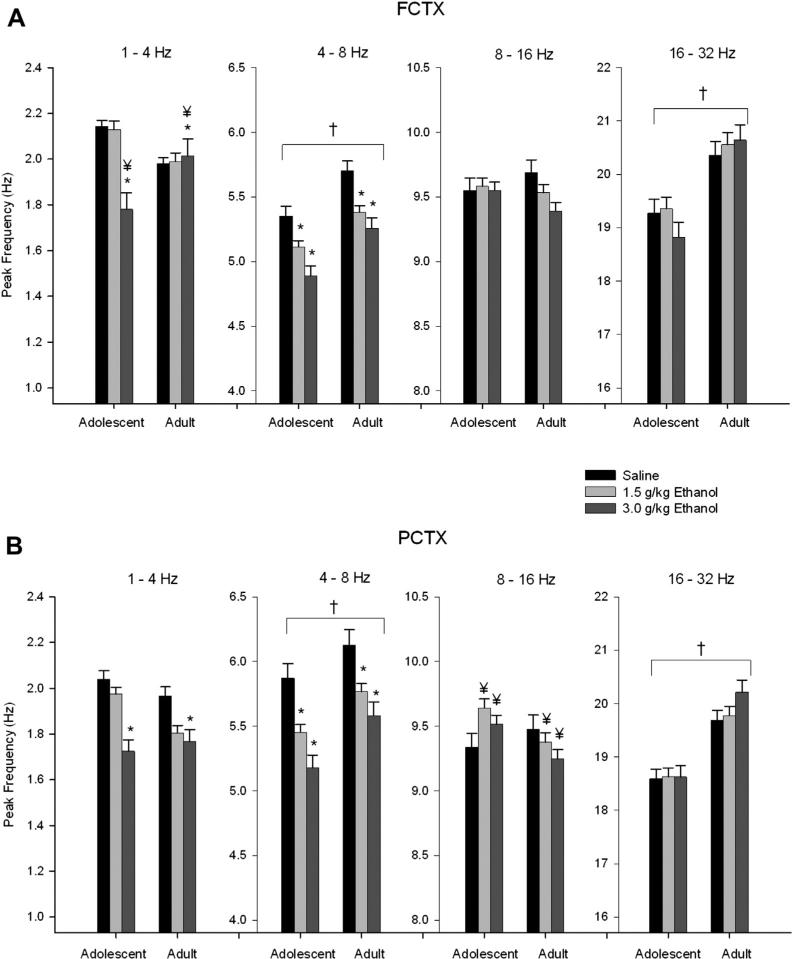
Six minutes of EEG was recorded 15 min following I.P. injections of saline and 2 doses of ethanol (1.5 and 3.0 g/kg). Spectral analyses of the records revealed in the 2 × 3 ANOVA that the frontal cortical power was found to be greater in adolescent rats than in adults (Group effect: F's = 13.99–30.14, df's = 1,33; p's all < 0.0008) in all frequency bands (Fig. 1A). Analyses of the peak frequency within the 4 frequency bands uncovered that adolescents have significantly lower peak frequency in the theta (4–8 Hz) and beta (16–32 Hz) bands (F = 26.16–32.53, df = 1,33; p < 0.00002) (Fig. 2A).
Fig. 1.
Acute effects of ethanol on electroencephalogram (EEG) power in adolescent and adult rats following acute saline/ethanol challenge. (A) Frontal cortex EEG in adolescent rats has greater power across all frequency bands compared to adults. Further post hoc analyses show a 3.0 g/kg ethanol dose effect in the delta (1–4 Hz), midrange (8–16 Hz), and beta (16–32 Hz) bands as well as a 1.5 g/kg dose effect in the beta band. (B) Power in the parietal cortex was found to be higher in adolescents than in adults across all frequency bands. Post hoc analyses show a 3.0 g/kg dose effect in all frequency bands, and in the beta band at the 1.5 g/kg dose. Age × ethanol interactions were seen at the 3.0 g/kg dose in the theta (4–8 Hz), midrange, and beta frequency bands. * indicates p < 0.05 ethanol effect. † indicates p < 0.05 age effect. ¥ indicates p < 0.05 age × ethanol interaction. Error bars = SEM.
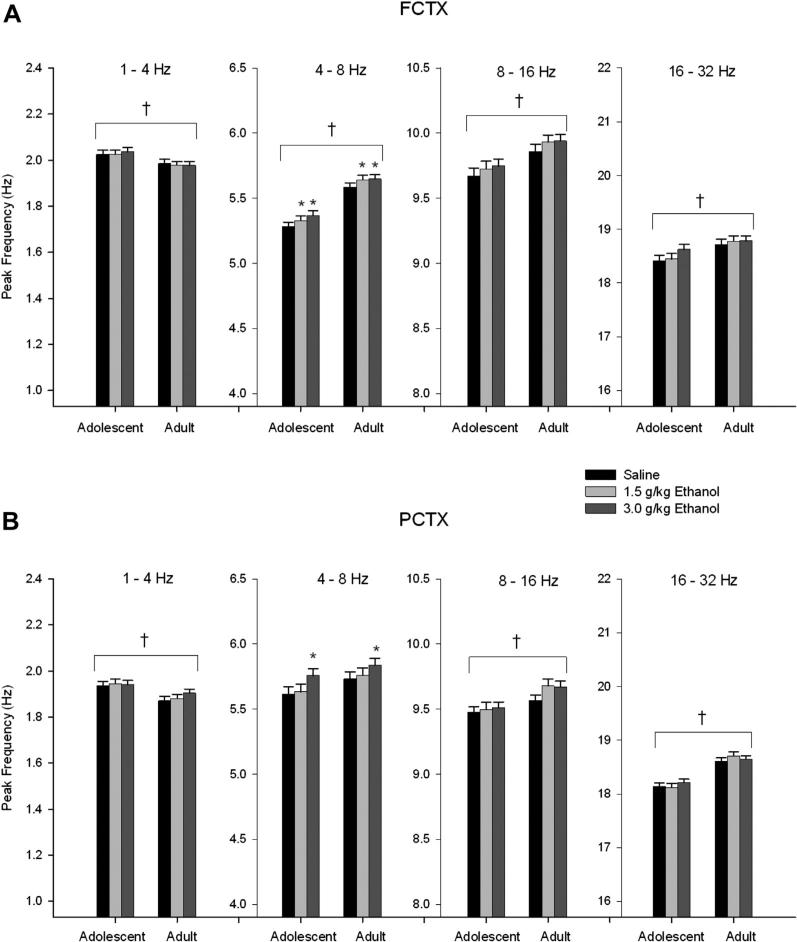
Fig. 2.
Acute effects of ethanol on electroencephalogram (EEG) peak frequency in adolescent and adult rats following acute saline/ethanol challenge. (A) Adolescents have significantly lower peak frequencies in the theta (4–8 Hz) and beta (16–32 Hz) bands of the frontal cortex compared to adults. Post hoc analyses show ethanol effects on peak frequency in the delta (1–4 Hz) and theta bands at the 3.0 g/kg dose, and in theta band at the 1.5 g/kg dose. Age × ethanol interaction was observed in the delta band for the 3.0 g/kg dose of ethanol. (B) Adolescents have lower peak frequency in the theta and beta bands in the parietal cortex compared to adults. Post hoc analyses show ethanol decreases peak frequency in the theta band at the 1.5 g/kg dose, and in delta and theta at the 3.0 g/kg dose. Age × ethanol interactions on peak frequency were observed in delta and midrange bands at 3.0 g/kg dose of ethanol as well as 1.5 g/kg in the midrange band. * indicates p < 0.05 ethanol effect. † indicates p < 0.05 age effect. ¥ indicates p < 0.05 age × ethanol interaction. Error bars = SEM.
Significant main effects of ethanol were also found for frontal cortical power in all frequency bands (F's = 6.94–20.64, df's 2,66; p's all < 0.003) except in the theta (4–8 Hz) band in the frontal cortex. Post hoc analyses revealed that ethanol caused a reduction in power in the midrange (8–16 Hz) (p < 0.002) and beta (16–32 Hz) (p < 0.000002) frequency ranges at the 3.0 g/kg dose as compared to saline. However, ethanol was found to produce a dose-dependent increase in power in the delta (1–4 Hz) band (F = 13.34, df = 2,66; p < 0.0002) at the 3.0 g/kg dose (post hoc, p < 0.0005), and a decrease in peak frequency values in the delta band (1–4 Hz) (F = 9.01, df = 2,66; p < 0.000001), at the 3.0 g/kg dose (post hoc, p < 0.001) and theta (4–8 Hz) band (F = 24.84, df = 2,66; p < 0.0001) at both the 1.5 g/kg (post hoc, p < 0.00005) and 3.0 g/kg (post hoc, p < 0.000002) doses. Decreases in peak frequency were also seen over the entire frequency range (1–50 Hz) at both the 1.5 g/kg (post hoc, p < 0.0007) and 3.0 g/kg (post hoc, p < 0.000001) doses.
An age (adolescent vs. adult) × ethanol dose (saline, 1.5, 3.0 g/kg) interaction (repeated-measures ANOVA) was also observed in the frontal cortex data, as seen in Fig. 2A. Adolescents showed a decrease in peak frequency in the delta range (1–4 Hz) whereas adults showed an increase (F = 12.59, df = 2,66; p < 0.0003), that was significant for the 3.0 g/kg dose (post hoc analyses, p < 0.0002).
Similar to the frontal cortex, a 2 × 3 repeated-measures ANOVA revealed that parietal cortical power was greater in adolescent rats as compared to adults in all bands (F's = 5.07–40.42, df's 1,32; p's all < 0.04) (Fig. 1B). Analyses of the peak frequency within the 4 frequency bands uncovered that adolescents have a lower peak frequency in the theta (4–8 Hz: F = 19.63, df = 1,32; p < 0.0002) and beta (16–32 Hz: F = 52.59, df = 1,32; p < 0.000001) band in the parietal cortex (Fig. 2B).
Further examination of the adolescent rats’ parietal cortex activity revealed significant ethanol effects on spectral power values in all bands (2 × 3 ANOVA: F's = 4.79–30.74, df's = 2,64; p's all < 0.015). Post hoc analyses revealed that ethanol caused a reduction in power in the theta (4–8 Hz) (3.0 dose: F = 4.79; p < 0.015), midrange (8–16 Hz) (3.0 g/kg dose p < 0.000001), and beta (16–32 Hz) (1.5 g/kg dose p < 0.015; 3.0 dose p < 0.000001) frequency ranges. However, ethanol was found to produce a dose-dependent increase in power in the delta (1–4 Hz) band (2 × 3 ANOVA: F = 6.69, df = 2,64; p < 0.006) at the 3.0 g/kg dose (post hoc, p < 0.008). Ethanol also produced decreases in peak frequency values in the delta (F = 20.68, df = 2,64; p < 0.00002) and theta (F = 19.76; df = 2,64; p < 0.000004) bands as well as over the entire frequency spectrum (1–50 Hz: F = 27.96, df = 2,64; p < 0.000001) in the parietal cortex, that were significant in post hoc analyses at both the 1.5 and 3.0 g/kg doses (post hoc p values ranged from p < 0.001 to p < 0.000002).
As in the frontal cortex, several age (adolescent vs. adult) × ethanol dose (saline, 1.5, 3.0 g/kg) interactions were observed in the parietal cortex power data as seen in Fig. 1B. In the theta frequency band, adults showed an increase in power whereas the adolescents showed a small dose-dependent decline (F = 13.77, df = 2,64; p < 0.00003). Post hoc analyses revealed that this effect was significant at the 3.0 g/kg dose (p < 0.000004). Adolescents showed more of a decline in EEG power produced by ethanol than the adults in the midrange (F = 6.79, df = 2,64; p < 0.002) and beta (F = 6.43, df = 2,64; p < 0.003) frequency band, that was significant at the 3.0 g/kg dose in post hoc analyses (8–16 Hz, p < 0.002; 16–32 Hz, p < 0.006). Additionally, ethanol produced a decrease in peak frequency in the adults and an increase in peak frequency in the adolescents in the midrange frequencies (F = 4.79, df = 2,64; p < 0.016) (post hoc analyses, 1.5 g/kg dose, p < 0.03; 3.0 g/kg dose, p < 0.02), and produced a larger decrease in peak frequency in the delta band in adolescents than adults (F = 3.47, df = 2,64; p < 0.05) (post hoc analyses, NS)(Fig. 2B). These results suggest that alcohol produced more signs of EEG sedation (e.g. increased theta power) in adults than in adolescents.
Characteristics of NREM sleep 20 h following acute saline/alcohol challenge
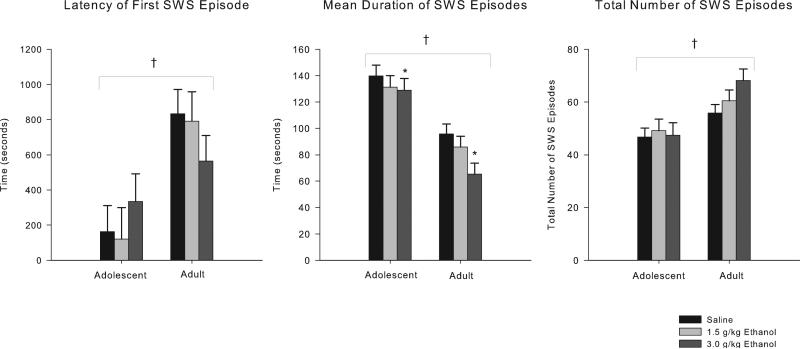
One hundred eighty (180) min of EEG were recorded 20 h following I.P. injections of saline and 2 doses of ethanol (1.5 and 3.0 g/kg). The records were visually scored for NREM sleep episodes. Thirty (30) rats survived all procedures in good health and had technically adequate recordings for all 3 doses (<25% artifact/noise, average all animals 3%) for the frontal recordings, and 29 records were available for the parietal recordings. As seen in Fig. 3, ANOVA analyses revealed that adolescents significantly differed from adults on all measures of slow-wave sleep (SWS) scored from the EEG record. Adolescents’ latency to the onset of the first SWS episode was significantly shorter (ANOVA: F = 19.6, df = 1,28; p < 0.0002; Mann–Whitney U Z = 3.74, p < 0.0002), the mean duration of their SWS episodes was longer (F = 33.49, df = 1,28; p < 0.000004), and the total number of SWS episodes was lower (F = 14.77, df = 1,28; p < 0.0007) compared to adults. Withdrawal from ethanol also affected SWS by reducing the mean duration of slow-wave episodes over the recording period (F = 4.82, df = 2,56; p < 0.02) (post hoc: 3.0 g/kg dose, p < 0.003). No significant main effects of ethanol were found on latency to the first slow-wave episode or the total number of slow-wave episodes, and no age × ethanol interactions were observed.
Fig. 3.
Characteristics of NREM sleep 20 h after acute saline/ethanol challenge. 2 × 3 ANOVA shows adolescents go to sleep sooner and have fewer, longer episodes of NREM sleep compared to adult rats. Post hoc analyses shows withdrawal from the 3.0 g/kg dose of ethanol reduced mean duration of slow-wave episodes over the 3-hour recording. * indicates p < 0.05 ethanol effect. † indicates p < 0.05 age effect. Error bars = SEM.
Frontal and parietal cortical EEG power 20 h following acute alcohol challenge
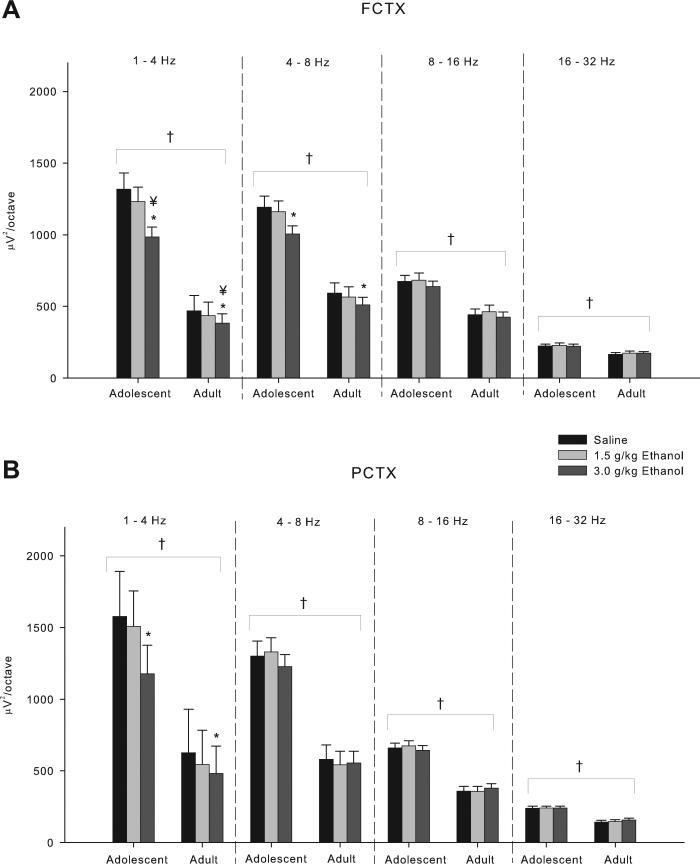
Spectral analyses of the records recorded 20 h after alcohol/saline administration in adolescent and adult rats revealed in ANOVA analyses that the frontal cortical power was found to be greater in adolescent rats than in adults (F's = 8.62–37.45, df's = 1,28; p's all < 0.007) in all frequency bands (Fig. 4A). Analyses of the peak frequency within the 4 frequency bands uncovered that adolescents have lower peak frequency in all frequency bands (F's = 5.2–50.20, df's = 1,28; p's all < 0.03) except delta (1–4 Hz), which is higher than adults (F = 4.65, df = 1,28; p < 0.04) (Fig. 5A). Significant main effects of 20 h of withdrawal from ethanol were also found for frontal cortical power in the delta (F = 11.97, df = 2,56; p < 0.0002) (post hoc analyses, 3.0 g/kg dose, p < 0.0004), theta (F = 13.17, df = 2,56; p < 0.00003) (post hoc analyses, 3.0 g/kg dose, p < 0.0002), and midrange (F = 3.30, df = 2,56; p < 0.05) (post hoc analyses, NS) bands. Twenty hours of withdrawal from acute ethanol administration caused a reduction in power in the delta and theta frequency ranges (Fig. 4A) and an increase in peak frequency in the theta range (F = 7.71, df = 2,56; p < 0.002) (post hoc analyses, 1.5 g/kg dose, p < 0.02; 3.0 g/kg dose, p < 0.002) (Fig. 5A). One age (adolescent vs. adult) × ethanol dose (saline, 1.5, 3.0 g/kg) interaction was also observed in the frontal cortex data collected 20 h after acute ethanol administration. Adolescents showed more of a decline in EEG delta power 20 h after ethanol administration than the adults (F = 4.32, df = 2,56; p < 0.02) (post hoc analyses, 3.0 g/kg dose, p < 0.03) (Fig. 4A).
Fig. 4.
Effects of acute ethanol on electroencephalogram (EEG) power during sleep, 20 h after ethanol/saline challenge. (A and B) Frontal and parietal cortical power was found to be higher in adolescent rats compared to adults in all frequency bands. (A) Post hoc analyses of ethanol effects in the frontal cortex show reductions in power in delta (1–4 Hz) and theta (4–8 Hz) frequency bands and age × ethanol interactions in the delta band for the 3.0 g/kg dose. (B) Post hoc analyses show delta band in parietal cortex was decreased by the 3.0 g/kg ethanol dose. * indicates p < 0.05 ethanol effect. † indicates p < 0.05 age effect. ¥ indicates p < 0.05 age × ethanol interaction. Error bars = SEM.
Fig. 5.
Effects of acute ethanol on electroencephalogram (EEG) peak frequency during sleep, 20 h after ethanol/saline challenge. (A) Adolescent rats have lower peak frequency in all bands compared to adults except in the delta (1–4 Hz) band, where they are higher than adults. Post hoc analyses found ethanol increased peak frequency in the theta (4–8 Hz) band at both the 1.5 g/kg and 3.0 g/kg dose in frontal cortex. (B) Age effects were also seen in delta, midrange (8–16 Hz) and beta (16–32 Hz) frequency bands in parietal cortex. Post hoc analyses show an increase in the theta band at the 3.0 g/kg ethanol dose. * indicates p < 0.05 ethanol effect. † indicates p < 0.05 age effect. Error bars = SEM.
Similar to the frontal cortex, ANOVA analyses of the parietal cortical power recorded 20 h after acute ethanol administration revealed differences between adolescents and adults. EEG power in the parietal cortex was found to be greater in adolescent rats as compared to adults in all bands (F's = 6.30–43.19, df's = 1,27; p's all < 0.02) (Fig. 4B). Analyses of the peak frequency within the 4 frequency bands uncovered that adolescents have lower peak frequencies in the midrange (F = 6.69, df = 1,27; p < 0.02) and beta (F = 34.36, df = 1,27; p < 0.000004) bands, and increases in the delta (F = 4.94, df = 1,27; p < 0.04) band in the parietal cortex as compared to adults (Fig. 5B). Further examination of the adolescent and adult rats’ parietal cortex activity revealed significant reductions in spectral power values following 20 h of withdrawal from ethanol in the delta (F = 6.08, df = 2,56; p < 0.02) (post hoc analyses, 3.0 g/kg dose, p < 0.02) band (Fig. 4B). Ethanol withdrawal also increased peak frequency values in the theta frequencies (F = 11.11, df = 2,56; p < 0.0002) (post hoc analyses, 3.0 g/kg dose, p < 0.0002), and over the entire frequency range (F = 9.56, df = 2,56; p < 0.0004) (post hoc analyses, 3.0 g/kg dose, p < 0.0003) in the parietal cortex (Fig. 5B). No age (adolescent vs. adult) × ethanol dose (saline, 1.5, 3.0 g/kg) interactions in the parietal cortex were observed.
Discussion
Previous studies from our laboratory have shown that ethanol exposure during adolescence produces long-term neurobehavioral consequences that could be related to an increased risk for developing alcoholism in adulthood (Criado & Ehlers, 2010a, 2010b, 2013; Criado, Liu, Ehlers, & Mathé, 2011; Ehlers & Criado, 2010; Ehlers, Criado, Wills, Liu, & Crews, 2011; Ehlers, Walker, Pian, Roth, & Slawecki, 2007; Pian, Criado, & Ehlers, 2008; Pian, Criado, Walker, et al., 2008; Slawecki, Betancourt, Cole, & Ehlers, 2001; Slawecki, Thorsell, & Ehlers, 2004). Studies in human subjects have indicated that the response to alcohol may be one of the factors that contribute to alcoholism risk (Schuckit, 1999; Schuckit & Smith, 1996). The present study found that acute administration of ethanol produced a different pattern of EEG response in adult rats than in adolescents. Specifically, ethanol produced a dose-dependent decrease in mean frequency in the delta frequency range in the adolescent rats, whereas it produced an increase in mean frequency in the frontal cortex in the adult rats. In the parietal cortex, adults showed a dose-dependent increase in power in the theta frequencies, whereas the adolescents showed a small dose-dependent decline. Adolescents also showed more of a decline in EEG power produced by ethanol than the adults in the midrange and beta frequencies in the parietal cortex. Taken together, these findings suggest that ethanol may cause more EEG measures of sedation (increased parietal theta) in adults than (0.75 g/kg) produce differential effects in adults as compared to adolescents in the beta frequencies (Pian, Criado, Walker, et al., 2008). These studies are also consistent with previous behavioral studies that have shown that adult rats are more sensitive to the acute administration of ethanol on a number of measures including ethanol-induced motor incoordination, sedation, and hypothermia (Ernst et al., 1976; Hollstedt et al., 1980; Little et al., 1996; Moy et al., 1998; Silveri & Spear, 1998, 2000; Varlinskaya & Spear, 2002). However, in the present study no differences between adolescents and adults were found on visual inspection of acute intoxication.
The mechanisms underlying the differences in sensitivity to the effects of ethanol on the waking cortical EEG in adult and adolescent rats remain unclear. Two candidate substrates for the developmental difference in ethanol sensitivity are the g-aminobutyric acid (GABA) and the N-methyl-d-aspartate (NMDA) receptor systems. It has been demonstrated that the activity of GABA and its receptors can be enhanced by ethanol (Grobin, Matthews, Devaud, & Morrow, 1998; Mihic, 1999; Proctor, Soldo, Allan, & Dunwiddie, 1992; Weiner, Zhang, & Carlen, 1994; Weiner, Gu, & Dunwiddie, 1997), and it has been shown that the functionality and maturity of the GABAergic system is less in adolescents compared to adults (Moy et al., 1998; Silveri & Spear, 2002). The over-expression of NMDA receptors in adolescent brains may be critical for synaptic plasticity changes that occur over adolescence (McDonald, Silverstein, & Johnston, 1989; Silveri & Spear, 2002). Silveri and Spear (1998) indicated that age-related developmental over-expression of the NMDA system may contribute to lower sensitivity to ethanol in adolescents as compared to adults. However, we have previously shown, using quantitative biochemical determinations of NMDAR subunits in adolescents and adults, that after 2 weeks of ethanol vapor exposure and 24 h and 2 weeks following withdrawal, that adolescents have lower subunit expression than adults, and that adults have a more robust change in subunit expression to ethanol exposure and withdrawal than adolescents (Pian, Criado, Milner, & Ehlers, 2010). Therefore, it appears that adolescents may have fewer ethanol-induced changes in NMDAR subunits as well as a decreased sensitivity to the EEG effects of acute alcohol. The developmental differences in ethanol-induced changes in EEG power observed in the present study may be due to the combination of age-related differential sensitivities of NMDAR subunits or a number of neural transmission systems known to be affected by ethanol.
Consistent with our previous findings (Pian, Criado, Walker, et al., 2008), in the present study we observed significantly higher overall frontal and parietal cortical power in a wide range of EEG frequencies in adolescent rats as compared to adult rats. The decline seen in EEG power from adolescence to adulthood could be a result of the age-related reductions in the number of active synapses that occur during the pruning process that occurs from adolescence to early adulthood (Feinberg, 1982; Huttenlocher, 1979; Huttenlocher & Dabholkar, 1997; Purves & Lichtman, 1980; Rakic, Bourgeois, Eckenhoff, Zecevic, & Goldman-Rakic, 1986; Whitford et al., 2007). Large age-related changes are seen in EEG power during brain development (Basar, Yordanova, Kolev, & Basar-Eroglu, 1997; Dustman, Shearer, & Emmerson, 1993; Katada, Ozaki, Suzuki, & Suhara, 1981). Consistent with our observations in rats, in the present study, higher cortical power in the EEG has also been reported in human adolescents relative to adults (Dustman, LaMarche, Cohn, Shearer, & Talone, 1985; Dustman, Shearer, & Emmerson, 1999; Ehlers, Wall, Garcia-Andrade, & Phillips, 2001).
Our findings of increased power in the slow-wave frequency bands in adolescent rats are also consistent with human studies (Gasser, Verleger, Bächer, & Sroka, 1988; Matsuura et al., 1985). Theoretically, active synapses responsible for the slow-wave frequency bands may be undergoing more extensive “rewiring” and synaptic pruning processes than active synapses responsible for high-frequency bands during adolescence. The slow-wave frequency bands are thought to arise primarily from highly synchronous local neural activity (Whitford et al., 2007) that is responsible for the large amplitude potentials associated with slow-wave activity (Steriade, Gloor, Llinas, Lopes de Silva, & Mesulam, 1990). Therefore, the loss in the number of synapses seen during adolescent “pruning” may be directly related to loss of EEG power in the slow-wave frequencies seen over development (Whitford et al., 2007). High-frequency activity is thought to arise primarily from asynchronous activity and is associated with low EEG power (Whitford et al., 2007). Consequently, when animals mature into adulthood, the power in the slow-wave frequencies is dramatically reduced, whereas high-frequency activity is less impacted, as supported by the findings in adult rats from the present study and our previous studies (Pian, Criado, Walker, et al., 2008).
The present study also found that acute administration of ethanol not only increased the power in the slow-wave frequency bands, but 20 h later, a decrease in slow waves was seen over the 3-h sleep cycle. Increases in power in the slow frequencies following acute ethanol drinking in human subjects have been observed previously (Ehlers, Wall, & Schuckit, 1989). Reductions in slow waves during the sleep cycle have also been reported to occur in protracted abstinence from long-term chronic ethanol vapor exposure in animal models (Criado et al., 2008; Ehlers & Slawecki, 2000) and in human alcoholics during abstinence (Irwin et al., 2000). Fragmentation of sleep has also been reported in alcohol-treated rats (Mukherjee & Simasko, 2009). Additionally, “binge-like” alcohol administration was found to be more disruptive of NREM sleep in alcohol-preferring rats (P-rats) than in non-alcohol preferring rats (NP) (Thakkar, Engemann, Sharma, Mohan, & Sahota, 2010). However, the spectral characteristics of the sleep EEG during acute alcohol withdrawal (hangover) have not been previously reported in either human or animal models. This study found that adolescent rats appear to be more sensitive to the suppression of slow-wave amplitude in the withdrawal/hangover period following a single acute dose of ethanol. This finding may be important since a strong relationship has been suggested between disruptions in sleep and substance abuse (Brower, 2001; Hasler et al., 2012) and risk taking (Telzer, Fuligni, Lieberman, & Galván, 2013) in adolescents, and relapse to drinking in abstinent adult alcoholics (Gillin et al., 1994). Other animal studies have provided data to support the idea that adolescents may be more sensitive to some of the symptoms of hangover and/or withdrawal. For instance, Slawecki and Roth (2004) found that hypoactivity developed more quickly in adolescents than in adults following alcohol withdrawal. Additionally, adolescents have been shown to have more high-frequency power in the parietal cortex following alcohol withdrawal than adults (Slawecki et al., 2006). However, anxiety, hyperthermia, and social suppression appear to all be less prominent in adolescent rats compared to adults following alcohol withdrawal (Brasser & Spear, 2002; Doremus et al., 2003; Doremus-Fitzwater & Spear, 2007; Slawecki & Roth, 2004; Varlinskaya & Spear, 2004). Taken together, these data suggest that sensitivity to ethanol withdrawal during adolescence can be increased or decreased depending on the symptom being assessed.
Since the present study found that adolescent rats, as compared to adults, demonstrate low sensitivity to acute ethanol administration in the theta frequency range and more susceptibility to slow-wave sleep disruption during hangover, it lends support to the idea that these traits may form part of the substrate of the increased risk for alcohol use disorders seen in adults who begin drinking in their early teenage years.
Acknowledgments
This work was supported by National Institute of Health (NIH) grants, U01 AA019969; R01 AA006059 to Cindy L. Ehlers from the National Institute on Alcohol Abuse and Alcoholism (NIAAA). The authors thank Shirley Sanchez for assistance in editing the manuscript and Phil Lau for help in statistical analyses. Dr. James Havstad developed the software used for EEG assessment.
References
- Andersen A, Due P, Holstein BE, Iversen L. Tracking drinking behaviour from age 15-19 years. Addiction. 2003;98:1505–1511. doi: 10.1046/j.1360-0443.2003.00496.x. [DOI] [PubMed] [Google Scholar]
- Basar E, Yordanova J, Kolev V, Basar-Eroglu C. Is the alpha rhythm a control parameter for brain responses? Biological Cybernetics. 1997;76:471–480. doi: 10.1007/s004220050360. [DOI] [PubMed] [Google Scholar]
- Brasser SM, Spear NE. Physiological and behavioral effects of acute ethanol hangover in juvenile, adolescent, and adult rats. Behavioral Neuroscience. 2002;116:305–320. doi: 10.1037//0735-7044.116.2.305. [DOI] [PubMed] [Google Scholar]
- Brower KJ. Alcohol's effects on sleep in alcoholics. Alcohol Research & Health. 2001;25:110–125. [PMC free article] [PubMed] [Google Scholar]
- Criado JR, Ehlers CL. Event-related oscillations in the parietal cortex of adult alcohol-preferring (P) and alcohol-nonpreferring rats (NP). Alcohol. 2010a;44:335–342. doi: 10.1016/j.alcohol.2010.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Criado JR, Ehlers CL. Effects of adolescent ethanol exposure on event-related oscillations (EROs) in the hippocampus of adult rats. Behavioural Brain Research. 2010b;210:164–170. doi: 10.1016/j.bbr.2010.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Criado JR, Ehlers CL. Effects of adolescent onset voluntary drinking followed by ethanol vapor exposure on subsequent ethanol consumption during protracted withdrawal in adult Wistar rats. Pharmacology, Biochemistry, and Behavior. 2013;103:622–630. doi: 10.1016/j.pbb.2012.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Criado JR, Liu T, Ehlers CL, Mathé AA. Prolonged chronic ethanol exposure alters neuropeptide Y and corticotropin-releasing factor levels in the brain of adult Wistar rats. Pharmacology, Biochemistry, and Behavior. 2011;99:104–111. doi: 10.1016/j.pbb.2011.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Criado JR, Wills DN, Walker BM, Ehlers CL. Effects of adolescent ethanol exposure on sleep in adult rats. Alcohol. 2008;42:631–639. doi: 10.1016/j.alcohol.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeWit DJ, Adlaf EM, Offord DR, Ogborne AC. Age at first alcohol use: a risk factor for the development of alcohol disorders. The American Journal of Psychiatry. 2000;157:745–750. doi: 10.1176/appi.ajp.157.5.745. [DOI] [PubMed] [Google Scholar]
- Doremus TL, Brunell SC, Varlinskaya EI, Spear LP. Anxiogenic effects during withdrawal from acute ethanol in adolescent and adult rats. Pharmacology, Biochemistry, and Behavior. 2003;75:411–418. doi: 10.1016/s0091-3057(03)00134-5. [DOI] [PubMed] [Google Scholar]
- Doremus-Fitzwater TL, Spear LP. Developmental differences in acute ethanol withdrawal in adolescent and adult rats. Alcoholism: Clinical and Experimental Research. 2007;31:1516–1527. doi: 10.1111/j.1530-0277.2007.00457.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dustman RE, LaMarche JA, Cohn NB, Shearer DE, Talone JM. Power spectral analysis and cortical coupling of EEG for young and old normal adults. Neurobiology of Aging. 1985;6:193–198. doi: 10.1016/0197-4580(85)90049-1. [DOI] [PubMed] [Google Scholar]
- Dustman RE, Shearer DE, Emmerson RY. EEG and event-related potentials in normal aging. Progress in Neurobiology. 1993;41:369–401. doi: 10.1016/0301-0082(93)90005-d. [DOI] [PubMed] [Google Scholar]
- Dustman RE, Shearer DE, Emmerson RY. Life-span changes in EEG spectral amplitude, amplitude variability and mean frequency. Clinical Neuro-physiology. 1999;110:1399–1409. doi: 10.1016/s1388-2457(99)00102-9. [DOI] [PubMed] [Google Scholar]
- Ehlers CL, Criado JR. Adolescent ethanol exposure: does it produce long-lasting electrophysiological effects? Alcohol. 2010;44:27–37. doi: 10.1016/j.alcohol.2009.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlers CL, Criado JR, Wills DN, Liu W, Crews FT. Periadolescent ethanol exposure reduces adult forebrain ChAT+IR neurons: correlation with behavioral pathology. Neuroscience. 2011;199:333–345. doi: 10.1016/j.neuroscience.2011.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlers CL, Havstad JW. Characterization of drug effects on the EEG by power spectral time series analysis. Psychopharmacology Bulletin. 1982;18:43–47. [Google Scholar]
- Ehlers CL, Slawecki CJ. Effects of chronic ethanol exposure on sleep in rats. Alcohol. 2000;20:173–179. doi: 10.1016/s0741-8329(99)00077-4. [DOI] [PubMed] [Google Scholar]
- Ehlers CL, Slutske WS, Gilder DA, Lau P, Wilhelmsen KC. Age at first intoxication and alcohol use disorders in Southwest California Indians. Alcoholism: Clinical and Experimental Research. 2006;30:1856–1865. doi: 10.1111/j.1530-0277.2006.00222.x. [DOI] [PubMed] [Google Scholar]
- Ehlers CL, Walker BM, Pian JP, Roth JL, Slawecki CJ. Increased alcohol drinking in isolate-housed alcohol-preferring rats. Behavioral Neuroscience. 2007;121:111–119. doi: 10.1037/0735-7044.121.1.111. [DOI] [PubMed] [Google Scholar]
- Ehlers CL, Wall TL, Garcia-Andrade C, Phillips E. Effects of age and parental history of alcoholism on EEG findings in mission Indian children and adolescents. Alcoholism: Clinical and Experimental Research. 2001;25:672–679. [PubMed] [Google Scholar]
- Ehlers CL, Wall TL, Schuckit MA. EEG spectral characteristics following ethanol administration in young men. Electroencephalography and Clinical Neurophysiology. 1989;73:179–187. doi: 10.1016/0013-4694(89)90118-1. [DOI] [PubMed] [Google Scholar]
- Ernst AJ, Dempster JP, Yee R, Dennis C, Nakano L. Alcohol toxicity, blood alcohol concentration and body water in young and adult rats. Journal of Studies on Alcohol. 1976;37:347–356. doi: 10.15288/jsa.1976.37.347. [DOI] [PubMed] [Google Scholar]
- Feinberg I. Schizophrenia: caused by a fault in programmed synaptic elimination during adolescence? Journal of Psychiatric Research. 1982;17:319–334. doi: 10.1016/0022-3956(82)90038-3. [DOI] [PubMed] [Google Scholar]
- Freund G. Alcohol withdrawal syndrome in mice. Archives of Neurology. 1969;21:315–320. doi: 10.1001/archneur.1969.00480150105013. [DOI] [PubMed] [Google Scholar]
- Gasser T, Verleger R, Bächer P, Sroka L. Development of the EEG of school-age children and adolescents. I. Analysis of band power. Electroencephalography and Clinical Neurophysiology. 1988;69:91–99. doi: 10.1016/0013-4694(88)90204-0. [DOI] [PubMed] [Google Scholar]
- Gillin JC, Smith TL, Irwin M, Butters N, De Modena A, Schuckit M. Increased pressure for rapid eye movement sleep at time of hospital admission predicts relapse in nondepressed patients with primary alcoholism at 3-month follow-up. Archives of General Psychiatry. 1994;51:189–197. doi: 10.1001/archpsyc.1994.03950030025003. [DOI] [PubMed] [Google Scholar]
- Grant BF, Dawson DA. Age at onset of alcohol use and its association with DSM-IV alcohol abuse and dependence: results from the National Longitudinal Alcohol Epidemiologic Survey. Journal of Substance Abuse. 1997;9:103–110. doi: 10.1016/s0899-3289(97)90009-2. [DOI] [PubMed] [Google Scholar]
- Grobin AC, Matthews DB, Devaud LL, Morrow AL. The role of GABA(A) receptors in the acute and chronic effects of ethanol. Psychopharmacology (Berl) 1998;139:2–19. doi: 10.1007/s002130050685. [DOI] [PubMed] [Google Scholar]
- Hasler BP, Clark DB. Circadian misalignment, reward-related brain function, and adolescent alcohol involvement. Alcoholism: Clinical and Experimental Research. 2013;37:558–565. doi: 10.1111/acer.12003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasler BP, Smith LJ, Cousins JC, Bootzin RR. Circadian rhythms, sleep, and substance abuse. Sleep Medicine Reviews. 2012;16:67–81. doi: 10.1016/j.smrv.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins JD, Graham JW, Maguin E, Abbott R, Hill KG, Catalano RF. Exploring the effects of age of alcohol use initiation and psychosocial risk factors on subsequent alcohol misuse. Journal of Studies on Alcohol. 1997;58:280–290. doi: 10.15288/jsa.1997.58.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollstedt C, Olsson O, Rydberg U. Effects of ethanol on the developing rat. II. Coordination as measured by the tilting-plane test. Medical Biology. 1980;58:164–168. [PubMed] [Google Scholar]
- Huttenlocher PR. Synaptic density in human frontal cortex – developmental changes and effects of aging. Brain Research. 1979;163:195–205. doi: 10.1016/0006-8993(79)90349-4. [DOI] [PubMed] [Google Scholar]
- Huttenlocher PR, Dabholkar AS. Regional differences in synaptogenesis in human cerebral cortex. The Journal of Comparative Neurology. 1997;387:167–178. doi: 10.1002/(sici)1096-9861(19971020)387:2<167::aid-cne1>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Iacono WG, Carlson SR, Malone SM, McGue M. P3 event-related potential amplitude and the risk for disinhibitory disorders in adolescent boys. Archives of General Psychiatry. 2002;59:750–757. doi: 10.1001/archpsyc.59.8.750. [DOI] [PubMed] [Google Scholar]
- Irwin M, Miller C, Gillin JC, De Modena A, Ehlers CL. Polysomno-graphic and spectral sleep EEG in primary alcoholics: an interaction between alcohol dependence and African-American ethnicity. Alcoholism: Clinical and Experimental Research. 2000;24:1376–1384. [PubMed] [Google Scholar]
- Jessor R, Jessor SL. Problem behavior and psychosocial development: A longitudinal study of youth. Academic Press; New York: 1977. [Google Scholar]
- Katada A, Ozaki H, Suzuki H, Suhara K. Developmental characteristics of normal and mentally retarded children's EEGs. Electroencephalography and Clinical Neurophysiology. 1981;52:192–201. doi: 10.1016/0013-4694(81)90166-8. [DOI] [PubMed] [Google Scholar]
- Little PJ, Kuhn CM, Wilson WA, Swartzwelder HS. Differential effects of ethanol in adolescent and adult rats. Alcoholism: Clinical and Experimental Research. 1996;20:1346–1351. doi: 10.1111/j.1530-0277.1996.tb01133.x. [DOI] [PubMed] [Google Scholar]
- Matsuura M, Yamamoto K, Fukuzawa H, Okubo Y, Uesugi H, Moriiwa M, et al. Age development and sex differences of various EEG elements in healthy children and adults – quantification by a computerized wave form recognition method. Electroencephalography and Clinical Neurophysiology. 1985;60:394–406. doi: 10.1016/0013-4694(85)91013-2. [DOI] [PubMed] [Google Scholar]
- McDonald JW, Silverstein FS, Johnston MV. Neuroprotective effects of MK-801, TCP, PCP and CPP against N-methyl-D-aspartate induced neurotoxicity in an in vivo perinatal rat model. Brain Research. 1989;490:33–40. doi: 10.1016/0006-8993(89)90427-7. [DOI] [PubMed] [Google Scholar]
- Mihic SJ. Acute effects of ethanol on GABAA and glycine receptor function. Neurochemistry International. 1999;35:115–123. doi: 10.1016/s0197-0186(99)00053-4. [DOI] [PubMed] [Google Scholar]
- Moy SS, Duncan GE, Knapp DJ, Breese GR. Sensitivity to ethanol across development in rats: comparison to [3H]zolpidem binding. Alcoholism: Clinical and Experimental Research. 1998;22:1485–1492. [PubMed] [Google Scholar]
- Mukherjee S, Simasko SM. Chronic alcohol treatment in rats alters sleep by fragmenting periods of vigilance cycling in the light period with extended wakenings. Behavioral Brain Research. 2009;198:113–124. doi: 10.1016/j.bbr.2008.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penning R, van Nuland M, Fliervoet LA, Olivier B, Verster JC. The pathology of alcohol hangover. Current Drug Abuse Reviews. 2010;3:68–75. doi: 10.2174/1874473711003020068. [DOI] [PubMed] [Google Scholar]
- Pian JP, Criado JR, Ehlers CL. Differential effects of acute alcohol on prepulse inhibition and event-related potentials in adolescent and adult Wistar rats. Alcoholism: Clinical and Experimental Research. 2008;32:2062–2073. doi: 10.1111/j.1530-0277.2008.00794.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pian JP, Criado JR, Milner R, Ehlers CL. N-methyl-d-aspartate receptor subunit expression in adult and adolescent brain following chronic ethanol exposure. Neuroscience. 2010;170:645–654. doi: 10.1016/j.neuroscience.2010.06.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pian JP, Criado JR, Walker BM, Ehlers CL. Differential effects of acute alcohol on EEG and sedative responses in adolescent and adult Wistar rats. Brain Research. 2008;1194:28–36. doi: 10.1016/j.brainres.2007.11.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piasecki TM, Alley KJ, Slutske WS, Wood PK, Sher KJ, Shiffman S, et al. Low sensitivity to alcohol: relations with hangover occurrence and susceptibility in an ecological momentary assessment investigation. Journal of Studies on Alcohol and Drugs. 2012;73:925–932. doi: 10.15288/jsad.2012.73.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piasecki TM, Robertson BM, Epler AJ. Hangover and risk for alcohol use disorders: existing evidence and potential mechanisms. Current Drug Abuse Reviews. 2010;3:92–102. doi: 10.2174/1874473711003020092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piasecki TM, Sher KJ, Slutske WS, Jackson KM. Hangover frequency and risk for alcohol use disorders: evidence from a longitudinal high-risk study. Journal of Abnormal Psychology. 2005;114:223–234. doi: 10.1037/0021-843X.114.2.223. [DOI] [PubMed] [Google Scholar]
- Proctor WR, Soldo BL, Allan AM, Dunwiddie TV. Ethanol enhances synaptically evoked GABAA receptor-mediated responses in cerebral cortical neurons in rat brain slices. Brain Research. 1992;595:220–227. doi: 10.1016/0006-8993(92)91053-h. [DOI] [PubMed] [Google Scholar]
- Purves D, Lichtman JW. Elimination of synapses in the developing nervous system. Science. 1980;210:153–157. doi: 10.1126/science.7414326. [DOI] [PubMed] [Google Scholar]
- Rakic P, Bourgeois JP, Eckenhoff MF, Zecevic N, Goldman-Rakic PS. Concurrent overproduction of synapses in diverse regions of the primate cerebral cortex. Science. 1986;232:232–235. doi: 10.1126/science.3952506. [DOI] [PubMed] [Google Scholar]
- Rohsenow DJ, Howland J, Arnedt JT, Almeida AB, Greece J, Minsky S, et al. Intoxication with bourbon versus vodka: effects on hangover, sleep, and next-day neurocognitive performance in young adults. Alcoholism: Clinical and Experimental Research. 2010;34:509–518. doi: 10.1111/j.1530-0277.2009.01116.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohsenow DJ, Howland J, Minsky SJ, Arnedt JT. Effects of heavy drinking by maritime academy cadets on hangover, perceived sleep, and next-day ship power plant operation. Journal of Studies on Alcohol. 2006;67:406–415. doi: 10.15288/jsa.2006.67.406. [DOI] [PubMed] [Google Scholar]
- Rohsenow DJ, Howland J, Winter M, Bliss CA, Littlefield CA, Heeren TC, et al. Hangover sensitivity after controlled alcohol administration as predictor of post-college drinking. Journal of Abnormal Psychology. 2012;121:270–275. doi: 10.1037/a0024706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose RJ, Dick DM, Viken RJ, Pulkkinen L, Kaprio J. Drinking or abstaining at age 14? A genetic epidemiological study. Alcoholism: Clinical and Experimental Research. 2001;25:1594–1604. [PubMed] [Google Scholar]
- Schuckit MA. Low level of response to alcohol as a predictor of future alcoholism. The American Journal of Psychiatry. 1994;151:184–189. doi: 10.1176/ajp.151.2.184. [DOI] [PubMed] [Google Scholar]
- Schuckit MA. New findings in the genetics of alcoholism. The Journal of the American Medical Association. 1999;281:1875–1876. doi: 10.1001/jama.281.20.1875. [DOI] [PubMed] [Google Scholar]
- Schuckit MA, Smith TL. An 8-year follow-up of 450 sons of alcoholic and control subjects. Archives of General Psychiatry. 1996;53:202–210. doi: 10.1001/archpsyc.1996.01830030020005. [DOI] [PubMed] [Google Scholar]
- Silveri MM, Spear LP. Decreased sensitivity to the hypnotic effects of ethanol early in ontogeny. Alcoholism: Clinical and Experimental Research. 1998;22:670–676. doi: 10.1111/j.1530-0277.1998.tb04310.x. [DOI] [PubMed] [Google Scholar]
- Silveri MM, Spear LP. Ontogeny of ethanol elimination and ethanol-induced hypothermia. Alcohol. 2000;20:45–53. doi: 10.1016/s0741-8329(99)00055-5. [DOI] [PubMed] [Google Scholar]
- Silveri MM, Spear LP. The effects of NMDA and GABAA pharmacological manipulations on ethanol sensitivity in immature and mature animals. Alcoholism: Clinical and Experimental Research. 2002;26:449–456. [PubMed] [Google Scholar]
- Slawecki CJ. Altered EEG responses to ethanol in adult rats exposed to ethanol during adolescence. Alcoholism: Clinical and Experimental Research. 2002;26:246–254. [PubMed] [Google Scholar]
- Slawecki CJ, Betancourt M, Cole M, Ehlers CL. Periadolescent alcohol exposure has lasting effects on adult neurophysiological function in rats. Brain Research. Developmental Brain Research. 2001;128:63–72. doi: 10.1016/s0165-3806(01)00150-x. [DOI] [PubMed] [Google Scholar]
- Slawecki CJ, Roth J. Comparison of the onset of hypoactivity and anxiety-like behavior during alcohol withdrawal in adolescent and adult rats. Alcoholism: Clinical and Experimental Research. 2004;28:598–607. doi: 10.1097/01.alc.0000122767.69206.1b. [DOI] [PubMed] [Google Scholar]
- Slawecki CJ, Roth J, Gilder A. Neurobehavioral profiles during the acute phase of ethanol withdrawal in adolescent and adult Sprague-Dawley rats. Behavioural Brain Research. 2006;170:41–51. doi: 10.1016/j.bbr.2006.01.023. [DOI] [PubMed] [Google Scholar]
- Slawecki CJ, Thorsell A, Ehlers CL. Long-term neurobehavioral effects of alcohol or nicotine exposure in adolescent animal models. Annals of the New York Academy of Sciences. 2004;1021:448–452. doi: 10.1196/annals.1308.062. [DOI] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neuroscience and Biobehavioral Reviews. 2000;24:417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Steriade M, Gloor P, Llinas RR, Lopes de Silva FH, Mesulam MM. Report of IFCN Committee on Basic Mechanisms. Basic mechanisms of cerebral rhythmic activities. Electroencephalography and Clinical Neurophysiology. 1990;76:481–508. doi: 10.1016/0013-4694(90)90001-z. [DOI] [PubMed] [Google Scholar]
- Swift R, Davidson D. Alcohol hangover: mechanisms and mediators. Alcohol Health and Research World. 1998;22:54–60. [PMC free article] [PubMed] [Google Scholar]
- Telzer EH, Fuligni AJ, Lieberman MD, Galván A. The effects of poor quality sleep on brain function and risk taking in adolescence. NeuroImage. 2013;71:275–283. doi: 10.1016/j.neuroimage.2013.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakkar MM, Engemann SC, Sharma R, Mohan RR, Sahota P. Sleep-wakefulness in alcohol preferring and non-preferring rats following binge alcohol administration. Neuroscience. 2010;170:22–27. doi: 10.1016/j.neuroscience.2010.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varlinskaya EI, Spear LP. Acute effects of ethanol on social behavior of adolescent and adult rats: role of familiarity of the test situation. Alcoholism: Clinical and Experimental Research. 2002;26:1502–1511. doi: 10.1097/01.ALC.0000034033.95701.E3. [DOI] [PubMed] [Google Scholar]
- Varlinskaya EI, Spear LP. Acute ethanol withdrawal (hangover) and social behavior in adolescent and adult male and female Sprague-Dawley rats. Alcoholism: Clinical and Experimental Research. 2004;28:40–50. doi: 10.1097/01.ALC.0000108655.51087.DF. [DOI] [PubMed] [Google Scholar]
- Weiner JL, Gu C, Dunwiddie TV. Differential ethanol sensitivity of subpopulations of GABAA synapses onto rat hippocampal CA1 pyramidal neurons. Journal of Neurophysiology. 1997;77:1306–1312. doi: 10.1152/jn.1997.77.3.1306. [DOI] [PubMed] [Google Scholar]
- Weiner JL, Zhang L, Carlen PL. Potentiation of GABAA-mediated synaptic current by ethanol in hippocampal CA1 neurons: possible role of protein kinase C. The Journal of Pharmacology and Experimental Therapeutics. 1994;268:1388–1395. [PubMed] [Google Scholar]
- Whitford TJ, Rennie CJ, Grieve SM, Clark CR, Gordon E, Williams LM. Brain maturation in adolescence: concurrent changes in neuroanatomy and neurophysiology. Human Brain Mapping. 2007;28:228–237. doi: 10.1002/hbm.20273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witt ED. Mechanisms of alcohol abuse and alcoholism in adolescents: a case for developing animal models. Behavioral and Neural Biology. 1994;62:168–177. doi: 10.1016/s0163-1047(05)80015-9. [DOI] [PubMed] [Google Scholar]