Abstract
Background
Changes in the multiple mechanisms that regulate glucose metabolism after gastric bypass (RYGB) are still being unveiled.
Objective
To compare the changes of glucose and pancreatic hormones [C-peptide, glucagon and pancreatic polypeptide (PP)] during a meal test (MTT) and steady-state insulin and free fatty acid (FFA) concentrations during euglycemic–hyperinsulinemic clamp 14 days and 6 months after RYGB in morbidly obese non-diabetic patients.
Setting
Academic Medical Center, United States.
Methods
Two groups were studied at baseline and at 14 days: RYGB followed by caloric restriction (RYGB, n=12) or equivalent caloric restriction alone (Diet, n=10), to control for energy intake and weight loss. The RYGB group was studied again at 6 months, to assess the changes after substantial weight loss. During MTT we determined the early and overall changes in glucose and pancreatic hormone concentrations, and during the clamp we assessed steady-state insulin and FFA concentrations.
Results
After 14 days, RYGB subjects had enhanced post-prandial glucose, C-peptide and glucagon responses and decreased post-prandial PP concentrations. Steady-state insulin concentrations were decreased at 14 days only in RYGB subjects, and FFA increased in both groups. Six months after RYGB and substantial weight loss, the decrease in insulin concentrations during clamp persisted, and there were further changes in post-prandial glucose and glucagon responses. FFA concentrations during clamp were significantly lower at 6 months, relative to pre-surgical values.
Conclusions
RYGB produces, in morbidly obese non-diabetic patients, early changes in post-meal glucose, C-peptide, glucagon and PP responses, and appears to enhance insulin clearance early after RYGB and improve insulin sensitivity in adipose tissue at 6 months post-surgery. The early changes cannot be explained by caloric restriction alone.
Keywords: Gastric bypass, gut hormones, incretins, insulin resistance, free fatty acids, insulin clearance, hyperinsulinemic euglycemic clamp, bariatric surgery, C-peptide, glucagon, glucose, type 2 diabetes
INTRODUCTION
Roux-en-Y gastric bypass (RYGB) is the most common bariatric surgical procedure used to treat morbid obesity1 and promotes changes in the regulation of glucose metabolism2. In addition to improving glucose homeostasis as a consequence of the robust weight loss that occurs with this procedure, other factors that are likely to contribute to the effects of RYGB on insulin secretion and action include the magnitude of caloric restriction, reduction in adipose tissue mass, altered gastrointestinal (GI) and pancreatic hormone responses such as changes in insulin, glucagon-like peptide 1 (GLP-1), glucose-dependent insulinotropic polypeptide (GIP), as well as changes in beta cell function, hepatic glucose metabolism and bile acids, among others2–4. Although previous studies have reported differences in many of these parameters after RYGB, the majority of these studies are cross-sectional and few have controlled for reduced energy intake. In addition, few studies have studied the effects of surgery on post-prandial glucose kinetics 5,6, glucagon 7 and pancreatic polypeptide (PP) responses 8, and insulin sensitivity of adipose tissue 9 as assessed by insulin-mediated suppression of free fatty acids (FFA). Lastly, the potential for changes of hepatic insulin clearance after RYGB has not been investigated. The current study is a follow-up from previously published study design, subjects, and other metabolic data10, with additional analysis of the effects of RYGB on glucose, C-peptide, glucagon, and PP responses during a meal tolerance test (MTT); and steady state circulating insulin and FFA concentrations during a euglycemic–hyperinsulinemic clamp. Metabolic studies were done before and 14 days after RYGB followed by caloric restriction or equivalent caloric restriction alone, as well as 6 months after RYGB.
MATERIAL AND METHODS
Morbidly obese non-diabetic patients, selected to undergo RYGB, were recruited at the University of California San Francisco’s (UCSF) Bariatric Surgery Program. They met the National Institutes of Health and UCSF Bariatric Surgery Program eligibility criteria for bariatric surgery as described previously10. Exclusion criteria included previous weight loss, foregut and/or hindgut surgery; and diagnosis of endocrine or chronic renal disease. This project was approved by the UCSF Committee on Human Research and San Francisco General Hospital Clinical Research Center (CRC) Advisory Committee. Written consent was obtained from each participant.
Group Allocation and Metabolic Evaluation
The two groups were studied at baseline and at 14 days: RYGB followed by caloric restriction (RYGB N=12) or equivalent caloric restriction alone (Diet N=10), to control for reduced energy intake and weight loss. As previously reported10, the two study groups did not differ with respect to baseline demographics (Female/Male Ratio: 9:3 RYGB, 6:4 Diet, p=0.65 and Age in years 47.4±8.7 RYGB, 40.2±13.4 Diet, p=0.16) and body composition [Weight (kg): 138.0±21.6 RYGB, 134.7±16.9 Diet, p=0.70; BMI (kg/m2): 48.4±6.8 RYGB, 48.3±6.6 Diet, p=0.99 and % Excess body weight: 55.4±6.4 RYGB, 55.3±6.8 Diet, p=0.96), and participants in both groups were markedly insulin resistant at baseline. The RYGB group was studied again at 6 months; to assess the longer term changes after more substantial weight loss had occurred.
All participants underwent the same baseline metabolic evaluation (visit 1, V1)10.
Meal Tolerance Test
On day 1, participants underwent a mixed meal tolerance test (MTT), in which they ingested a standardized 282 kcal, 100 mL liquid meal containing 50% carbohydrate, 20% protein, and 30% fat with 9.9 g of simple sugars. Participants consumed this meal within a maximum of 20 minutes. Venous blood samples were drawn at 0, +5, +15, +30, +60 +120, and +180 minutes relative to the end of the meal. The samples were processed on site and stored at −70°C for subsequent batch analysis of glucose, C-peptide, glucagon, and PP.
Euglycemic–Hyperinsulinemic Clamp
On day 2, after an overnight fast, whole-body insulin sensitivity and insulin and FFA concentrations were measured during the steady state interval (60–120 minutes) of a euglycemic-hyperinsulinemic clamp as described previously 10,11. Insulin (Humulin R, Eli Lilly, Indianapolis, IN, USA), bound to albumin, was administered intravenously at a rate of 40 mU/m2/min for 120 min. Blood was drawn by intravenous catheter in a heated vein, and whole-blood glucose concentrations were measured in real time at 5-min intervals (YSI STAT 2300 glucose analyzer, Yellow Springs, OH). Infusion of 20% dextrose was adjusted to maintain a whole-blood glucose level of 90 mg/dL.
Surgery
The participants assigned to immediate surgery were discharged from the CRC and admitted for surgery the next day. The RYGB was performed in a standardized fashion by one author (GC) as previously described 10. In brief, RYGB was performed laparoscopically, a 30-mL gastric pouch created and connected to an alimentary limb of 100 cm and a biliopancreatic limb of 50 cm.
Participants were then followed as outpatients for 14 days, during which they consumed a standardized low calorie diet: Optifast HP (Novartis Nutrition Corporation), which provides 800 kcal/day (25% carbohydrate, 48% protein, and 27% fat).
Follow-up in Patients Undergoing Diet Alone
After completing the baseline evaluation and discharge from the CRC, participants assigned to the Diet group started the 14-day diet period at home, consuming the identical standardized diet and with the same follow-up procedures as described for the RYGB group above. Physical activity was not assessed in either group.
Follow-up Metabolic Assessments (Visit 2 and Visit 3)
After 14 days of hypocaloric feeding, all participants were readmitted to the CRC (visit 2, V2) and underwent the same metabolic assessments that were performed at V1. As previously reported10, after 14 days of caloric restriction with or without RYGB, the magnitude of weight loss and changes in body composition did not differ significantly between groups (Weight loss (kg): 9.9±2.4 RYGB, 8.2±2.3 Diet, p=0.11; % Excess weight loss: 12.7±2.4 RYGB, 10.9±2.8 Diet, p=0.12 and % of weight loss as fat: 40.4±16.2 RYGB, 29.9±16.8 Diet, p=0.22) They were then discharged and continued their standard medical treatment. Six participants in the Diet group underwent RYGB after the V2 assessment. A total of 12 participants (nine who were originally assigned to RYGB and three originally assigned to Diet who subsequently underwent RYGB) had a third inpatient evaluation 6 months after RYGB (visit 3, V3). Six months after undergoing RYGB substantial weight loss was achieved in all participants (35.7±5.2 kg; EWL=49.7%, p<0.01 vs. V1), of which 72.1% was fat10. The remaining participants in the Diet group went on to have bariatric surgery and were not studied further.
Laboratory Analyses
Whole-blood and plasma glucose levels were measured by the glucose oxidase method (YSI 2300 STAT-Plus Glucose Analyzer, YSI Inc., Yellow Springs, OH, USA). Serum insulin, C-peptide and glucagon concentrations were measured by radioimmunoassay (Millipore, St. Charles, MO, USA). Pancreatic polypeptide concentrations were measured by radioimmunoassay (Alpco, Salem, NH, USA). Free fatty acid concentrations were measured by enzymatic assay (Wako Diagnostics, Richmond, VA, USA).
Statistical Analysis
Data are summarized as mean and standard deviation unless otherwise stated. The unadjusted association of proportions between groups was determined by chi-square test. Changes in continuous variables from V1 to V2, V1 to V3 and from V2 to V3 were compared between and within groups using two-sided and paired t tests respectively. The early changes in glucose and pancreatic hormone concentrations during the MTT were calculated by linear regression of the estimated slope representing the change (increase or decrease) of glucose and hormone concentrations between 0 and 15 minutes (Slope 0–15). To study the overall changes in glucose and hormone concentrations in response to MTT, we calculated, using the trapezoidal rule, the area under the curve from 0 to 180 minutes during the MTT (AUC 0–180). In addition, the rate of glucose disappearance from the bloodstream was calculated by linear regression of the estimated slope representing the change in glucose from 30 minutes after the start of the meal to the lowest level of glucose during MTT (glucose disappearance). Average insulin and FFA concentrations during the steady-state interval (60–120 minutes) of the clamp were calculated. Calculation of excess weight loss (EWL) used Metropolitan Life Insurance tables to determine ideal weight 12. Statistical significance was considered to be p<0.05. SPSS, version 13.0.1 (SPSS Inc, Chicago, IL, USA), was used for all statistical analyses.
RESULTS
Baseline Evaluation (V1)
Prior to surgical and diet interventions, glucose and pancreatic hormone concentrations during the MTT and insulin and FFA concentrations during the clamp were generally similar for both groups (Figures 1 and 2), with the exception of C-peptide concentrations, which were slightly higher in the Diet group.
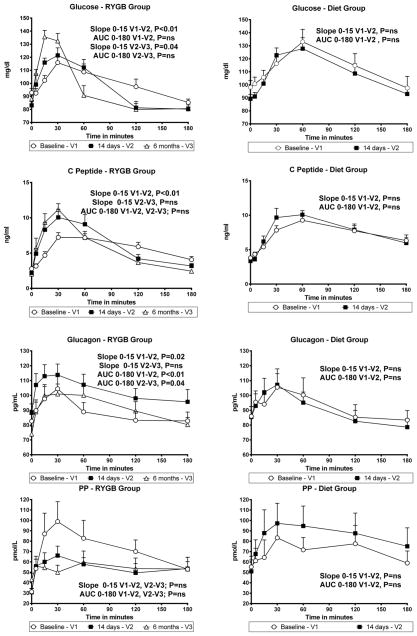
Figure 1.
Glucose, C-peptide, Glucagon and PP Concentrations after a Meal Tolerance Test at Baseline, 14 days (RYGB and Diet), and 6 months after (RYGB only).
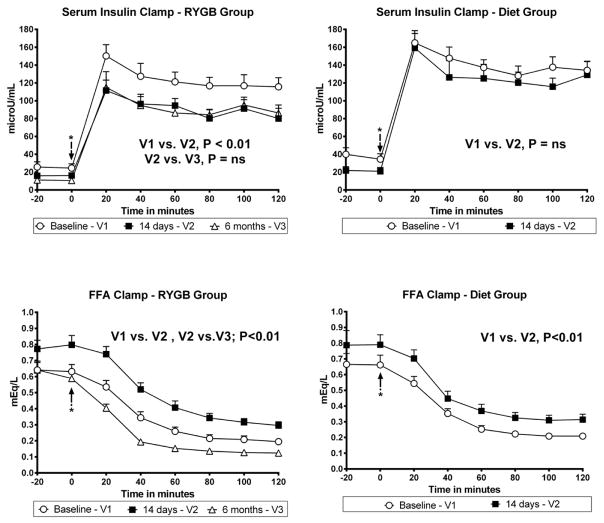
Figure 2.
Mean Insulin Concentrations and Free Fatty Acid Concentrations during Euglycemic Hyperinsulinemic Clamp at Baseline, 14 days (RYGB and Diet), and 6 months after (RYGB only). (* Marks Clamp procedure start time)
Changes at 14 days (RYGB and Diet, V2) and at 6 months (RYGB, V3)
Glucose Concentrations during the MTT
After 14 days, the early increase of plasma glucose concentrations (Slope 0–15 min) was significantly greater than at baseline in the RYGB group but not with Diet alone (Figure 1). Similarly, the rate of glucose disappearance (Slope 30–120 min) was significantly accelerated in the RYGB group (difference in Slope 30–120 between V1 and V2 = −0.26±0.05 mg/dl/min, p=0.02), but remained unchanged in the Diet group (−0.07±0.05 mg/dl/min, p=0.59). The overall AUC 0–180 for glucose did not change in either group. Six months after RYGB there was a further increase in the early change in plasma glucose concentrations (Figure 1) and in glucose disappearance.
Pancreatic Hormone Concentrations During the MTT
C-peptide
After 14 days, the early changes in plasma C-peptide concentrations during the MTT were significantly greater than at baseline in the RYGB group, whereas changes in C-peptide concentrations were similar in the Diet group (Figure 1). There were no significant changes in AUC 0–180 for C-peptide concentrations in either group between baseline and day 14. There were no further significant changes in C-peptide slopes or AUC 0–180 six months after RYGB (Figure 1).
Glucagon
After 14 days, both the early change and AUC 0–180 for glucagon increased significantly in the RYGB group, but remained unchanged in the Diet group (Figure 1). Six months after RYGB, AUC 0–180 for glucagon decreased significantly from responses measured at 14 days and returned to concentrations similar to those observed at baseline (Figure 1). In addition, 6 months after RYGB, fasting glucagon concentrations also decreased significantly from the concentrations measured at 14 days (88.3 pg/mL to 73.9 pg/mL, p=0.03)
Pancreatic Polypeptide (PP)
Early increases of PP concentrations did not change significantly in either group at 14 days or 6 months compared with baseline (Figure 1). However, there was a trend for a decrease in the AUC 0–180 14 days after RYGB (−19.2±37.2 pmol/L, p=0.14 vs. baseline), and a trend toward an increase in the Diet group (17.7±24.1, p=0.08 vs. baseline); thus the difference in AUC 0–180 between groups at 14 days was statistically significant (p=0.02). There were no further significant changes of slopes or the AUC 0–180 for PP, 6 months after RYGB (Figure 1).
Steady-State Insulin and Free Fatty Acid Concentrations During Euglycemic–Hyperinsulinemic Clamp
After 14 days, the average steady-state insulin concentration during the final hour of the clamp decreased significantly in the RYGB group, but was unchanged in the Diet group (Figure 2). The decrease of steady-state insulin concentrations persisted 6 months after RYGB. FFA concentrations increased significantly and to a similar extent under fasting conditions and during the clamp in both groups after 14 days. FFA concentrations decreased significantly 6 months after RYGB (Figure 2).
DISCUSSION
In this study, we observed that RYGB accompanied by caloric restriction was associated with changes in post-prandial glucose kinetics and concentrations of C-peptide, glucagon, and PP in response to a meal, as well as changes in steady-state insulin concentrations during a clamp that were not observed after caloric restriction alone. We also demonstrated that, after substantial weight loss had occurred, there were further changes of postprandial glucose kinetics and glucagon responses; and that the augmented postprandial C-peptide responses observed at 14 days persisted 6 months after surgery. Lastly, FFA concentrations were significantly lower at 6 months, relative to pre-surgical and 14-day values.
A major strength of this study is that the metabolic assessments were performed at baseline and after only 14 days in both groups, while assigned similar diets, to minimize the effects of different degrees of caloric restriction or weight loss. The follow-up study performed 6 months after RYGB then allowed us to study the effects associated with more substantial weight loss. Other studies have described the effects of RYGB on glucose homeostasis, but many of the improvements may also be associated with the profound negative energy balance and/or weight loss that ensue after surgery13. These considerations are important as reductions of fasting insulin and glucose concentrations14,15 and hepatic glucose production16 have been well documented during periods of negative energy balance induced by hypocaloric feeding and following weight loss17. Our study provides additional evidence for the presence of unique changes promoted by RYGB that are independent of marked caloric restriction and modest, but rapid weight loss early after surgery.
The changes of postprandial glucose kinetics we observed after RYGB are in accord with a cross-sectional study by Rodieux et al.5 that compared non-diabetic, weight-stable women who had undergone RYGB to those who underwent adjustable gastric banding, and matched control subjects. Meal-associated glucose kinetics were assessed using labeled glucose. RYGB subjects showed a more rapid appearance of orally-ingested glucose in the systemic circulation and a shorter duration of postprandial hyperglycemia than those who had undergone banding or the unoperated controls. These findings are similar with those observed in our study, as RYGB subjects had an early post-prandial increase of plasma glucose followed by an accelerated rate of plasma glucose disappearance after MTT both at 14 days and 6 months. We also reported that, at both 14 days and at 6 months after RYGB, there was an early increase in the C-peptide responses to meal ingestion; however the integrated overall response (AUC) was unchanged from baseline concentrations. These results provide additional evidence that RYGB modify the dynamic of the beta cell secretion by promoting an earlier and accentuated release of post-prandial C-peptide. In addition, our data support the hypothesis that these changes occur partly in response the early post-prandial increase of plasma glucose, as c-peptide and insulin secretion is partly regulated by the glucose available to the pancreatic beta cell6,18,19.
A novel finding here reportedis the paradoxical increase in post-prandial glucagon responses at 14 days post-surgery and reduced PP responses in the RYGB group only, changes that were not sustained 6 months after surgery. This was not an expected finding, as we and others have reported accentuated post-prandial GLP-1 responses after RYGB, and a known effect of GLP-1 is inhibition of glucagon secretion 20. Others have reported, though without a diet control group, an increase of postprandial glucagon excursions early after RYGB21 and a decrease in fasting glucagon concentrations after RYGB and substantial weight loss22, a finding similar to what was noted in our study. The increase, observed 14 days after RYGB, likely reflects an inability of glucose to adequately suppress glucagon release after the meal. It has been suggested that this could be due to “pancreatic type processing” of proglucagon in the intestinal L-cell with subsequent glucagon release23. The assay used to measure pancreatic glucagon in this study does not cross-react with oxyntomodulin (enteroglucagon) which has been reported to increase after RYGB23,24. Another possible explanation would be an increased activation of the parasympathetic input to the pancreatic islets, as parasympathetic activation increases glucagon secretion via release of acetylcholine and neuropeptides such as vasoactive intestinal polypeptide from cholinergic and peptidergic nerve terminals25. However, this mechanism appears unlikely since PP responses following the test meal were not increased, but were instead reduced after RYGB. PP is an islet hormone that is well known to be closely regulated by the activity of the parasympathetic input to the islet25. The observation that plasma glucagon responses to MTT were increased 14 days after RYGB makes suppression of glucagon an unlikely explanation for the improvement of postprandial glucose metabolism immediately after surgery. However, fasting glucagon concentrations did decrease progressively with ensuing weight loss in the months following RYGB in our study and also as has been previously reported 22, and this could be an additional long-term benefit of RYGB on glucose metabolism.
An important and interesting finding of our study was that steady-state insulin concentrations during the clamp, decreased in the RYGB group to a greater extent than in the Diet group. This result suggests that insulin is cleared more rapidly following RYGB, an effect that persisted after 6 months. This finding points to the possibility that the liver (as the central organ regulating glucose storage and production) is likely an important target for the early improvements of glucose metabolism following RYGB. In addition, studies by others have demonstrated an association between liver fat and the rate of insulin clearance26 and that liver fat decreases with RYGB and caloric restriction27. Thus, increased insulin clearance following RYGB may occur as a result of differential reduction in total liver fat content after RYGB. This is supported by the observation by others that the liver is responsible for more than 80% of insulin clearance in the human body and that the main driver of liver insulin clearance rates is liver fat content26,28,29. A possible, but untested hypothesis, is a direct effect of the increased postprandial GLP-1 levels on liver fat content30,31,32.
Lastly, FFA concentrations increased significantly and to a similar extent during the clamp in both groups at 14 days. The increase of fasting FFA concentrations after 14 days is consistent with increased fasting lipolysis in individuals in negative energy balance and has been reported to occur in other studies of the short-term effects of RYGB 22. The higher FFA concentrations during the clamp could result from either a blunting of the ability of insulin to suppress lipolysis or decreased FFA clearance (e.g., fat oxidation). At 6 months after surgery, fasting FFA concentrations returned to baseline levels, likely due to the subjects being in much less marked negative energy balance. Our study also demonstrated that FFA concentrations during the clamp were significantly lower after 6 months, relative to pre-surgical values, suggesting improved insulin sensitivity in adipose tissue at 6 months.
It is important to note that we studied only non-diabetic morbidly obese patients. Diabetic patients may have worse beta cell function and different hepatic and peripheral dysfunctions in gluco-regulatory mechanisms and thus likely respond differently to some interventions. Other limitations of our study include slight but not statistically significant imbalance between groups in gender distribution, weight loss as fat after 14 days, and possible differences in diet absorption, that when combined may have impacted the results at 14 days.
CONCLUSIONS
Despite these limitations, we conclude that, in morbidly obese non-diabetics, RYGB is associated with early and persistent changes in post-prandial glucose kinetics and pancreatic hormone concentrations. In addition, our data suggest enhanced hepatic insulin clearance early after RYGB, as well as improved insulin sensitivity in adipose tissue at 6 months. These findings, taken together with the other documented changes in GI hormones concentrations, hepatic glucose metabolism, hepatic and peripheral insulin resistance3,4,6, provide additional evidence that the short and long-term metabolic and endocrine effects of RYGB, caloric restriction and weight loss have specific and independent effects in the many organs and tissues that are involved in glycemic control, including the gut, liver, pancreas, adipose tissue and muscle; and that these combined effects then contribute to the global improvements in glucose metabolism following RYGB.
Acknowledgments
This research was supported by Grant Number KL2 RR024130 from the National Center for Research Resources (NCRR), a component of the NIH and NIH Roadmap for Medical Research (GMC), and by NIH/NCRR UCSF-CTSI Grant Number UL1 RR024131. Dr. Havel’s research program receives support from NIH grants HL-075675, HL-091333, AT-003545, DK-097307, DK-095980 and a Multicampus Award (#142691) from the University of California, Office of the President. We are also grateful for the assistance of the SFGH-CRC nursing, dietary, and laboratory staff, especially Laurie Herraiz, RD, Veronica Monti, RD, and Viva Tai, MPH, RD; James Graham, of the University of California Davis, who coordinated the hormone analyses and Ruxandra Ciovica, MD and Sofia Peeva, BS for their contributions to study design and implementation, patient recruitment and data tabulation and analyses.
Footnotes
Author Contributions: G.M.C. obtained funding, designed the study, researched data, wrote the manuscript. C.R. designed the study, researched data, reviewed/edited the manuscript. P.J.H. researched data, reviewed/edited the manuscript. M.R. designed the study, researched data, reviewed/edited the manuscript. J.M.S. advised on study design, reviewed/edited the manuscript. M.S. contributed to study design, researched data, reviewed/edited the manuscript. K.M. contributed to study design, researched data, reviewed/edited the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Buchwald H, Estok R, Fahrbach K, et al. Weight and type 2 diabetes after bariatric surgery: systematic review and meta-analysis. Am J Med. 2009;122:248–56. e5. doi: 10.1016/j.amjmed.2008.09.041. [DOI] [PubMed] [Google Scholar]
- 2.Ferrannini E, Mingrone G. Impact of different bariatric surgical procedures on insulin action and beta-cell function in type 2 diabetes. Diabetes Care. 2009;32:514–20. doi: 10.2337/dc08-1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Salehi M, Aulinger B, Prigeon RL, D’Alessio DA. Effect of endogenous GLP-1 on insulin secretion in type 2 diabetes. Diabetes. 2010;59:1330–7. doi: 10.2337/db09-1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morinigo R, Casamitjana R, Delgado S, et al. Insulin resistance, inflammation, and the metabolic syndrome following Roux-en-Y gastric bypass surgery in severely obese subjects. Diabetes Care. 2007;30:1906–8. doi: 10.2337/dc07-0189. [DOI] [PubMed] [Google Scholar]
- 5.Rodieux F, Giusti V, D’Alessio DA, Suter M, Tappy L. Effects of gastric bypass and gastric banding on glucose kinetics and gut hormone release. Obesity (Silver Spring) 2008;16:298–305. doi: 10.1038/oby.2007.83. [DOI] [PubMed] [Google Scholar]
- 6.Anderwald CH, Tura A, Promintzer-Schifferl M, et al. Alterations in gastrointestinal, endocrine, and metabolic processes after bariatric Roux-en-Y gastric bypass surgery. Diabetes Care. 2012;35:2580–7. doi: 10.2337/dc12-0197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jacobsen SH, Olesen SC, Dirksen C, et al. Changes in gastrointestinal hormone responses, insulin sensitivity, and beta-cell function within 2 weeks after gastric bypass in non-diabetic subjects. Obes Surg. 2012;22:1084–96. doi: 10.1007/s11695-012-0621-4. [DOI] [PubMed] [Google Scholar]
- 8.Ramon JM, Salvans S, Crous X, et al. Effect of Roux-en-Y gastric bypass vs sleeve gastrectomy on glucose and gut hormones: a prospective randomised trial. J Gastrointest Surg. 2012;16:1116–22. doi: 10.1007/s11605-012-1855-0. [DOI] [PubMed] [Google Scholar]
- 9.Curry TB, Roberts SK, Basu R, et al. Gastric bypass surgery is associated with near-normal insulin suppression of lipolysis in nondiabetic individuals. Am J Physiol Endocrinol Metab. 2011;300:E746–51. doi: 10.1152/ajpendo.00596.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Campos GM, Rabl C, Peeva S, et al. Improvement in peripheral glucose uptake after gastric bypass surgery is observed only after substantial weight loss has occurred and correlates with the magnitude of weight lost. J Gastrointest Surg. 2010;14:15–23. doi: 10.1007/s11605-009-1060-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol. 1979;237:E214–23. doi: 10.1152/ajpendo.1979.237.3.E214. [DOI] [PubMed] [Google Scholar]
- 12.Deitel M, Gawdat K, Melissas J. Reporting weight loss 2007. Obes Surg. 2007;17:565–8. doi: 10.1007/s11695-007-9116-0. [DOI] [PubMed] [Google Scholar]
- 13.Laferrere B. Effect of gastric bypass surgery on the incretins. Diabetes Metab. 2009;35:513–7. doi: 10.1016/S1262-3636(09)73458-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Henry RR, Scheaffer L, Olefsky JM. Glycemic effects of intensive caloric restriction and isocaloric refeeding in noninsulin-dependent diabetes mellitus. J Clin Endocrinol Metab. 1985;61:917–25. doi: 10.1210/jcem-61-5-917. [DOI] [PubMed] [Google Scholar]
- 15.Schwartz MW, Seeley RJ. Seminars in medicine of the Beth Israel Deaconess Medical Center. Neuroendocrine responses to starvation and weight loss. N Engl J Med. 1997;336:1802–11. doi: 10.1056/NEJM199706193362507. [DOI] [PubMed] [Google Scholar]
- 16.Schwarz JM, Neese RA, Turner S, Dare D, Hellerstein MK. Short-term alterations in carbohydrate energy intake in humans. Striking effects on hepatic glucose production, de novo lipogenesis, lipolysis, and whole-body fuel selection. J Clin Invest. 1995;96:2735–43. doi: 10.1172/JCI118342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Petersen KF, Dufour S, Befroy D, Lehrke M, Hendler RE, Shulman GI. Reversal of nonalcoholic hepatic steatosis, hepatic insulin resistance, and hyperglycemia by moderate weight reduction in patients with type 2 diabetes. Diabetes. 2005;54:603–8. doi: 10.2337/diabetes.54.3.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ma J, Rayner CK, Jones KL, Horowitz M. Insulin secretion in healthy subjects and patients with Type 2 diabetes--role of the gastrointestinal tract. Best Pract Res Clin Endocrinol Metab. 2009;23:413–24. doi: 10.1016/j.beem.2009.03.009. [DOI] [PubMed] [Google Scholar]
- 19.Baggio LL, Drucker DJ. Biology of incretins: GLP-1 and GIP. Gastroenterology. 2007;132:2131–57. doi: 10.1053/j.gastro.2007.03.054. [DOI] [PubMed] [Google Scholar]
- 20.Holst JJ, Gromada J. Role of incretin hormones in the regulation of insulin secretion in diabetic and nondiabetic humans. Am J Physiol Endocrinol Metab. 2004;287:E199–206. doi: 10.1152/ajpendo.00545.2003. [DOI] [PubMed] [Google Scholar]
- 21.Jorgensen NB, Jacobsen SH, Dirksen C, et al. Acute and long-term effects of Roux-en-Y gastric bypass on glucose metabolism in subjects with Type 2 diabetes and normal glucose tolerance. Am J Physiol Endocrinol Metab. 2012;303:E122–31. doi: 10.1152/ajpendo.00073.2012. [DOI] [PubMed] [Google Scholar]
- 22.Swarbrick MM, Stanhope KL, Austrheim-Smith IT, et al. Longitudinal changes in pancreatic and adipocyte hormones following Roux-en-Y gastric bypass surgery. Diabetologia. 2008;51:1901–11. doi: 10.1007/s00125-008-1118-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Falken Y, Hellstrom PM, Holst JJ, Naslund E. Changes in glucose homeostasis after Roux-en-Y gastric bypass surgery for obesity at day three, two months, and one year after surgery: role of gut peptides. J Clin Endocrinol Metab. 2011;96:2227–35. doi: 10.1210/jc.2010-2876. [DOI] [PubMed] [Google Scholar]
- 24.Laferrere B, Swerdlow N, Bawa B, et al. Rise of oxyntomodulin in response to oral glucose after gastric bypass surgery in patients with type 2 diabetes. J Clin Endocrinol Metab. 2010;95:4072–6. doi: 10.1210/jc.2009-2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Havel PJ, Taborsky GJ., Jr The contribution of the autonomic nervous system to changes of glucagon and insulin secretion during hypoglycemic stress. Endocr Rev. 1989;10:332–50. doi: 10.1210/edrv-10-3-332. [DOI] [PubMed] [Google Scholar]
- 26.Kotronen A, Seppala-Lindroos A, Bergholm R, Yki-Jarvinen H. Tissue specificity of insulin resistance in humans: fat in the liver rather than muscle is associated with features of the metabolic syndrome. Diabetologia. 2008;51:130–8. doi: 10.1007/s00125-007-0867-x. [DOI] [PubMed] [Google Scholar]
- 27.Rabl C, Campos GM. The impact of bariatric surgery on nonalcoholic steatohepatitis. Semin Liver Dis. 2012;32:80–91. doi: 10.1055/s-0032-1306428. [DOI] [PubMed] [Google Scholar]
- 28.Kotronen A, Juurinen L, Tiikkainen M, Vehkavaara S, Yki-Jarvinen H. Increased liver fat, impaired insulin clearance, and hepatic and adipose tissue insulin resistance in type 2 diabetes. Gastroenterology. 2008;135:122–30. doi: 10.1053/j.gastro.2008.03.021. [DOI] [PubMed] [Google Scholar]
- 29.Kotronen A, Vehkavaara S, Seppala-Lindroos A, Bergholm R, Yki-Jarvinen H. Effect of liver fat on insulin clearance. Am J Physiol Endocrinol Metab. 2007;293:E1709–15. doi: 10.1152/ajpendo.00444.2007. [DOI] [PubMed] [Google Scholar]
- 30.Cummings BP, Stanhope KL, Graham JL, et al. Chronic administration of the glucagon-like peptide-1 analog, liraglutide, delays the onset of diabetes and lowers triglycerides in UCD-T2DM rats. Diabetes. 2010;59:2653–61. doi: 10.2337/db09-1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gupta NA, Mells J, Dunham RM, et al. Glucagon-like peptide-1 receptor is present on human hepatocytes and has a direct role in decreasing hepatic steatosis in vitro by modulating elements of the insulin signaling pathway. Hepatology. 2010;51:1584–92. doi: 10.1002/hep.23569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Svegliati-Baroni G, Saccomanno S, Rychlicki C, et al. Glucagon-like peptide-1 receptor activation stimulates hepatic lipid oxidation and restores hepatic signalling alteration induced by a high-fat diet in nonalcoholic steatohepatitis. Liver Int. 2011;31:1285–97. doi: 10.1111/j.1478-3231.2011.02462.x. [DOI] [PubMed] [Google Scholar]