Abstract
The adult prostate gland grows and develops under hormonal control while its physiological functions are controlled by the autonomic nervous system. The prostate gland receives sympathetic input via the hypogastric nerve and parasympathetic input via the pelvic nerve. In addition, the hypogastric and pelvic nerves also provide sensory inputs to the gland. This review provides a summary of the innervation of the adult prostate gland and describes the changes which occur with age and disease. Growth and development of the prostate gland is age dependent as is the occurrence of both benign prostate disease and prostate cancer. In parallel, the activity and influence of both the sympathetic and parasympathetic nervous system changes with age. The influence of the sympathetic nervous system on benign prostatic hyperplasia is well documented and this review considers the possibility of a link between changes in autonomic innervation and prostate cancer progression.
Keywords: autonomic nervous system, acetylcholine, adrenoceptors, muscarinic receptors, noradrenaline, parasympathetic nervous system, sympathetic nervous system
The adult prostate gland grows and develops in an age-dependent manner. In aged males this development gives rise to abnormalities which are benign [benign prostatic hyperplasia (BPH)] and/or malignant (prostate cancer). Seemingly in parallel, autonomic nervous system activity changes in men with age, and this has been associated with diseases such as hypertension and BPH. This review describes the innervation of the prostate gland through the stages of adult life and explores the possibility that changes in autonomic nervous system activity may contribute to prostate cancer initiation and/or progression.
Fetal and Prepubescent Prostate Development
Morphogenesis of the human prostate gland occurs around the tenth week of gestation when circulating fetal androgen levels stimulate the differentiation of the endodermal urogenital sinus, causing the formation of solid epithelial outgrowths (prostatic buds).1 The prostatic buds rapidly lengthen, arborize, cannulate and cytodifferentiate into basal and luminal epithelium.1 The newly formed tubuloalveolar ducts grow and spread throughout the urogenital mesenchyme, which concurrently differentiates and matures into the smooth muscle-containing prostatic stroma. The growth and maturation of the tubuloalveolar ducts and stroma is dependant on androgens as well as the interaction between the urogenital mesenchyme and epithelial growths.1 By the thirteenth week of gestation, there are approximately 70 primary ducts surrounding the developing urethra and by birth ductal branching is complete.1 The pre-pubertal prostate is small, weighing approximately 2 g, and due to the low levels of testosterone, growth of the prostate during this period is limited.2
Prior to puberty, the prostate gland is quiescent and presumably not influenced by the autonomic nervous system. At the beginning of puberty, secretion of androgens from the testes cause the prostate to undergo a period of rapid development and growth ultimately reaching its full size and mature morphology by 18–20 y.2
The Young Adult Prostate Gland
The young adult prostate weighs approximately 20 g and is the largest of the male accessory reproductive organs. It is an alobular structure found posterior to the bladder that completely encapsulates the prostatic urethra and ejaculatory ducts.2 The glandular elements of the prostate are made up of branching tubuloalveolar ducts with numerous secretory acini, surrounded by a thin fibromuscular stroma. The glandular elements or zones, which produce and drain prostatic secretions into the urethra, account for approximately 70% of the total prostate bulk with the fibromuscular stroma, comprising of connective tissue and smooth muscle, making up the remaining 30%.3
While testosterone is the primary circulating androgen produced by the testes, in peripheral tissues such as the prostate, testosterone is converted locally to dihydrotestosterone (DHT) by the action of the enzyme 5α-reductase.4 DHT is more potent than testosterone and has a higher affinity for the nuclear androgen receptor.5 Activation of the androgen receptor, via various mechanisms, results in cell proliferation and growth.1 In addition to androgens, growth and proliferation of the prostatic stroma is mediated by estrogens, particularly estradiol acting at the ERα estrogen receptor.6 Estradiol is formed locally in the prostate from the conversion of testosterone by aromatase, which like 5α-reductase is localized primarily in the prostatic stroma.7 Furthermore, as with the development of the fetal prostate, reciprocal stroma-epithelial (mesenchyme-epithelial) interactions mediated by paracrine factors, in part under the influence of androgens and estrogens, play a vital role in the growth of the prostate.1 Following the spike in androgen levels during puberty, circulating androgen levels stabilize around 20 y of age. Stabilization of androgen levels corresponds to a period of slow prostatic growth until approximately the age of 50.8
Innervation of the Adult Prostate Gland
Intact neuronal inputs and contractile mechanisms of prostatic smooth muscle are essential for the proper functioning of the prostate, as sympathetically mediated contractions of the prostatic smooth muscle expel prostatic fluid from the prostate into the ejaculate. The prostate is innervated by a rich supply of mixed autonomic postganglionic neurons that arise from the pelvic (inferior hypogastric) plexus, containing neuronal inputs from both sympathetic and parasympathetic neurons. The preganglionic sympathetic neurons arise from the lumbar spinal cord and descend to the pelvic plexus via the hypogastric nerve, whereas preganglionic parasympathetic neurons join the pelvic plexus from the pelvic nerve arising from the sacral spinal cord segment.9,10
Consistent with the role of adrenergic nerves mediating contraction of the prostatic smooth muscle, the prostatic stroma is richly innervated with short noradrenergic nerves arising from the pelvic plexus that are absent from the prostatic glandular epithelium.11 In the prostate, noradrenaline released from noradrenergic nerves activates G protein-coupled α1-adrenoceptors, which results in smooth muscle contraction via an increase in intracellular calcium. In early in vitro contractile studies, prostatic smooth muscle contraction could be elicited by exogenously applied α-adrenoceptor agonists and such effects could be inhibited or blocked by non-specific α-adrenoceptor antagonists such as phentolamine (inhibits α-adrenoceptors but exhibits no selectivity toward α1- or α2-adrenoceptors)12,13 or non-specific α1-adrenoceptor antagonists such as prazosin (inhibits α1-adrenoceptors but exhibits no selectivity toward α1A-, α1B or α1D-adrenoceptor subtypes).14 As delineated by molecular and cloning studies, the α1-adrenoceptor family consists of three subtypes: the α1A-adrenoceptor, the α1B-adrenoceptor and the α1D-adrenoceptor.15 Experiments investigating mRNA expression and α1-adrenoceptor density have indicated that the α1A-adrenoceptor is the dominant subtype expressed in the prostate of various species, including humans, and that the α1A-adrenoceptor is primarily localized to the prostatic stroma.16,17 Pharmacological characterization studies have identified that a functional phenotype of the α1A-adrenoceptor subtype, the α1L-adrenoceptor, mediates the adrenergic contractile response in the human,18,19 canine,20 rabbit,21 guinea pig,22 rat23 and mouse24 prostates, with the α1B-adrenoceptor and α1D-adrenoceptor subtypes having little or no involvement in smooth muscle contraction.19,25,26 Importantly, α1L-adrenoceptors also mediate the contractile response to endogenously released noradrenaline in electrical field stimulation experiments.19,27
In prostatic smooth muscle, stimulation of the Gq/11 protein-coupled α1A-adrenoceptors (α1L-adrenoceptors) results in the prototypical activation of phospholipase C and the subsequent production of the second messengers inositol 1,4,5-triphosphate and diacylglyercol. These in turn mediate smooth muscle contraction via activation of Ca2+-dependent and Rho kinase-dependent Ca2+-sensitization signaling pathways.28 However, recent studies have shown that activation of c-jun N-terminal kinase29 as well as phosphorylation of caldesmon30 are involved in smooth muscle contraction of the prostate following α1-adrenoceptor stimulation, indicating that further intracellular pathways are involved in α1-adrenoceptor mediated contraction of the prostate.
In addition to α1A-adrenoceptors, which directly mediate prostatic smooth muscle contraction, both α2-adrenoceptors and β-adrenoceptors are also found in the prostate. The human prostate contains a population of α2-adrenoceptors with a density comparable to31 or lesser than that of α1-adrenoceptors25,32 and are localized primarily in the glandular epithelium and vascular tissue with sparse stromal distribution.32,33 Functionally, α2-adrenoceptors reduce nerve-mediated contractions of the prostate via pre-junctional inhibition of neuronal noradrenaline release19,31 and appear to be without a direct post-junctional role in contraction of the prostate.25,34 Similarly, β-adrenoceptors have been found in the human, pig and rat prostates35-37 and have been shown to inhibit α1-adrenoceptor-mediated contractile responses in various experimental species as well as in the human prostate.36,38-41 β-adrenoceptors inhibit α1-adrenoceptor-mediated contraction via post-junctional activation of adenylate cyclase and accumulation of cyclic adenosine monophosphate (cAMP) resulting in relaxation of prostatic smooth muscle.39 This response is most likely mediated by β2-adrenoceptors; however β1-adrenoceptors and β3-adrenoceptors have also been implicated.37,38,40 Recently, further interplay between α1-adrenoceptors and β-adrenoceptors in the prostate has been proposed, as activation of α1-adrenoceptors results in the phosphorylation of β-adrenoceptors, possibly via mechanisms involving G protein-coupled receptor kinases. Such an effect may enhance the α1-adrenoceptor-mediated contractile response.42
In addition to mediating contraction, adrenergic innervation plays a role in the growth of the prostate. In rodents, sympathectomy of the hypogastric nerve causes a reduction in prostatic weight in rats,43 whereas chronic administration of α1-adrenoceptor agonists induces proliferation and hyperplasia.44,45 Multiple α1-adrenoceptor antagonists have been shown to suppress stromal and epithelial cell growth in cell culture;46 however this does not appear to be the result of specific blockade of the receptor and does not appear to translate to a clinical reduction in prostate volume.47 In the rat, prostatic hyperplasia induced by phenylephrine activation of α1-adrenoceptors has been strongly linked to activation of inflammatory pathways and the transforming growth factor β (TGFβ) signaling cascade.45 Furthermore, human studies have shown that α1-adrenoceptors in the prostate couple to non-contractile intracellular protein kinases involved in growth, proliferation and apoptosis,48 indicating that activation of α1-adrenoceptors in the human prostate couple to multiple pathways, some of which may be involved in proliferation and growth of the prostate.
Cholinergic innervation is found in both the stromal and glandular epithelial regions of the human,11,49,50 guinea pig27 and rat51 prostates. Responses elicited by cholinergic innervation in the prostate are mediated by G protein-coupled muscarinic receptors of which there are five subtypes. These are the M1, M3 and M5 muscarinic receptors, which couple to Gq/11 proteins, and the M2 and M4 muscarinic receptors, which couple to Gi/o proteins.52 The endogenous agonist for all five subtypes is acetylcholine.52 In various species, muscarinic receptors are primarily localized in the glandular epithelium; however some muscarinic receptor expression is also found in the prostatic stroma suggesting a dual role in secretion and contraction. In agreement with the primary localization of muscarinic receptors on the glandular epithelium, stimulation of the cholinergic nerves or direct activation of muscarinic receptors results in the production of prostatic secretions.53,54
Despite muscarinic receptors being located primarily in the glandular epithelium, they play a direct role in post-junctional contraction in the prostate, as in vitro contraction of isolated prostates elicited by electrical field stimulation or exogenous agonists can be inhibited by muscarinic receptor antagonists in the human,12,31,55 canine,41,56 rabbit,57,58 guinea pig,38,58,59 rat58,59 and mouse60,61 prostates. However, the magnitude of cholinergic contractions in the prostate is less than that observed for α1-adrenoceptor stimulation.62 Additionally, muscarinic receptors regulate nerve mediated contractions by pre-junctional inhibition31 or facilitation63 of neurotransmitter release. The muscarinic receptor subtype responsible for contraction differs between species. M2 muscarinic receptors mediate contraction in the canine prostate56 and are also the predominant subtype in human stromal cells where they inhibit the accumulation of cAMP.64 In the guinea pig prostate, M1 muscarinic receptors facilitate nerve mediated contraction,63 whereas contraction of the rat and mouse prostates is mediated by M3 muscarinic receptors,61,65 which are localized on the prostatic smooth muscle.51
In the prostate, activation of muscarinic receptors would likely result in the activation of the prototypical signaling pathways which are phospholipase C for M1, M3 and M5 and adenylate cyclase for M2 and M4 muscarinic receptors, respectively. Only a few studies have investigated the signaling pathways for the muscarinic receptor subtypes, which mediate prostatic contraction. Studies in cancer cell lines indicate that activation of the muscarinic receptors present in prostate smooth muscle can result in cAMP accumulation or phosphatidylinositol turnover.64 However, recently, Rho kinase-dependent Ca2+ sensitization signaling pathways have been implicated in the muscarinic receptor-mediated contraction of the rat prostate.66
As is the case in most physiological systems, the sympathetic and parasympathetic nervous systems appear to oppose each other in the form of a physiological balancing mechanism. This appears to be the case also in the control of prostatic growth as parasympathectomy in rats leads to increased prostate weight.43
In addition to adrenergic and cholinergic innervation numerous other non-adrenergic, non-cholinergic neurotransmitters that can elicit or modulate contraction are found in the prostate. While these mechanisms have been shown to play a role in prostate contraction, their physiological role is uncertain and in general their contribution to physiological contraction is much less than that of noradrenaline acting at α1A-adrenoceptors.
Adenosine 5′-triphosphate (ATP) is a known sympathetic co-transmitter with noradrenaline in rat and guinea pig prostates and mediates contraction via post-junctional P2X1 purinoceptors,27,38,67,68 localized on the prostatic smooth muscle.67-69 However, this effect seems to be species-dependent as ATP does not contribute to nerve mediated contractile responses in the mouse prostate60,70 despite immunolocalization of P2X1-purinoceptors in the prostatic smooth muscle.70 Following neuronal release, ectonucleotidases hydrolyse ATP to adenosine, which in turn can activate adenosine receptors. In the mouse prostate, pre-junctional A2A adenosine receptors contribute to nerve mediated contraction by facilitating noradrenaline release71 whereas in the rat prostate, pre-junctional A1 adenosine receptors inhibit excitatory neurotransmitter release.72 Furthermore, A1 and A2A adenosine receptors have a post-junctional role in contraction as the α1-adrenoceptor-mediated response in cultured human prostatic stromal cells is enhanced or inhibited by A1 or A2A adenosine receptors via the inhibition and stimulation of adenylate cyclase, respectively.73
Neuropeptide Y is co-localized in all adrenergic nerves in pelvic ganglia and is also found co-localized in some cholinergic nerves.10 Dense neuropeptide Y innervation is found throughout the prostatic stroma of humans,50 guinea pigs, rats74 and mice.70,75 However, there are conflicting reports concerning the expression of neuropeptide Y receptors in the prostates of humans76 and rats.77 Curiously, despite dense innervation to the stroma and roles in modulating neurotransmitter release in other genitourinary tissues, most studies have found that neuropeptide Y does not appear to regulate or mediate contraction of the prostate63,76,78 and may therefore instead be involved in prostate growth.79 However, one study did find that in the human prostate, exogenous application of high concentrations of neuropeptide Y inhibits nerve-mediated contractions and relaxes noradrenaline pre-contracted prostate preparations.80
In various species the prostate receives a rich supply of vasoactive intestinal polypeptide (VIP) containing nerves, which are co-localized predominantly in cholinergic nerves innervating the prostatic glandular epithelium.10,11,75 Receptors for VIP have been found in the prostate;81 however due to their glandular distribution, VIP does not appear to be involved in contraction and instead may have roles in secretion or prostatic growth.82
In contrast to neuropeptide Y and VIP, which have little if any role in the contraction of the prostate, other peptides are known to regulate or elicit prostatic contraction. The sensory neuropeptide calcitonin gene-related peptide (CGRP) has been found in nerves located within the stroma and/or glandular epithelium of numerous species70,80,83-85 as well as neuroendocrine cells in the human prostate.86 Functionally, exogenous CGRP has been shown to relax phenylephrine mediated contractions78 or inhibit nerve mediated contractions87 in the rat prostate. Furthermore, tachykinins also modulate prostate contraction. Neurokinin A and substance P have been found distributed sparsely in nerve fibers supplying the prostatic smooth muscle of human,50 sheep,88 canine,85 guinea pig, rat89,90 and mouse prostates.70 Exogenous application of tachykinin agonists induces prostate contraction via neurokinin NK2 receptors in the human prostate,91 whereas neurokinin NK1 and NK2 receptors mediate contraction to exogenous agonists in cultured canine prostatic stromal cells.85 Conversly, no direct contractile effects have been observed in the rat78 or pig58 prostates in response to substance P. In the guinea pig prostate, however, substance P and neurokinin A potentiate contractions elictited by nerve stimulation.90
While not mediating contraction, nitrergic neuronal mechanisms regulate the tone of the prostatic smooth muscle by mediating relaxation. The prostate receives a dense innervation of nitric oxide synthase containing nerves, which are localized throughout the prostatic stroma and glandular epithelium.92 In addition, nitric oxide synthase is commonly co-localized in both noradrenergic and cholinergic nerves.80,84 Electrical field stimulation relaxes in vitro prostate preparations that have been pre-contracted with noradrenaline in the human, canine,93 rabbit58,94 and guinea pig58 tissue. This nerve-mediated relaxation can be blocked by inhibition of nitric oxide synthase or enhanced in the presence of nitric oxide donors.58,93,94 The exogenous addition of nitric oxide donors also relaxes precontracted prostate tissues.58,80,93,95 Furthermore, when the adrenergic and cholinergic components of nerve mediated contraction are blocked, electrical field stimulation relaxes prostatic smooth muscle, which can be inhibited or enhanced by nitric oxide synthase inhibitors or nitric oxide donors, respectively.93 Phosphodiesterase (PDE) enzymes, which are involved in the hydrolysis of cyclic nucleotides produced by the action of nitric oxide, are also found in the human prostate.95 Moreover, inhibitors of the PDE4 and PDE5 isoforms have been shown to relax prostatic tissues precontracted with noradrenaline or endothelin-1.95
Age-Related Changes in Prostate Innervation
At approximately age 50, the steady growth of the prostate slowly accelerates.8 In parallel, a number of studies have shown an age-related decrease in the innervation to the prostate over this time.50,96 At the same time, an increase in α1A-adrenoceptor mRNA expression has been observed in aged human prostate.97,98 This is in contrast to the aged rat prostate where α1A-adrenoceptor mRNA expression is lower.99 In the aged human prostate the contractile response to exogenously adminstered α1-adrenoceptor agonists remains the same36 or is increased.55 However, in similar studies of the aged rat prostate, a decrease in α1-adrenoceptor density99 and distribution33 was observed, which resulted in a reduced contractile response mediated by α1-adrenoceptor agonists.99 While the reason for the difference between species is unclear, it might be due to differences in age. Rats used in the previous studies were 18–22 mo of age, corresponding to an approximate human age of only 45–55 y old.100
Research in our laboratory has also noted that prostates taken from 12-mo-old α1A-adrenoceptor knockout mice are smaller than those taken from wild-type litter mate controls at the same age (Fig. 1). As previously mentioned, sympathetic innervation is known to play a role in the growth of the rat prostate, as surgical sympathectomy of the pelvic ganglia43 reduces the size of the prostate, while sympathetic stimulation results in prostate growth.44 Furthermore, in patients with spinal cord injury resulting in severe paralysis, smaller prostate size is observed.101 In rats, the in vivo administration of non-selective α1-adrenoceptor antagonists results in a reduction in prostatic weight102 as well as cellular proliferation.103 This effect appears to be dose dependent, as low doses of α1-adrenoceptor antagonist have no effect on prostatic weight.104 Our observations with knockout mice (Fig. 1) indicate that the α1A-adrenoceptor subtype is not only responsible for the nerve mediated contractile response but in the mouse prostate is responsible for sympathetically mediated growth as well.
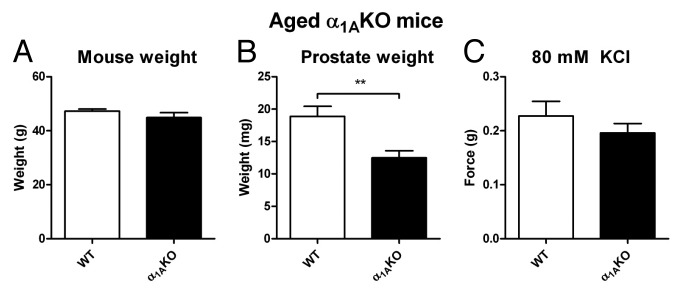
Figure 1. Aged α1AKO mice. Comparison of mean mouse weight (A), isolated prostate weight (B) and mean contractile response to 80 mM KCl Krebs-Henseleit solution (C) from aged wild-type (WT, open columns) and α1A-adrenoceptor knockout mice (α1AKO, black columns). Columns represent mean weight/contractile force ± S.E.M., n = 10–14. p-values, **p < 0.01 vs. control, were calculated by an unpaired t-test and represent the probability of genotype affecting prostate weight in the aged mouse.
Cholinergic innervation of the human prostate was shown not to change with age.96 Similarly, in the aged human prostate31 as well as the aged canine105 and rabbit106 prostates, no change in muscarinic receptor density was observed. Whereas, in the aged rat prostate, M1–3 muscarinic receptor mRNA decreased107 as did M3 muscarinic receptor density.65,107 However, studies using antibodies in the rat prostate, showed an increase in M2 muscarinic receptors in the rat prostate with age.33 Therefore the effect of age on the cholinergic innervation in the aged prostate appears to be highly subtype and species dependent. This is of note as a possible synergistic adrenergic-cholinergic action has previously been observed in the human prostate.31
Many inhibitory mechanisms of prostate contractility have also been studied with regard to aging. An increase in α2-adrenoceptor density has been observed with age in the human,25 rat33 and rabbit106 prostates. In the prostate, α2-adrenoceptors are primarily responsible for inhibition of noradrenaline release and do not play a direct contractile role,17 therefore, the implications of this phenomenon are unknown and could be complex. Expression of β-adrenoceptors, which are capable of relaxing electrical field stimulation and agonist mediated contraction,17 have also been shown to be reduced in the aged rat prostate.33 Furthermore, a decrease in the activity of adenylate cyclase activated by the β-adrenoceptor agonist isoproterenol occurs in the aged rat prostate.108 In contrast, β-adrenoceptor expression does not change in the aged rabbit prostate.106 Additionally, nitrergic innervation and nitric oxide mediated relaxation is decreased in the aged rabbit94 and guinea pig109 prostates. Therefore, a reduction in the inhibitory mechanisms of contraction in the prostate with age may play a role in the development of lower urinary tract symptoms (LUTS) associated with BPH.
BPH
BPH is a consequence of age- and androgen-dependant growth of the human prostate and affects approximately 50% of men by the age of 60 and 90% by 90 y of age.2 BPH is a purely histological description of the prostate and should not be confused with LUTS associated with or secondary to BPH.110 While rarely life threatening, LUTS affect approximately 50% of men aged 50 to 80 y111 and refers to a number of symptoms that can be found in either men or women that can severely affect their quality of life.112 LUTS may be caused by BPH, however other factors such as detrusor over- or under-activity may also result in such symptoms.113 The LUTS can be categorized as related to urine storage, urine voiding and post-micturition. Urine storage symptoms relate to urgency and frequency of urination, nocturia and incontinence. Urine voiding-related symptoms include hesitancy, poor flow, intermittency and straining, while post-micturition symptoms include post-void dribble and a sense of incomplete emptying.110 Due to the high prevalence of LUTS accompanying BPH, there are significant costs associated with treatment that may be expected to rise with the aging population.114
BPH is characterized by a progressive nodular increase in the number of epithelial and stromal cells, which occurs initially in the transition and periurethral zones of the human prostate.115 Hyperplasia of the stoma is predominant and results in a greater proportion of smooth muscle relative to the glandular epithelium and an increase in the muscular tone of the hyperplasic prostate.116 BPH can lead to benign prostatic enlargement and in turn benign prostatic obstruction of the urethra. Benign prostatic obstruction is one cause of bladder outlet obstruction; others include bladder neck obstruction and urethral stricture,117 which increases bladder pressure and increases urethral resistance impairing the flow of urine from the bladder, leading to the voiding symptoms associated with LUTS.118 Bladder outlet obstruction due to BPH may also result in storage symptoms by inducing overactivity of the bladder detrusor.119
Effects of BPH on Prostate Innervation
In parallel with BPH development, α1-adrenoceptor density in the human hyperplasic prostate is increased120 or remains the same as the normal prostate.25,36 Consequently there is an increase in the tone of the prostatic smooth muscle, which constricts the urethra, contributing to the LUTS associated with BPH. In the hyperplasic prostate the increase in tone of the prostate is mediated, in part, by neuronal noradrenaline acting at α1L-adrenoceptors to cause smooth muscle contraction and this mechanism forms the basis for the treatment of BPH with α1-adrenoceptor antagonists. However, prostatic smooth muscle contraction is also mediated by numerous other receptor systems,74,121 such as acetylcholine acting at muscarinic receptors or ATP acting at purinoceptors. Therefore, blockade of these receptors, particularly muscarinic receptors, has been hypothesized as a suitable additional target for a better pharmacological treatment for BPH.122 Changes with age in these, or other mediators of prostatic smooth muscle contraction, may play a role in the development of LUTS associated with BPH by increasing prostatic tone.
Given the important role of α1-adrenoceptors in the treatment of BPH, many studies have previously investigated the effect of age or disease on adrenergic contractile mechanisms, which demonstrated significant variation between species and experimental conditions. In BPH as with age, total innervation as well as adrenergic innervation of the human prostate gland decreases.50,96 Adrenoceptor studies of the human prostate show contrasting effects. α1A-adrenoceptor mRNA was increased in the diseased human prostate,97 as was observed with age in normal human prostate.98 At the receptor level, α1-adrenoceptor density in the hyperplasic human prostate is increased120 or remains the same as in normal prostate.25,36 Studies of the contractile response in human prostate mirror those investigating α1-adrenoceptor density. Whereby, the contractile response of human hyperplasic prostate to exogenously applied α1-adrenoceptor agonists were shown to remain the same31,36,55 or were increased31 compared with normal prostate.
In contrast to the adrenergic component of contraction, only a few studies have investigated the effect of age or disease on the cholinergic component of contraction in the prostate. As observed with the adrenergic component, previous studies show differences between species and experiments. Unlike in normal aged prostates, in hyperplasic prostates, acetylcholinesterase staining decreased.49,50 However, as seen in the aged prostate, hyperplasic human prostate31 shows no change in muscarinic receptor density. Conversely, an upregulation of the M3 muscarinic receptor, but not the M1 or M2 muscarinic receptor subtypes, was observed in the human hyperplasic prostate.123 Finally, acetylcholine was shown to potentate the noradrenergically mediated contractile response in the hyperplasic human prostate,31 but not in un-diseased tissue.31 In general, the effect of BPH on the cholinergic contractile response in the prostate gland is still poorly understood.
Changes in inhibitory inputs of prostatic contraction have also been widely investigated with respect to BPH. Expression of β-adrenoceptors, which are capable of relaxing electrical field stimulation and agonist mediated contraction,17 were shown to be decreased in the hyperplasic human prostates.36 Furthermore, a decrease in the activity of adenylate cyclase activated by the β-adrenoceptor agonist isoproterenol occurs in the hyperplasic human prostate.36 These studies implicate a reduction in the β-adrenoceptor inhibitory mechanisms of contraction in the prostate as playing a role in the development of LUTS associated with BPH. In contrast, in the hyperplasic human prostate expression of α2-adrenoceptors was shown to be increased.25,31
Prostate Cancer
Approximately 20,000 men are diagnosed with localized (organ-confined) prostate cancer in Australia each year. In many patients the tumors are slow-growing and are not associated directly with mortality. Therefore these men can be successfully treated with conventional treatment regimes including surgical removal of the prostate (radical prostatectomy) or radiotherapy. If the patient relapses and the cancer returns, they are treated with androgen ablation/deprivation therapy, which can reduce the tumor and circulating prostate specific antigen (PSA) to undetectable levels. However, in most cases the cancer will eventually recur in an androgen independent/hormone refractory/castration resistant form that usually results in lethal bone metastasis.124 Chemotherapeutic regimes for advanced prostate cancer are often still used but generally result in only a small increase in survival time.125,126
The strongest risk factor for the development of prostate cancer in men is age. The chance of developing the disease rises rapidly after the age of 50 with 1 in 11 Australian men developing prostate cancer by the age of 70. The sympathetic nervous system is implicated in disorders of the aging male such as hypertension and BPH. In both of these age-related disorders, the sympathetic nervous system shows signs of overactivity and symptoms can be controlled by the use of therapeutic drugs which block the effects mediated by adrenoceptors (e.g., α1-adrenoceptor antagonists, β-blockers).
In humans, α1-adrenoceptor antagonists used in the treatment of BPH have been shown to induce prostate apoptosis.98,103,127 This apoptotis inducing effect of α1-adrenoceptor antagonists has also been shown in prostate cancer cell lines in vitro46,128,129 and in vivo in mice bearing a tumor following subcutaneous xenograft injection of PC3128 or LNCaP129 cells. The antitumorigenic effect of the α1-adrenoceptor antagonists was originally postulated to be due to antiangiogenic effects on the prostate vasculature;130 however, their apoptotic effects on prostate cancer cell lines in culture46,128 suggest an alternate mechanism of action. Nevertheless, several reports indicate that only the quinazoline based α1-adrenoceptor antagonists have anti-apoptotic efficacy against prostate cancer cells and that this is by an α1-adrenoceptor-independent mechanism.130 However, in vivo data generated from our laboratory using α1A adrenoceptor knockout mice indicates that there is also a mechanism which involves the sympathetic nervous system and more specifically α1-adrenoceptors (Fig. 1). In support of this, it has previously been shown that activating the α1-adrenoceptor signaling pathway induces proliferation of human prostatic stromal cells.131
β-adrenoceptors have also been implicated in metastasis development in models of prostate and other cancers. In vitro studies have shown that active migration of tumor cells can be induced by noradrenaline using cell lines derived from colon, breast or prostate cancer cells.132 Furthermore, this tumor cell migration could be inhibited by the β-adrenoceptor antagonists propranolol or ICI 118,551.132 Noradrenaline induced development of metastasis following in vivo xenograft of PC3 cells in BALB/c nude mice can also be inhibited by the β-blocker propranolol.133 Indeed it has recently been shown that inducing stress in an in vivo mouse xenograft model of prostate cancer can artificially activate the sympathetic nervous system to stimulate β-adrenoceptor induced metastatic effects.134 This metastatic effect was mediated by increased levels of circulating adrenaline and could be inhibited by β-adrenoceptor antagonists. With regard to parasympathetic cholinergic mechanisms, stimulation of muscarinic receptors of the M3 subtype have been shown to stimulate proliferation of LNCaP prostate cancer cells as well as benign and cancerous primary prostate cells.135 Similarly, in studies of the cancerous rat prostate, an increase in M3 muscarinic receptor density was observed.136 This suggests that M3 muscarinic receptors may play a significant role in prostate cancer tumor growth and possibly also androgen-independent tumor progression.
It has previously been hypothesized that there is an association between increased sympathetic activity and prostate cancer,137 and the scientific literature abounds with circumstantial evidence for the involvement of the sympathetic nervous system in prostate cancer progression. For instance obesity, which is associated with high sympathetic tone,138 is also associated with increased prostate cancer risk,139 as is cardiovascular disease.140,141 In clinical studies on schizophrenia patients, there is a significant association of reduced risk of prostate cancer among patients treated with neuroleptic medication,142 particularly those who had been treated with high dose phenothiazine neuroleptics such as chlorpromazine.142 Although these drugs primarily target dopamine receptors, high doses of this class of neuroleptics are known to antagonize α1-adrenoceptors, so it is notable that this relationship was seen only in patients treated with a high cumulative dose. Diabetes, on the other hand, which is associated with sympathetic neuropathy, is associated with a lower risk for late stage prostate cancer but no association in early stages.143 Similarly, lower body sympathetic dysfunction due to spinal cord injury is associated with a low incidence of prostate cancer diagnosis.144 Finally, the most striking clinical evidence comes from a study on antihypertensives which indicated that β-blockers and the long-term use of α-adrenoceptor antagonists may prevent prostate cancer whereas other classes of antihypertensives such as calcium channel blockers or angiotensin-converting enzyme inhibitors do not influence prostate cancer risk.145
In clinical trials, a study conducted on 4,070 men in the U.S. found that men exposed to α1-adrenoceptor antagonists have a 1.5 times lower risk of developing prostate cancer than unexposed men.146 This study only looked at men taking the quinazoline based antagonists doxazosin, prazosin and terazosin and therefore observed effects may be due to non-adrenoceptor mechanisms. Conversely, evidence from a larger observational cohort study of 23,320 Finnish men showed that overall prostate cancer risk, while not reduced among α-adrenoceptor antagonist users compared with non users, showed a decreased incidence of high grade tumors,147 indicating a decrease in tumor aggression. This second study looked at the effects of the widely used sulphonamide based α1-adrenoceptor antagonist tamsulosin as well as the quinazoline based antagonist alfuzosin. The use of the non-quinazoline tamsulosin in this study is likely to be the cause of this novel effect on tumor aggression, which is likely to be α1-adrenoceptor mediated. The evidence found in this second epidemiological study is consistent with the sympathetic nervous system, which is often overactive in the aging male, contributing to the progression of prostate cancer. The former study, on the other hand, is consistent with the previously reported antiproliferative α1-adrenoceptor independent effects of the quinazoline-based compounds.
Interpretation of antitumorigenic effects in prostate cancer cell lines is difficult since the expression of the different adrenoceptor and muscarinic receptor subtypes in the different prostate cancer cell lines is not well characterized. In addition, these commonly studied cell lines are likely to be phenotypically different under different conditions in differerent laboratories. For example, based on single-cell RNA sequencing data, the β2-adrenoceptor is expressed at significant levels in the PC3 and LNCaP prostate cancer cell lines while the M3 muscarinic receptor is expressed only in PC3 cells148 while the α1A-adrenoceptor is not expressed in either of these cell lines. However, there are inconsistencies between the sequencing data and functional studies on α1A-adrenoceptors and muscarinic receptors. Two papers have suggested a role for the α1A-adrenoceptor in proliferation of LNCaP cells149 and chemoresistance of DU145 cells;150 however receptor detection was based on α1A-drenoceptors antibodies, which are notorious for lack of specificity.151 The muscarinic receptor agonist carbachol likewise stimulates proliferation of LNCaP cells despite an apparent lack of mRNA for any of the relevant acetylcholine receptor subtypes.135
Conclusions
Prostate disease and autonomic nervous system activity appear to change with age in a parallel and similar manner. Further research into whether this association is coincidental or not may elucidate the physiological mechanisms involved in clinical observations such as those that show prostate cancer patients who have been exposed to α1-adrenoceptor antagonists for the treatment of BPH have a lower incidence of high grade tumors.147 Validation of such a physiological mechanism will indicate a novel molecular target for chemotherapy of metastatic androgen-independent prostate cancer. This will in turn drive the development of new treatment strategies for these forms of currently treatment resistant prostate cancers. In addition, α1-adrenoceptor antagonists, β-blockers and muscarinic receptor antagonists are already currently available, reasonably well-tolerated and very effective in the treatment of LUTS and hypertension. Understanding these signaling pathways may provide evidence for the immediate use of such a treatment strategy by clinicians to produce a survival advantage for advanced prostate cancer patients.
Glossary
Abbreviations:
- ATP
adenosine 5′-triphosphate
- BPH
benign prostatic hyperplasia
- cAMP
cyclic adenosine monophosphate
- CGRP
calcitonin gene-related peptide
- DHT
dihydrotestosterone
- LUTS
lower urinary tract symptoms
- PDE
phosphodiesterase
- PSA
prostate specific antigen
- TGFβ
transforming growth factor β
- VIP
vasoactive intestinal polypeptide
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/organogenesis/article/24843
References
- 1.Cunha GR, Donjacour AA, Cooke PS, Mee S, Bigsby RM, Higgins SJ, et al. The endocrinology and developmental biology of the prostate. Endocr Rev. 1987;8:338–62. doi: 10.1210/edrv-8-3-338. [DOI] [PubMed] [Google Scholar]
- 2.Berry SJ, Coffey DS, Walsh PC, Ewing LL. The development of human benign prostatic hyperplasia with age. J Urol. 1984;132:474–9. doi: 10.1016/s0022-5347(17)49698-4. [DOI] [PubMed] [Google Scholar]
- 3.McNeal JE. Normal histology of the prostate. Am J Surg Pathol. 1988;12:619–33. doi: 10.1097/00000478-198808000-00003. [DOI] [PubMed] [Google Scholar]
- 4.Bruchovsky N, Wilson JD. The conversion of testosterone to 5-alpha-androstan-17-beta-ol-3-one by rat prostate in vivo and in vitro. J Biol Chem. 1968;243:2012–21. [PubMed] [Google Scholar]
- 5.Fang S, Anderson KM, Liao S. Receptor proteins for androgens. On the role of specific proteins in selective retention of 17-beta-hydroxy-5-alpha-androstan-3-one by rat ventral prostate in vivo and in vitro. J Biol Chem. 1969;244:6584–95. [PubMed] [Google Scholar]
- 6.Prins GS, Korach KS. The role of estrogens and estrogen receptors in normal prostate growth and disease. Steroids. 2008;73:233–44. doi: 10.1016/j.steroids.2007.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Matzkin H, Soloway MS. Immunohistochemical evidence of the existence and localization of aromatase in human prostatic tissues. Prostate. 1992;21:309–14. doi: 10.1002/pros.2990210407. [DOI] [PubMed] [Google Scholar]
- 8.Swyer GI. Post-natal growth changes in the human prostate. J Anat. 1944;78:130–45. [PMC free article] [PubMed] [Google Scholar]
- 9.Keast JR. Visualization and immunohistochemical characterization of sympathetic and parasympathetic neurons in the male rat major pelvic ganglion. Neuroscience. 1995;66:655–62. doi: 10.1016/0306-4522(94)00595-V. [DOI] [PubMed] [Google Scholar]
- 10.Keast JR. Plasticity of pelvic autonomic ganglia and urogenital innervation. Int Rev Cytol. 2006;248:141–208. doi: 10.1016/S0074-7696(06)48003-7. [DOI] [PubMed] [Google Scholar]
- 11.Vaalasti A, Hervonen A. Autonomic innervation of the human prostate. Invest Urol. 1980;17:293–7. [PubMed] [Google Scholar]
- 12.Caine M, Raz S, Zeigler M. Adrenergic and cholinergic receptors in the human prostate, prostatic capsule and bladder neck. Br J Urol. 1975;47:193–202. doi: 10.1111/j.1464-410X.1975.tb03947.x. [DOI] [PubMed] [Google Scholar]
- 13.Raz S, Zeigler M, Caine M. Pharmacological receptors in the prostate. Br J Urol. 1973;45:663–7. doi: 10.1111/j.1464-410X.1973.tb12237.x. [DOI] [PubMed] [Google Scholar]
- 14.Shapiro A, Mazouz B, Caine M. The alpha-adrenergic blocking effect of prazosin on the human prostate. Urol Res. 1981;9:17–20. doi: 10.1007/BF00256833. [DOI] [PubMed] [Google Scholar]
- 15.Hieble JP, Bylund DB, Clarke DE, Eikenburg DC, Langer SZ, Lefkowitz RJ, et al. International Union of Pharmacology. X. Recommendation for nomenclature of alpha 1-adrenoceptors: consensus update. Pharmacol Rev. 1995;47:267–70. [PubMed] [Google Scholar]
- 16.Testa R, Guarneri L, Ibba M, Strada G, Poggesi E, Taddei C, et al. Characterization of alpha 1-adrenoceptor subtypes in prostate and prostatic urethra of rat, rabbit, dog and man. Eur J Pharmacol. 1993;249:307–15. doi: 10.1016/0014-2999(93)90527-O. [DOI] [PubMed] [Google Scholar]
- 17.Michel MC, Vrydag W. Alpha1-, alpha2- and beta-adrenoceptors in the urinary bladder, urethra and prostate. Br J Pharmacol. 2006;147(Suppl 2):S88–119. doi: 10.1038/sj.bjp.0706619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Muramatsu I, Oshita M, Ohmura T, Kigoshi S, Akino H, Gobara M, et al. Pharmacological characterization of alpha 1-adrenoceptor subtypes in the human prostate: functional and binding studies. Br J Urol. 1994;74:572–8. doi: 10.1111/j.1464-410X.1994.tb09186.x. [DOI] [PubMed] [Google Scholar]
- 19.Guh JH, Chueh SC, Ko FN, Teng CM. Characterization of alpha 1-adrenoceptor subtypes in tension response of human prostate to electrical field stimulation. Br J Pharmacol. 1995;115:142–6. doi: 10.1111/j.1476-5381.1995.tb16331.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ohmura T, Sakamoto S, Hayashi H, Kigoshi S, Muramatsu I. Identification of alpha 1-adrenoceptor subtypes in the dog prostate. Urol Res. 1993;21:211–5. doi: 10.1007/BF00590038. [DOI] [PubMed] [Google Scholar]
- 21.Hiraoka Y, Ohmura T, Sakamoto S, Hayashi H, Muramatsu I. Identification of alpha 1-adrenoceptor subtypes in the rabbit prostate. J Auton Pharmacol. 1995;15:271–8. doi: 10.1111/j.1474-8673.1995.tb00310.x. [DOI] [PubMed] [Google Scholar]
- 22.Pennefather JN, Lau WA, Chin C, Story ME, Ventura S. alpha(1L)-adrenoceptors mediate noradrenaline-induced contractions of the guinea-pig prostate stroma. Eur J Pharmacol. 1999;384:25–30. doi: 10.1016/S0014-2999(99)00667-6. [DOI] [PubMed] [Google Scholar]
- 23.Hiraoka Y, Ohmura T, Oshita M, Watanabe Y, Morikawa K, Nagata O, et al. Binding and functional characterization of alpha1-adrenoceptor subtypes in the rat prostate. Eur J Pharmacol. 1999;366:119–26. doi: 10.1016/S0014-2999(98)00895-4. [DOI] [PubMed] [Google Scholar]
- 24.Gray KT, Ventura S. α1L-adrenoceptors mediate contractions of the isolated mouse prostate. Eur J Pharmacol. 2006;540:155–61. doi: 10.1016/j.ejphar.2006.04.016. [DOI] [PubMed] [Google Scholar]
- 25.Chapple CR, Aubry ML, James S, Greengrass PM, Burnstock G, Turner-Warwick RT, et al. Characterisation of human prostatic adrenoceptors using pharmacology receptor binding and localisation. Br J Urol. 1989;63:487–96. doi: 10.1111/j.1464-410X.1989.tb05942.x. [DOI] [PubMed] [Google Scholar]
- 26.Chapple CR, Burt RP, Andersson PO, Greengrass P, Wyllie M, Marshall I. Alpha 1-adrenoceptor subtypes in the human prostate. Br J Urol. 1994;74:585–9. doi: 10.1111/j.1464-410X.1994.tb09188.x. [DOI] [PubMed] [Google Scholar]
- 27.Lau WAK, Ventura S, Pennefather JN. Pharmacology of neurotransmission to the smooth muscle of the rat and the guinea-pig prostate glands. J Auton Pharmacol. 1998;18:349–56. doi: 10.1046/j.1365-2680.1998.1860349.x. [DOI] [PubMed] [Google Scholar]
- 28.Christ GJ, Andersson KE. Rho-kinase and effects of Rho-kinase inhibition on the lower urinary tract. Neurourol Urodyn. 2007;26(Suppl):948–54. doi: 10.1002/nau.20475. [DOI] [PubMed] [Google Scholar]
- 29.Strittmatter F, Walther S, Gratzke C, Göttinger J, Beckmann C, Roosen A, et al. Inhibition of adrenergic human prostate smooth muscle contraction by the inhibitors of c-Jun N-terminal kinase, SP600125 and BI-78D3. Br J Pharmacol. 2012;166:1926–35. doi: 10.1111/j.1476-5381.2012.01919.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Walther S, Strittmatter F, Roosen A, Heinzer F, Rutz B, Stief CG, et al. Expression and alpha1-adrenoceptor regulation of caldesmon in human prostate smooth muscle. Urology. 2012;79:e5–12. doi: 10.1016/j.urology.2011.10.053. [DOI] [PubMed] [Google Scholar]
- 31.Hedlund H, Andersson KE, Larsson B. Alpha-adrenoceptors and muscarinic receptors in the isolated human prostate. J Urol. 1985;134:1291–8. doi: 10.1016/s0022-5347(17)47714-7. [DOI] [PubMed] [Google Scholar]
- 32.James S, Chapple CR, Phillips MI, Greengrass PM, Davey MJ, Turner-Warwick RT, et al. Autoradiographic analysis of alpha-adrenoceptors and muscarinic cholinergic receptors in the hyperplastic human prostate. J Urol. 1989;142:438–44. doi: 10.1016/s0022-5347(17)38780-3. [DOI] [PubMed] [Google Scholar]
- 33.Slater M, Barden JA, Murphy CR. Tyrosine kinase A, autonomic and transmitter receptors, but not innervation, are upregulated in the aging rat prostate. Acta Histochem. 2000;102:427–38. doi: 10.1078/0065-1281-00565. [DOI] [PubMed] [Google Scholar]
- 34.Hieble JP, Caine M, Zalaznik E. In vitro characterization of the alpha-adrenoceptors in human prostate. Eur J Pharmacol. 1985;107:111–7. doi: 10.1016/0014-2999(85)90048-2. [DOI] [PubMed] [Google Scholar]
- 35.Poyet P, Gagne B, Lavoie M, Labrie F. Characteristics of the beta-adrenergic receptor in the rat ventral prostate using [125I]cyanopindolol. Mol Cell Endocrinol. 1986;48:59–67. doi: 10.1016/0303-7207(86)90166-8. [DOI] [PubMed] [Google Scholar]
- 36.Tsujii T, Azuma H, Yamaguchi T, Oshima H. A possible role of decreased relaxation mediated by beta-adrenoceptors in bladder outlet obstruction by benign prostatic hyperplasia. Br J Pharmacol. 1992;107:803–7. doi: 10.1111/j.1476-5381.1992.tb14527.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Goepel M, Wittmann A, Rübben H, Michel MC. Comparison of adrenoceptor subtype expression in porcine and human bladder and prostate. Urol Res. 1997;25:199–206. doi: 10.1007/BF00941983. [DOI] [PubMed] [Google Scholar]
- 38.Haynes JM, Hill SJ. Beta-adrenoceptor-mediated inhibition of alpha 1-adrenoceptor-mediated and field stimulation-induced contractile responses in the prostate of the guinea pig. Br J Pharmacol. 1997;122:1067–74. doi: 10.1038/sj.bjp.0701494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Drescher P, Eckert RE, Madsen PO. Smooth muscle contractility in prostatic hyperplasia: role of cyclic adenosine monophosphate. Prostate. 1994;25:76–80. doi: 10.1002/pros.2990250204. [DOI] [PubMed] [Google Scholar]
- 40.Kalodimos PJ, Ventura S. Beta2-adrenoceptor-mediated inhibition of field stimulation induced contractile responses of the smooth muscle of the rat prostate gland. Eur J Pharmacol. 2001;431:81–9. doi: 10.1016/S0014-2999(01)01414-5. [DOI] [PubMed] [Google Scholar]
- 41.Normandin DE, Lodge NJ. Pharmacological characterization of the isolated canine prostate. J Urol. 1996;155:1758–61. doi: 10.1016/S0022-5347(01)66193-7. [DOI] [PubMed] [Google Scholar]
- 42.Hennenberg M, Strittmatter F, Walther S, Hedlund P, Andersson KE, Stief CG, et al. α1-adrenoceptor activation induces phosphorylation of β2-adrenoceptors in human prostate tissue. BJU Int. 2011;108:922–8. doi: 10.1111/j.1464-410X.2010.10021.x. [DOI] [PubMed] [Google Scholar]
- 43.McVary KT, Razzaq A, Lee C, Venegas MF, Rademaker A, McKenna KE. Growth of the rat prostate gland is facilitated by the autonomic nervous system. Biol Reprod. 1994;51:99–107. doi: 10.1095/biolreprod51.1.99. [DOI] [PubMed] [Google Scholar]
- 44.Golomb E, Kruglikova A, Dvir D, Parnes N, Abramovici A. Induction of atypical prostatic hyperplasia in rats by sympathomimetic stimulation. Prostate. 1998;34:214–21. doi: 10.1002/(SICI)1097-0045(19980215)34:3<214::AID-PROS9>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 45.Kim J, Yanagihara Y, Kikugawa T, Ji M, Tanji N, Masayoshi Y, et al. A signaling network in phenylephrine-induced benign prostatic hyperplasia. Endocrinology. 2009;150:3576–83. doi: 10.1210/en.2008-1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kyprianou N, Benning CM. Suppression of human prostate cancer cell growth by α1-adrenoceptor antagonists doxazosin and terazosin via induction of apoptosis. Cancer Res. 2000;60:4550–5. [PubMed] [Google Scholar]
- 47.Roehrborn CG. Three months’ treatment with the alpha1-blocker alfuzosin does not affect total or transition zone volume of the prostate. Prostate Cancer Prostatic Dis. 2006;9:121–5. doi: 10.1038/sj.pcan.4500849. [DOI] [PubMed] [Google Scholar]
- 48.Kanagawa K, Sugimura K, Kuratsukuri K, Ikemoto S, Kishimoto T, Nakatani T. Norepinephrine activates P44 and P42 MAPK in human prostate stromal and smooth muscle cells but not in epithelial cells. Prostate. 2003;56:313–8. doi: 10.1002/pros.10267. [DOI] [PubMed] [Google Scholar]
- 49.Dunzendorfer U, Jonas D, Weber W. The autonomic innervation of the human prostate. Histochemistry of acetylcholinesterase in the normal and pathologic states. Urol Res. 1976;4:29–31. doi: 10.1007/BF00256133. [DOI] [PubMed] [Google Scholar]
- 50.Chapple CR, Crowe R, Gilpin SA, Gosling J, Burnstock G. The innervation of the human prostate gland--the changes associated with benign enlargement. J Urol. 1991;146:1637–44. doi: 10.1016/s0022-5347(17)38203-4. [DOI] [PubMed] [Google Scholar]
- 51.Nadelhaft I. Cholinergic axons in the rat prostate and neurons in the pelvic ganglion. Brain Res. 2003;989:52–7. doi: 10.1016/S0006-8993(03)03353-5. [DOI] [PubMed] [Google Scholar]
- 52.Caulfield MP, Birdsall NJM. International Union of Pharmacology. XVII. Classification of muscarinic acetylcholine receptors. Pharmacol Rev. 1998;50:279–90. [PubMed] [Google Scholar]
- 53.Smith ER. The stimulation of canine prostatic secretion by parasympathomimetic agents. J Pharmacol Exp Ther. 1968;164:312–6. [PubMed] [Google Scholar]
- 54.Smith ER, Lebeaux MI. The mediation of the canine prostatic secretion provoked by hypogastric nerve stimulation. Invest Urol. 1970;7:313–8. [PubMed] [Google Scholar]
- 55.Gup DI, Shapiro E, Baumann M, Lepor H. Contractile properties of human prostate adenomas and the development of infravesical obstruction. Prostate. 1989;15:105–14. doi: 10.1002/pros.2990150204. [DOI] [PubMed] [Google Scholar]
- 56.Fernández JLG, Rivera L, López PG, Recio P, Vela-Navarrete R, García-Sacristán A. Characterization of the muscarinic receptor mediating contraction of the dog prostate. J Auton Pharmacol. 1998;18:205–11. doi: 10.1046/j.1365-2680.1998.18486.x. [DOI] [PubMed] [Google Scholar]
- 57.Seki N, Suzuki H. Electrical and mechanical activity of rabbit prostate smooth muscles in response to nerve stimulation. J Physiol. 1989;419:651–63. doi: 10.1113/jphysiol.1989.sp017891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Najbar-Kaszkiel AT, Di Iulio JL, Li CG, Rand MJ. Characterisation of excitatory and inhibitory transmitter systems in prostate glands of rats, guinea pigs, rabbits and pigs. Eur J Pharmacol. 1997;337:251–8. doi: 10.1016/S0014-2999(97)01270-3. [DOI] [PubMed] [Google Scholar]
- 59.Cohen ML, Drey K. Contractile responses in bladder body, bladder neck and prostate from rat, guinea pig and cat. J Pharmacol Exp Ther. 1989;248:1063–8. [PubMed] [Google Scholar]
- 60.White CW, Short JL, Haynes JM, Evans RJ, Ventura S. The residual nonadrenergic contractile response to nerve stimulation of the mouse prostate is mediated by acetylcholine but not ATP in a comparison with the mouse vas deferens. J Pharmacol Exp Ther. 2010;335:489–96. doi: 10.1124/jpet.110.172130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.White CW, Short JL, Haynes JM, Matsui M, Ventura S. Contractions of the mouse prostate elicited by acetylcholine are mediated by M(3) muscarinic receptors. J Pharmacol Exp Ther. 2011;339:870–7. doi: 10.1124/jpet.111.186841. [DOI] [PubMed] [Google Scholar]
- 62.Ventura S, Pennefather JN, Mitchelson F. Cholinergic innervation and function in the prostate gland. Pharmacol Ther. 2002;94:93–112. doi: 10.1016/S0163-7258(02)00174-2. [DOI] [PubMed] [Google Scholar]
- 63.Lau WAK, Pennefather JN, Mitchelson FJ. Cholinergic facilitation of neurotransmission to the smooth muscle of the guinea-pig prostate gland. Br J Pharmacol. 2000;130:1013–20. doi: 10.1038/sj.bjp.0703409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yazawa H, Saita Y, Iida E, Honma Y, Morita T, Honda K. Characterization of muscarinic cholinoceptor in primary culture of smooth muscle cells from human prostate. J Urol. 1994;152:2173–7. doi: 10.1016/s0022-5347(17)32346-7. [DOI] [PubMed] [Google Scholar]
- 65.Yazawa H, Honda K. The M3-muscarinic cholinoceptor subtype in rat prostate and its down regulation by aging. Jpn J Pharmacol. 1993;61:319–24. doi: 10.1254/jjp.61.319. [DOI] [PubMed] [Google Scholar]
- 66.Saito M, Ohmasa F, Shomori K, Dimitriadis F, Ohiwa H, Shimizu S, et al. Rhos and Rho kinases in the rat prostate: their possible functional roles and distributions. Mol Cell Biochem. 2011;358:207–13. doi: 10.1007/s11010-011-0936-9. [DOI] [PubMed] [Google Scholar]
- 67.Ventura S, Dewalagama RK, Lau LCL. Adenosine 5′-triphosphate (ATP) is an excitatory cotransmitter with noradrenaline to the smooth muscle of the rat prostate gland. Br J Pharmacol. 2003;138:1277–84. doi: 10.1038/sj.bjp.0705167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Buljubasich R, Ventura S. Adenosine 5′-triphosphate and noradrenaline are excitatory cotransmitters to the fibromuscular stroma of the guinea pig prostate gland. Eur J Pharmacol. 2004;499:335–44. doi: 10.1016/j.ejphar.2004.07.080. [DOI] [PubMed] [Google Scholar]
- 69.Lee HY, Bardini M, Burnstock G. P2X receptor immunoreactivity in the male genital organs of the rat. Cell Tissue Res. 2000;300:321–30. doi: 10.1007/s004410000207. [DOI] [PubMed] [Google Scholar]
- 70.Gray KT, Ventura S. Evaluation of the mouse prostate as a suitable model for the study of human prostate function. J Pharmacol Toxicol Methods. 2005;51:41–50. doi: 10.1016/j.vascn.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 71.Gray KT, Short JL, Ledent C, Ventura S. Targeted disruption of the A2A adenosine receptor reduces in-vitro prostate contractility in mature mice. Eur J Pharmacol. 2008;592:151–7. doi: 10.1016/j.ejphar.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 72.Preston A, Lau WA, Pennefather JN, Ventura S. Effects of adenine nucleosides and nucleotides on neuromuscular transmission to the prostatic stroma of the rat. Br J Pharmacol. 2000;131:1073–80. doi: 10.1038/sj.bjp.0703652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Preston A, Frydenberg M, Haynes JM. A1 and A2A adenosine receptor modulation of alpha 1-adrenoceptor-mediated contractility in human cultured prostatic stromal cells. Br J Pharmacol. 2004;141:302–10. doi: 10.1038/sj.bjp.0705535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pennefather JN, Lau WAK, Mitchelson F, Ventura S. The autonomic and sensory innervation of the smooth muscle of the prostate gland: a review of pharmacological and histological studies. J Auton Pharmacol. 2000;20:193–206. doi: 10.1046/j.1365-2680.2000.00195.x. [DOI] [PubMed] [Google Scholar]
- 75.Wanigasekara Y, Kepper ME, Keast JR. Immunohistochemical characterisation of pelvic autonomic ganglia in male mice. Cell Tissue Res. 2003;311:175–85. doi: 10.1007/s00441-002-0673-1. [DOI] [PubMed] [Google Scholar]
- 76.Davis B, Goepel M, Bein S, Chess-Williams R, Chapple CR, Michel MC. Lack of neuropeptide Y receptor detection in human bladder and prostate. BJU Int. 2000;85:918–24. doi: 10.1046/j.1464-410x.2000.00573.x. [DOI] [PubMed] [Google Scholar]
- 77.Kopp J, Zhang X, Hökfelt T. Neuropeptide Y1 receptors in the rat genital tract. Regul Pept. 1997;70:149–60. doi: 10.1016/S0167-0115(97)00028-1. [DOI] [PubMed] [Google Scholar]
- 78.Watts SW, Cohen ML. Effect of bombesin, bradykinin, substance P and CGRP in prostate, bladder body and neck. Peptides. 1991;12:1057–62. doi: 10.1016/0196-9781(91)90060-3. [DOI] [PubMed] [Google Scholar]
- 79.Ruscica M, Dozio E, Boghossian S, Bovo G, Martos Riaño V, Motta M, et al. Activation of the Y1 receptor by neuropeptide Y regulates the growth of prostate cancer cells. Endocrinology. 2006;147:1466–73. doi: 10.1210/en.2005-0925. [DOI] [PubMed] [Google Scholar]
- 80.Hedlund P, Ekström P, Larsson B, Alm P, Andersson KE. Heme oxygenase and NO-synthase in the human prostate--relation to adrenergic, cholinergic and peptide-containing nerves. J Auton Nerv Syst. 1997;63:115–26. doi: 10.1016/S0165-1838(96)00139-7. [DOI] [PubMed] [Google Scholar]
- 81.Juarranz MG, De Neef P, Robberecht P. Vasoactive intestinal polypeptide receptor VPAC(1) subtype is predominant in rat prostate membranes. Prostate. 1999;41:1–6. doi: 10.1002/(SICI)1097-0045(19990915)41:1<1::AID-PROS1>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 82.Smith ER, Miller TB, Wilson MM, Appel MC. Effects of vasoactive intestinal peptide on canine prostatic contraction and secretion. Am J Physiol. 1984;247:R701–8. doi: 10.1152/ajpregu.1984.247.4.R701. [DOI] [PubMed] [Google Scholar]
- 83.Crowe R, Chapple CR, Burnstock G. The human prostate gland: a histochemical and immunohistochemical study of neuropeptides, serotonin, dopamine beta-hydroxylase and acetylcholinesterase in autonomic nerves and ganglia. Br J Urol. 1991;68:53–61. doi: 10.1111/j.1464-410X.1991.tb15257.x. [DOI] [PubMed] [Google Scholar]
- 84.Jen PYP, Dixon JS, Gearhart JP, Gosling JA. Nitric oxide synthase and tyrosine hydroxylase are colocalized in nerves supplying the postnatal human male genitourinary organs. J Urol. 1996;155:1117–21. doi: 10.1016/S0022-5347(01)66403-6. [DOI] [PubMed] [Google Scholar]
- 85.Walden PD, Marinese D, Srinivasan D, Tzoumaka E, Syyong HT, Ford AP, et al. Effect of neurokinins on canine prostate cell physiology. Prostate. 2005;63:358–68. doi: 10.1002/pros.20195. [DOI] [PubMed] [Google Scholar]
- 86.Abrahamsson PA, Dizeyi N, Alm P, di Sant’Agnese PA, Deftos LJ, Aumüller G. Calcitonin and calcitonin gene-related peptide in the human prostate gland. Prostate. 2000;44:181–6. doi: 10.1002/1097-0045(20000801)44:3<181::AID-PROS1>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 87.Ventura S, Lau WAK, Buljubasich S, Pennefather JN. Calcitonin gene-related peptide (CGRP) inhibits contractions of the prostatic stroma of the rat but not the guinea-pig. Regul Pept. 2000;91:63–73. doi: 10.1016/S0167-0115(00)00118-X. [DOI] [PubMed] [Google Scholar]
- 88.Arciszewski MB. Distribution of calcitonin gene-related peptide (CGRP), substance P (SP) and galanin (GAL) immunoreactive nerve fibers in the seminal vesicle and prostate of the male sheep. Ann Anat. 2004;186:83–7. doi: 10.1016/S0940-9602(04)80130-4. [DOI] [PubMed] [Google Scholar]
- 89.Crowe R, Milner P, Lincoln J, Burnstock G. Histochemical and biochemical investigation of adrenergic, cholinergic and peptidergic innervation of the rat ventral prostate 8 weeks after streptozotocin-induced diabetes. J Auton Nerv Syst. 1987;20:103–12. doi: 10.1016/0165-1838(87)90107-X. [DOI] [PubMed] [Google Scholar]
- 90.Buljubasich S, Lau WA, Pennefather JN, Ventura S. An immunohistochemical and pharmacological study of tachykinins in the rat and guinea-pig prostate glands. Eur J Pharmacol. 1999;380:137–44. doi: 10.1016/S0014-2999(99)00524-5. [DOI] [PubMed] [Google Scholar]
- 91.Palea S, Corsi M, Artibani W, Ostardo E, Pietra C. Pharmacological characterization of tachykinin NK2 receptors on isolated human urinary bladder, prostatic urethra and prostate. J Pharmacol Exp Ther. 1996;277:700–5. [PubMed] [Google Scholar]
- 92.Burnett AL, Maguire MP, Chamness SL, Ricker DD, Takeda M, Lepor H, et al. Characterization and localization of nitric oxide synthase in the human prostate. Urology. 1995;45:435–9. doi: 10.1016/S0090-4295(99)80012-0. [DOI] [PubMed] [Google Scholar]
- 93.Takeda M, Tang R, Shapiro E, Burnett AL, Lepor H. Effects of nitric oxide on human and canine prostates. Urology. 1995;45:440–6. doi: 10.1016/S0090-4295(99)80013-2. [DOI] [PubMed] [Google Scholar]
- 94.Aikawa K, Yokota T, Okamura H, Yamaguchi O. Endogenous nitric oxide-mediated relaxation and nitrinergic innervation in the rabbit prostate: the changes with aging. Prostate. 2001;48:40–6. doi: 10.1002/pros.1079. [DOI] [PubMed] [Google Scholar]
- 95.Uckert S, Küthe A, Jonas UDO, Stief CG. Characterization and functional relevance of cyclic nucleotide phosphodiesterase isoenzymes of the human prostate. J Urol. 2001;166:2484–90. doi: 10.1016/S0022-5347(05)65621-2. [DOI] [PubMed] [Google Scholar]
- 96.Chow PH, Dockery P, Cheung A. Innervation of accessory sex glands in the adult male golden hamster and quantitative changes of nerve densities with age. Andrologia. 1997;29:331–42. doi: 10.1111/j.1439-0272.1997.tb00327.x. [DOI] [PubMed] [Google Scholar]
- 97.Moriyama N, Yamaguchi T, Takeuchi T, Sakamoto E, Ueki T, Tsujimoto G, et al. Semiquantitative evaluation of α1A-adrenoceptor subtype mRNA in human hypertrophied and non-hypertrophied prostates: Regional comparison. Life Sci. 1998;64:201–10. doi: 10.1016/S0024-3205(98)00552-9. [DOI] [PubMed] [Google Scholar]
- 98.Kojima Y, Sasaki S, Shinoura H, Hayashi Y, Tsujimoto G, Kohri K. Quantification of alpha1-adrenoceptor subtypes by real-time RT-PCR and correlation with age and prostate volume in benign prostatic hyperplasia patients. Prostate. 2006;66:761–7. doi: 10.1002/pros.20399. [DOI] [PubMed] [Google Scholar]
- 99.Yono M, Foster HE, Jr., Weiss RM, Latifpour J. Age related changes in the functional, biochemical and molecular properties of α1-adrenoceptors in the rat genitourinary tract. J Urol. 2006;176:1214–9. doi: 10.1016/j.juro.2006.04.038. [DOI] [PubMed] [Google Scholar]
- 100.Quinn R. Comparing rat’s to human’s age: how old is my rat in people years? Nutrition. 2005;21:775–7. doi: 10.1016/j.nut.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 101.Frisbie JH, Kumar S, Aguilera EJ, Yalla S. Prostate atrophy and spinal cord lesions. Spinal Cord. 2006;44:24–7. doi: 10.1038/sj.sc.3101804. [DOI] [PubMed] [Google Scholar]
- 102.Justulin LA, Jr., Delella FK, Felisbino SL. Doxazosin reduces cell proliferation and increases collagen fibers in rat prostatic lobes. Cell Tissue Res. 2008;332:171–83. doi: 10.1007/s00441-007-0559-3. [DOI] [PubMed] [Google Scholar]
- 103.Kojima Y, Sasaki S, Oda N, Koshimizu TA, Hayashi Y, Kiniwa M, et al. Prostate growth inhibition by subtype-selective alpha(1)-adrenoceptor antagonist naftopidil in benign prostatic hyperplasia. Prostate. 2009;69:1521–8. doi: 10.1002/pros.21003. [DOI] [PubMed] [Google Scholar]
- 104.Mitropoulos D, Kyroudi-Voulgari A, Christelli E, Zervas A, Karayannacos P. Terazosin treatment suppresses basic fibroblast growth factor expression in the rat ventral prostate. Clin Invest Med. 2009;32:E1–7. doi: 10.25011/cim.v32i1.5080. [DOI] [PubMed] [Google Scholar]
- 105.Lepor H, Berry SJ. Decreased prostatic secretory function in canine benign prostatic hyperplasia is not due to decreased levels of muscarinic cholinergic receptors. J Urol. 1984;131:803–5. doi: 10.1016/s0022-5347(17)50628-x. [DOI] [PubMed] [Google Scholar]
- 106.Kondo S, Tashima Y, Morita T. Adrenergic and cholinergic muscarinic receptors in the prostate of young and old rabbits. Urol Int. 1992;49:201–5. doi: 10.1159/000282426. [DOI] [PubMed] [Google Scholar]
- 107.Saito M, Kazuyama E, Shimizu S, Dimitriadis F, Kinoshita Y, Masuda E, et al. Muscarinic receptors and their mRNAs in type 2 Goto-Kakizaki diabetic rat prostate. Prostate. 2010;70:1533–9. doi: 10.1002/pros.21188. [DOI] [PubMed] [Google Scholar]
- 108.Chen C, Ishikawa Y, Amano I, Eguchi T, Ishida H. Age-dependent changes in response of rat prostatic tissues to isoproterenol and forskolin: changes with sexual maturation in function of G proteins. Mech Ageing Dev. 1995;81:1–13. doi: 10.1016/0047-6374(94)01577-9. [DOI] [PubMed] [Google Scholar]
- 109.Dey A, Lang RJ, Exintaris B. Nitric oxide signaling pathways involved in the inhibition of spontaneous activity in the guinea pig prostate. J Urol. 2012;187:2254–60. doi: 10.1016/j.juro.2012.01.072. [DOI] [PubMed] [Google Scholar]
- 110.Abrams P, Cardozo L, Fall M, Griffiths D, Rosier P, Ulmsten U, et al. Standardisation Sub-committee of the International Continence Society The standardisation of terminology of lower urinary tract function: report from the Standardisation Sub-committee of the International Continence Society. Neurourol Urodyn. 2002;21:167–78. doi: 10.1002/nau.10052. [DOI] [PubMed] [Google Scholar]
- 111.Rosen R, Altwein J, Boyle P, Kirby RS, Lukacs B, Meuleman E, et al. Lower urinary tract symptoms and male sexual dysfunction: the multinational survey of the aging male (MSAM-7) Eur Urol. 2003;44:637–49. doi: 10.1016/j.eururo.2003.08.015. [DOI] [PubMed] [Google Scholar]
- 112.Barry MJ. Evaluation of symptoms and quality of life in men with benign prostatic hyperplasia. Urology. 2001;58(Suppl 1):25–32, discussion 32. doi: 10.1016/S0090-4295(01)01300-0. [DOI] [PubMed] [Google Scholar]
- 113.Chapple CR, Roehrborn CG. A shifted paradigm for the further understanding, evaluation, and treatment of lower urinary tract symptoms in men: focus on the bladder. Eur Urol. 2006;49:651–8. doi: 10.1016/j.eururo.2006.02.018. [DOI] [PubMed] [Google Scholar]
- 114.Kirby RS, Kirby M, Fitzpatrick JM. Benign prostatic hyperplasia: counting the cost of its management. BJU Int. 2010;105:901–2. doi: 10.1111/j.1464-410X.2010.09274.x. [DOI] [PubMed] [Google Scholar]
- 115.McNeal JE. Origin and evolution of benign prostatic enlargement. Invest Urol. 1978;15:340–5. [PubMed] [Google Scholar]
- 116.Bartsch G, Müller HR, Oberholzer M, Rohr HP. Light microscopic stereological analysis of the normal human prostate and of benign prostatic hyperplasia. J Urol. 1979;122:487–91. doi: 10.1016/s0022-5347(17)56476-9. [DOI] [PubMed] [Google Scholar]
- 117.Dmochowski RR. Bladder outlet obstruction: etiology and evaluation. Rev Urol. 2005;7(Suppl 6):S3–13. [PMC free article] [PubMed] [Google Scholar]
- 118.Lepor H. Pathophysiology of lower urinary tract symptoms in the aging male population. Rev Urol. 2005;7(Suppl 7):S3–11. [PMC free article] [PubMed] [Google Scholar]
- 119.Oelke M, Baard J, Wijkstra H, de la Rosette JJ, Jonas U, Höfner K. Age and bladder outlet obstruction are independently associated with detrusor overactivity in patients with benign prostatic hyperplasia. Eur Urol. 2008;54:419–26. doi: 10.1016/j.eururo.2008.02.017. [DOI] [PubMed] [Google Scholar]
- 120.Yamada S, Ashizawa N, Ushijima H, Nakayama K, Hayashi E, Honda K. Alpha-1 adrenoceptors in human prostate: characterization and alteration in benign prostatic hypertrophy. J Pharmacol Exp Ther. 1987;242:326–30. [PubMed] [Google Scholar]
- 121.Haynes JM, Ventura S. Current models of human prostate contractility. Clin Exp Pharmacol Physiol. 2005;32:797–804. doi: 10.1111/j.1440-1681.2005.04268.x. [DOI] [PubMed] [Google Scholar]
- 122.Ventura S, Oliver Vl, White CW, Xie JH, Haynes JM, Exintaris B. Novel drug targets for the pharmacotherapy of benign prostatic hyperplasia (BPH) Br J Pharmacol. 2011;163:891–907. doi: 10.1111/j.1476-5381.2011.01332.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Song W, Yuan M, Zhao S. Variation of M3 muscarinic receptor expression in different prostate tissues and its significance. Saudi Med J. 2009;30:1010–6. [PubMed] [Google Scholar]
- 124.Logothetis CJ, Lin SH. Osteoblasts in prostate cancer metastasis to bone. Nat Rev Cancer. 2005;5:21–8. doi: 10.1038/nrc1528. [DOI] [PubMed] [Google Scholar]
- 125.Petrylak DP, Tangen CM, Hussain MH, Lara PN, Jr., Jones JA, Taplin ME, et al. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med. 2004;351:1513–20. doi: 10.1056/NEJMoa041318. [DOI] [PubMed] [Google Scholar]
- 126.Tannock IF, de Wit R, Berry WR, Horti J, Pluzanska A, Chi KN, et al. TAX 327 Investigators Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351:1502–12. doi: 10.1056/NEJMoa040720. [DOI] [PubMed] [Google Scholar]
- 127.Kyprianou N, Litvak JP, Borkowski A, Alexander R, Jacobs SC. Induction of prostate apoptosis by doxazosin in benign prostatic hyperplasia. J Urol. 1998;159:1810–5. doi: 10.1016/S0022-5347(01)63162-8. [DOI] [PubMed] [Google Scholar]
- 128.Giardinà D, Martarelli D, Sagratini G, Angeli P, Ballinari D, Gulini U, et al. Doxazosin-related alpha1-adrenoceptor antagonists with prostate antitumor activity. J Med Chem. 2009;52:4951–4. doi: 10.1021/jm8016046. [DOI] [PubMed] [Google Scholar]
- 129.Liu CM, Lo YC, Tai MH, Wu BN, Wu WJ, Chou YH, et al. Piperazine-designed alpha 1A/alpha 1D-adrenoceptor blocker KMUP-1 and doxazosin provide down-regulation of androgen receptor and PSA in prostatic LNCaP cells growth and specifically in xenografts. Prostate. 2009;69:610–23. doi: 10.1002/pros.20919. [DOI] [PubMed] [Google Scholar]
- 130.Tahmatzopoulos A, Rowland RG, Kyprianou N. The role of alpha-blockers in the management of prostate cancer. Expert Opin Pharmacother. 2004;5:1279–85. doi: 10.1517/14656566.5.6.1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Haynes JM, Frydenberg M, Majewski H. Testosterone- and phorbol ester-stimulated proliferation in human cultured prostatic stromal cells. Cell Signal. 2001;13:703–9. doi: 10.1016/S0898-6568(01)00205-4. [DOI] [PubMed] [Google Scholar]
- 132.Masur K, Niggemann B, Zanker KS, Entschladen F. Norepinephrine-induced migration of SW 480 colon carcinoma cells is inhibited by beta-blockers. Cancer Res. 2001;61:2866–9. [PubMed] [Google Scholar]
- 133.Palm D, Lang K, Niggemann B, Drell TL, 4th, Masur K, Zaenker KS, et al. The norepinephrine-driven metastasis development of PC-3 human prostate cancer cells in BALB/c nude mice is inhibited by beta-blockers. Int J Cancer. 2006;118:2744–9. doi: 10.1002/ijc.21723. [DOI] [PubMed] [Google Scholar]
- 134.Hassan S, Karpova Y, Baiz D, Yancey D, Pullikuth A, Flores A, et al. Behavioral stress accelerates prostate cancer development in mice. J Clin Invest. 2013;123:874–86. doi: 10.1172/JCI63324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Rayford W, Noble MJ, Austenfeld MA, Weigel J, Mebust WK, Shah GV. Muscarinic cholinergic receptors promote growth of human prostate cancer cells. Prostate. 1997;30:160–6. doi: 10.1002/(SICI)1097-0045(19970215)30:3<160::AID-PROS3>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 136.Batra S, Christensson PI, Hartley-Asp B. Characterization of muscarinic cholinergic receptors in membrane preparations from rat prostatic adenocarcinoma. Prostate. 1990;17:261–8. doi: 10.1002/pros.2990170402. [DOI] [PubMed] [Google Scholar]
- 137.Rao J, Yang J, Liu Z, Wang L, Yin Z, Liu L, et al. Hypothetic association between greater sympathetic activity and prostate cancer. Med Hypotheses. 2008;71:442–3. doi: 10.1016/j.mehy.2008.03.039. [DOI] [PubMed] [Google Scholar]
- 138.Esler M, Rumantir M, Kaye D, Lambert G. The sympathetic neurobiology of essential hypertension: disparate influences of obesity, stress, and noradrenaline transporter dysfunction? Am J Hypertens. 2001;14:139S–46S. doi: 10.1016/S0895-7061(01)02081-7. [DOI] [PubMed] [Google Scholar]
- 139.Moyad MA. Is obesity a risk factor for prostate cancer, and does it even matter? A hypothesis and different perspective. Urology. 2002;59(Suppl 1):41–50. doi: 10.1016/S0090-4295(01)01175-X. [DOI] [PubMed] [Google Scholar]
- 140.Gann PH, Daviglus ML, Dyer AR, Stamler J. Heart rate and prostate cancer mortality: results of a prospective analysis. Cancer Epidemiol Biomarkers Prev. 1995;4:611–6. [PubMed] [Google Scholar]
- 141.Fitzpatrick AL, Daling JR, Furberg CD, Kronmal RA, Weissfeld JL. Hypertension, heart rate, use of antihypertensives, and incident prostate cancer. Ann Epidemiol. 2001;11:534–42. doi: 10.1016/S1047-2797(01)00246-0. [DOI] [PubMed] [Google Scholar]
- 142.Mortensen PB. Neuroleptic medication and reduced risk of prostate cancer in schizophrenic patients. Acta Psychiatr Scand. 1992;85:390–3. doi: 10.1111/j.1600-0447.1992.tb10325.x. [DOI] [PubMed] [Google Scholar]
- 143.Rosenberg DJ, Neugut AI, Ahsan H, Shea S. Diabetes mellitus and the risk of prostate cancer. Cancer Invest. 2002;20:157–65. doi: 10.1081/CNV-120001141. [DOI] [PubMed] [Google Scholar]
- 144.Scott PA, Sr., Perkash I, Mode D, Wolfe VA, Terris MK. Prostate cancer diagnosed in spinal cord-injured patients is more commonly advanced stage than in able-bodied patients. Urology. 2004;63:509–12. doi: 10.1016/j.urology.2003.10.022. [DOI] [PubMed] [Google Scholar]
- 145.Perron L, Bairati I, Harel F, Meyer F. Antihypertensive drug use and the risk of prostate cancer (Canada) Cancer Causes Control. 2004;15:535–41. doi: 10.1023/B:CACO.0000036152.58271.5e. [DOI] [PubMed] [Google Scholar]
- 146.Harris AM, Warner BW, Wilson JM, Becker A, Rowland RG, Conner W, et al. Effect of alpha1-adrenoceptor antagonist exposure on prostate cancer incidence: an observational cohort study. J Urol. 2007;178:2176–80. doi: 10.1016/j.juro.2007.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Murtola TJ, Tammela TL, Määttänen L, Ala-Opas M, Stenman UH, Auvinen A. Prostate cancer incidence among finasteride and alpha-blocker users in the Finnish Prostate Cancer Screening Trial. Br J Cancer. 2009;101:843–8. doi: 10.1038/sj.bjc.6605188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Ramsköld D, Luo S, Wang YC, Li R, Deng Q, Faridani OR, et al. Full-length mRNA-Seq from single-cell levels of RNA and individual circulating tumor cells. Nat Biotechnol. 2012;30:777–82. doi: 10.1038/nbt.2282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Thebault S, Roudbaraki M, Sydorenko V, Shuba Y, Lemonnier L, Slomianny C, et al. Alpha1-adrenergic receptors activate Ca(2+)-permeable cationic channels in prostate cancer epithelial cells. J Clin Invest. 2003;111:1691–701. doi: 10.1172/JCI16293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Katsogiannou M, El Boustany C, Gackiere F, Delcourt P, Athias A, Mariot P, et al. Caveolae contribute to the apoptosis resistance induced by the alpha(1A)-adrenoceptor in androgen-independent prostate cancer cells. PLoS One. 2009;4:e7068. doi: 10.1371/journal.pone.0007068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Jensen BC, Swigart PM, Simpson PC. Ten commercial antibodies for alpha-1-adrenergic receptor subtypes are nonspecific. Naunyn Schmiedebergs Arch Pharmacol. 2009;379:409–12. doi: 10.1007/s00210-008-0368-6. [DOI] [PMC free article] [PubMed] [Google Scholar]


