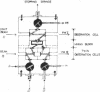
Abstract
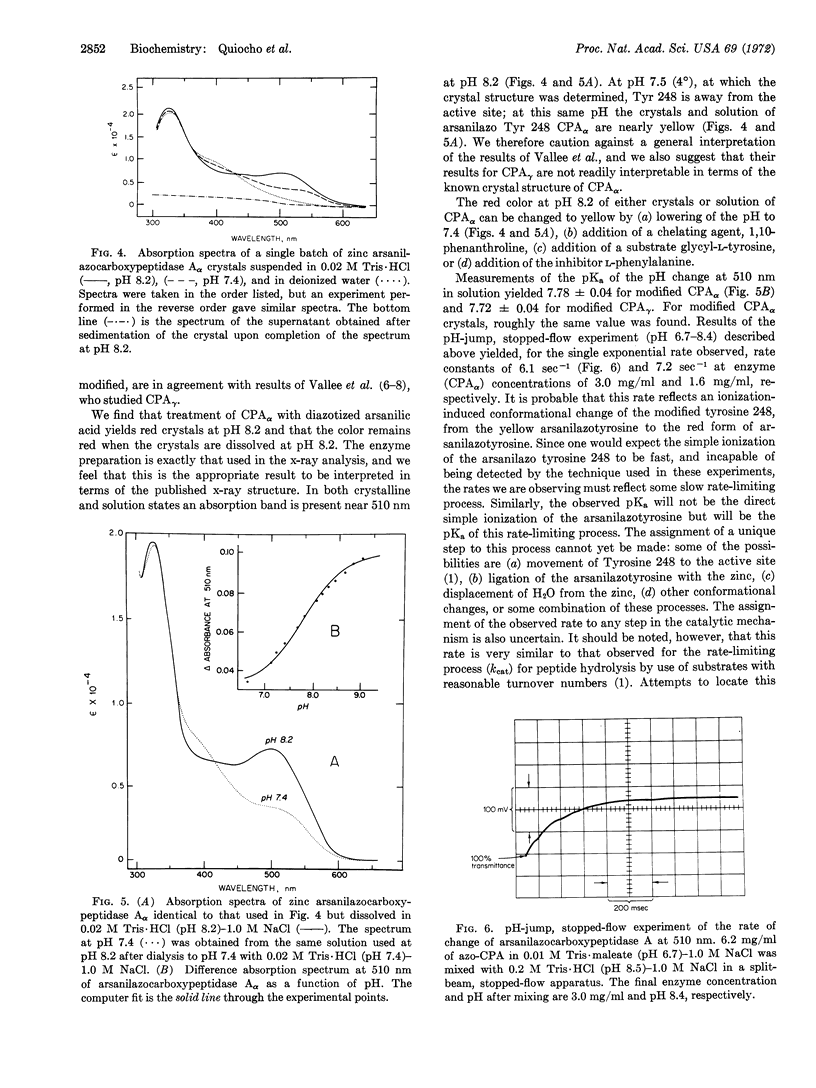
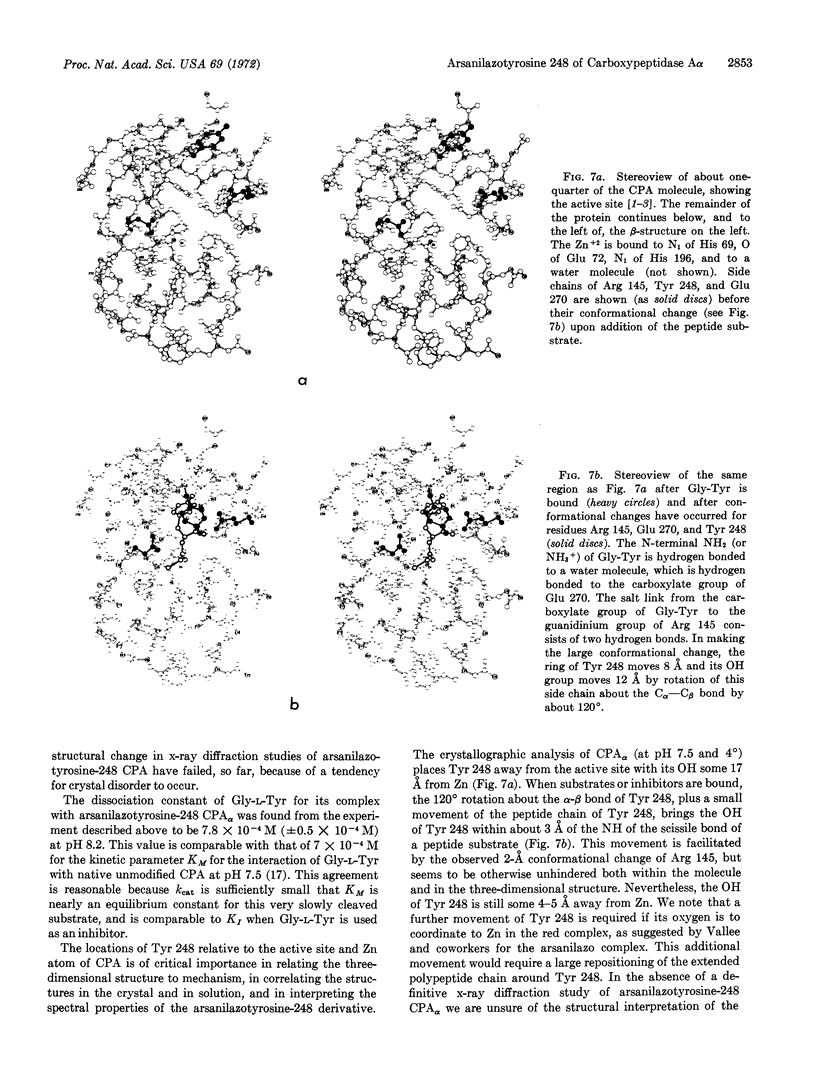
Modification of carboxypeptidase Aγ crystals (Anson) with diazotized arsanilic acid specifically labels tyrosine 248; at pH 8.2 the modified enzyme gives yellow crystals, but a red solution. It has been suggested that arsanilazotyrosine 248 forms a complex with the Zn cofactor accounting for the red color in solution, but that a complex is not formed in the crystal. However, the crystal structure of carboxypeptidase Aγ is unknown. We show here that crystals of carboxypeptidase Aα, whose crystal structure has been determined, are red both in solution and in the crystalline state (at pH 8.2) after modification with diazotized arsanilic acid. These new data are of importance in relating the structure in the crystalline state to the catalytic mechanisms, as based on the x-ray diffraction evidence.
The activity of carboxypeptidase A in the crystal and in solution has a ratio of only 1/3 for the α form, in contrast to the ratio of 1/300 for the γ form, with carbobenzoxyglycyl-L-phenylalanine as a substrate.
A pH-jump experiment monitored by stopped-flow kinetics in a split-beam apparatus has revealed a single exponential rate when a solution of arsanilazotyrosine 248 carboxypeptidase Aα at pH 6.7 (yellow) is increased to pH 8.5 (red). The rate constants obtained in this experiment are 6.1 sec-1 at 3.0 mg/ml and 7.2 sec-1 at 1.6 mg/ml concentration of enzyme.
Keywords: enzyme activity, stopped-flow pH-jump
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson R. G., Nickless G. Heterocyclic azo dyestuffs in analytical chemistry. A review. Analyst. 1967 Apr;92(93):207–238. doi: 10.1039/an9679200207. [DOI] [PubMed] [Google Scholar]
- DUYSENS L. N. The flattening of the absorption spectrum of suspensions, as compared to that of solutions. Biochim Biophys Acta. 1956 Jan;19(1):1–12. doi: 10.1016/0006-3002(56)90380-8. [DOI] [PubMed] [Google Scholar]
- FOLK J. E., SCHIRMER E. W. THE PORCINE PANCREATIC CARBOXYPEPTIDASE A SYSTEM. I. THREE FORMS OF THE ACTIVE ENZYME. J Biol Chem. 1963 Dec;238:3884–3894. [PubMed] [Google Scholar]
- Johansen J. T., Livingston D. M., Vallee B. L. Chemical modification of carboxypeptidase A crystals. Azo coupling with tyrosine-248. Biochemistry. 1972 Jul 4;11(14):2584–2588. doi: 10.1021/bi00764a005. [DOI] [PubMed] [Google Scholar]
- Johansen J. T., Vallee B. L. Differences between the conformation of arsanilazotyrosine 248 of carboxypeptidase A in the crystalline state and in solution. Proc Natl Acad Sci U S A. 1971 Oct;68(10):2532–2535. doi: 10.1073/pnas.68.10.2532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipscomb W. N., Hartsuck J. A., Reeke G. N., Jr, Quiocho F. A., Bethge P. H., Ludwig M. L., Steitz T. A., Muirhead H., Coppola J. C. The structure of carboxypeptidase A. VII. The 2.0-angstrom resolution studies of the enzyme and of its complex with glycyltyrosine, and mechanistic deductions. Brookhaven Symp Biol. 1968 Jun;21(1):24–90. [PubMed] [Google Scholar]
- NEURATH H. MECHANISM OF ZYMOGEN ACTIVATION. Fed Proc. 1964 Jan-Feb;23:1–7. [PubMed] [Google Scholar]
- Reeke G. N., Hartsuck J. A., Ludwig M. L., Quiocho F. A., Steitz T. A., Lipscomb W. N. The structure of carboxypeptidase a, vi. Some results at 2.0-a resolution, and the complex with glycyl-tyrosine at 2.8-a resolution. Proc Natl Acad Sci U S A. 1967 Dec;58(6):2220–2226. doi: 10.1073/pnas.58.6.2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TABACHNICK M., SOBOTKA H. Azoproteins. II. A spectrophotometric study of the coupling of diazotized arsanilic acid with proteins. J Biol Chem. 1960 Apr;235:1051–1054. [PubMed] [Google Scholar]
- Vallee B. L., Riordan J. F., Johansen J. T., Livingston D. M. Spectro-chemical probes for protein conformation and function. Cold Spring Harb Symp Quant Biol. 1972;36:517–531. doi: 10.1101/sqb.1972.036.01.066. [DOI] [PubMed] [Google Scholar]