Abstract
Process control of protein therapeutic manufacturing is central to ensuring the product is both safe and efficacious for patients. In this work, we investigate the cause of pink color variability in development lots of monoclonal antibody (mAb) and Fc-fusion proteins. Results show pink-colored product generated during manufacturing is due to association of hydroxocobalamin (OH-Cbl), a form of vitamin B12. OH-Cbl is not part of the product manufacturing process; however we found cyanocobalamin (CN-Cbl) in cell culture media converts to OH-Cbl in the presence of light. OH-Cbl can be released from mAb and Fc-fusion proteins by conversion with potassium cyanide to CN-Cbl, which does not bind. By exploiting the differential binding of CN-Cbl and OH-Cbl, we developed a rapid and specific assay to accurately measure B12 levels in purified protein. Analysis of multiple products and lots using this technique gives insight into color variability during manufacturing.
Keywords: vitamin B12, cobalamin, color, monoclonal antibody, process control
Introduction
Successful control of biopharmaceutical manufacturing is critical for ensuring product quality and lot-to-lot consistency of therapeutic proteins. Although some amount of heterogeneity can be expected because of the complexity of large molecules, rigorous testing and characterization are used to define product consistency throughout process development. Product appearance, i.e., the state and color of a product, is a required specification for release and can be a simple test to identify product or process impurities.1 Although color may not affect safety or efficacy, even moderate color variation in a therapeutic product can indicate process inconsistency and possibly present complications for blinding studies during clinical trials.
Although proteins commonly have some color due to light scattering at high concentrations, variability in color observed during manufacturing may be from process-related impurities.2,3 Cell culture media is a common source of colored components in biotherapeutic manufacturing, but these are normally cleared during downstream processing.4 Host cell proteins, however, have been observed to co-purify due to protein interactions.5 Similarly, vitamins in cell culture media have shown high affinity to monoclonal antibodies (mAbs) and may co-purify, resulting in a colored final product.6,7 Both vitamin B2 (riboflavin) and vitamin B9 (folic acid) are yellow, while vitamin B12 (cobalamin) is a vibrant pink and can dominate the color of the media.8
Vitamin B12, an essential vitamin for eukaryotes, has been described as the most complex of the B vitamins. It contains a corrin-ring surrounding a cobalt atom that is coordinated axially, by a 5,6-dimethylbenzimidazole nucleotide tail in one plane and a variable R-group in the opposite position (Fig. 1). There are four naturally occurring forms of vitamin B12 that are distinguished by different R-groups: cyanocobalamin (CN-Cbl), hydroxocobalamin (OH-Cbl), methylcobalamin (Me-Cbl), and adenosylcobalamin (Ado-Cbl). CN-Cbl is the most stable of the vitamin B12 forms and is ubiquitous in commercially-available products such as vitamin supplements and cell culture media. The other three vitamin B12 forms are less stable, with ligand affinity in the order of OH- < Ado- < Me-Cbl. Me- and Ado-Cbl are biologically active forms of vitamin B12, and are used as cofactors for methionine synthase and methyl-malonyl CoA mutase.9,10
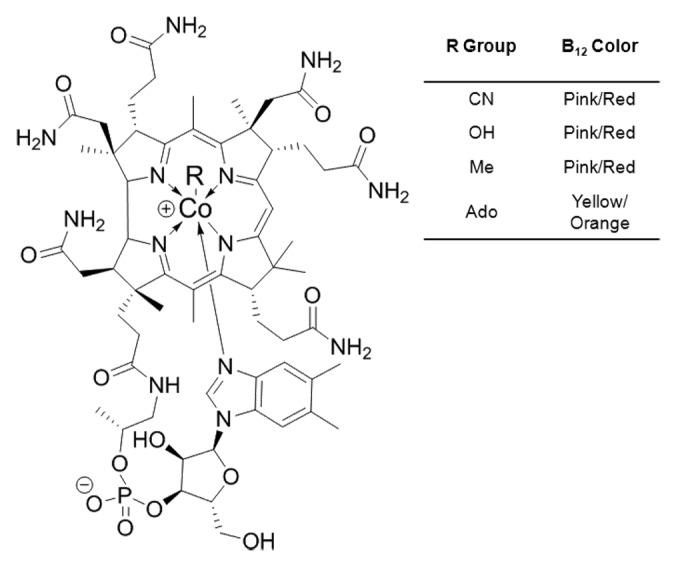
Figure 1. The structure of vitamin B12 with identification of R groups and associated color.
We investigated the cause of variable pink coloration of several purified mAbs and Fc-fusion proteins. Vitamin B12 used during manufacturing was a likely source of pink color, and a combination of binding experiments and media photo stability studies identified OH-Cbl as the specific B12 form responsible for pink color in product. Differential association between the B12 forms was further leveraged to develop a rapid and simple sample pretreatment for accurate quantification. Analysis of multiple proteins and process conditions provides insight into the sporadic occurrence of pink coloration and suggests a path for eliminating future color variability.
Results
Proteins are well-known to have yellow color originating from a variety of sources; however, during process development, several mAbs and Fc-fusion proteins were observed to have pink color.7,11 The occurrences spanned multiple products, processes, sites and manufacturing scales over several years, and the color varied in intensities. Pink coloration usually did not occur with every production run, making investigation of the root cause problematic. In the most striking example, material purified from bioreactors run side-by-side under seemingly identical conditions produced pink and non-pink protein (Fig. 2). Only one of the two product lots was pink, which suggested the presence of a pink contaminant. Removal of the pink color using conventional protein purification techniques including affinity and size exclusion chromatography was unsuccessful, indicating co-purification of the pink contaminant.

Figure 2. Two purified development lots of Fc-fusion protein. Both lots were produced within normal operating conditions, but had vastly different colors. Identifying markings on the containers have been intentionally obscured.
The main pink-colored component in the manufacturing process is CN-Cbl, a component of cell culture media. The appearance of pink in multiple molecules and the persistence of this color through purification led to the hypothesis that CN-Cbl binds to product, and the binding should be ubiquitous, energetically favorable, and have a rapid association. Repeated attempts to turn proteins pink by incubation with CN-Cbl, however, were unsuccessful. This included titration of CN-Cbl with uncolored lots of products that had shown pink variability. When combined, these observations suggest CN-Cbl is not responsible for the pink coloration.
Identification of the pink colored contaminant
While CN-Cbl does not directly associate with protein, it was possible that a different form of vitamin B12, such as OH-, Ado- or Me-Cbl, could bind. Like CN-Cbl, OH- and Me-Cbl are pink-colored and thus likely candidates.10 The lack of CN-Cbl-protein association was leveraged to determine if the pink-colored contaminant was a form of B12 other than CN-Cbl. A dilute potassium cyanide (KCN) solution is known to convert all B12 forms to CN-Cbl and is frequently used during B12 analyses.12 Therefore, a pink Fc-fusion protein was incubated in the presence of KCN to convert all vitamin B12 to CN-Cbl, then buffer-exchanged to remove disassociated CN-Cbl. After repeated washing, the protein-containing retentate and the filtrate were examined visually for pink color. In the presence of KCN, the retentate was colorless and the filtrate was pink, indicating that, by conversion to CN-Cbl, the pink contaminant had been released from the protein (Fig. 3). These results indicate a pink form of vitamin B12, other than CN-Cbl, is associated with the Fc-fusion protein.

Figure 3. Cartoon and pictures of vitamin B12 disassociation from a Fc-fusion protein. Incubation of Fc-fusion protein with (A) or without (B) KCN followed by washing.
To identify which form of vitamin B12 binds protein, samples of an uncolored IgG2 mAb were incubated with CN-, Me-, OH- or Ado-Cbl standards. Post-incubation, the IgG2/vitamin B12 solution was buffer-exchanged as before, and the retentate and filtrate visually inspected. Incubation without B12 or with CN-Cbl resulted in colorless protein, while incubation with Ado-Cbl, Me-Cbl or OH-Cbl resulted in protein with some degree of pink coloration (Fig. 4A and B). The association of Me-Cbl and Ado-Cbl was unexpected because these forms have been shown to specifically bind the Asp-X-His-X-X-Gly motif, which is not present in the proteins used here.13 To gain further insight, the B12 standards used in the experiment were analyzed by RP-UPLC with UV and MS detection. Each B12 form had a distinct retention time, with OH-Cbl eluting first, followed by CN-Cbl, Ado-Cbl, and Me-Cbl (Fig. 5A and B). The Ado-Cbl and the Me-Cbl chromatograms, however, also contained OH-Cbl, as confirmed by mass spectrometry. The B12 standards were purchased as >99% pure, so the presence of OH-Cbl was likely caused by Ado- and Me-Cbl degradation to OH-Cbl.14 This is further supported by the observation of lighter pink color and lower quantified B12 levels for protein incubated with Ado- and Me-Cbl compared with the OH-Cbl sample, even though all standards were prepared and used at the same concentration (Fig. 4B). OH-Cbl was also incubated in the presence of IgG1, IgG2 and Fc-fusion proteins; after removal of excess vitamin, all were visibly pink (Fig. 4C). Taken together, these data strongly suggest that OH-Cbl is the pink-colored contaminant.

Figure 4. Cartoon (A) and pictures of vitamin B12 association with an uncolored IgG2 (B). Numerical values are quantification of vitamin B12 associated with protein. Incubation of uncolored IgG1, IgG2, and Fc-Fusion proteins with and without OH-Cbl (C).
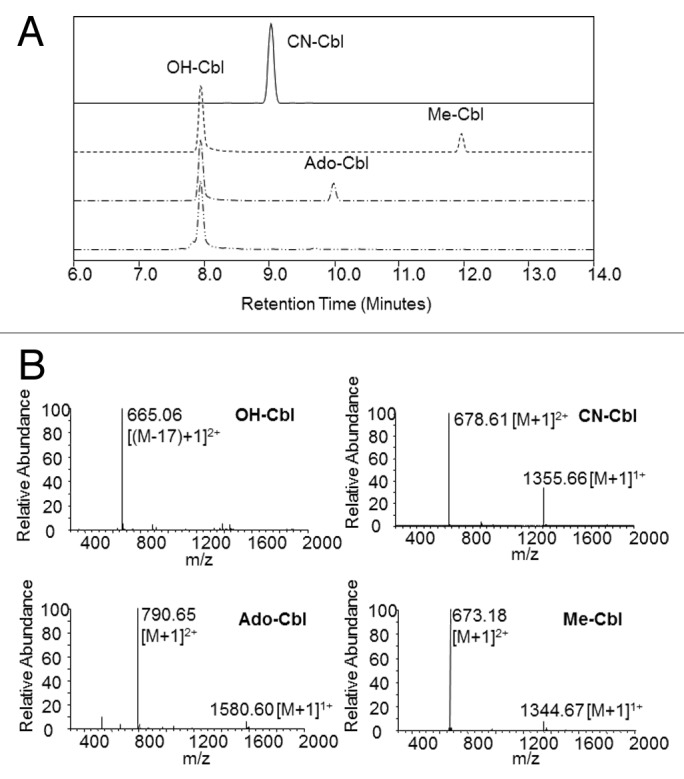
Figure 5.(A) RP-UPLC separation of CN-Cbl (solid), Me-Cbl (dashed), Ado-Cbl (dash-dot) and OH-Cbl (dash-dot-dot) with 360 nm detection. (B) Identification of standard by MS detection.
Since localization of OH-Cbl binding on mAb protein may provide insight into the mechanism of association, an IgG2 was incubated with OH-Cbl and subjected to limited proteolysis. Fc and F(ab’)2 fragments generated by IdeS digestion were separated by non-denaturing size exclusion chromatography (SEC) to maintain any OH-Cbl/protein associations. UV detection at both 280 nm and 360 nm was used to identify OH-Cbl association. While the UV 280 nm signal was unchanged by the presence of OH-Cbl, Fc and F(ab’)2 fragments showed an increase in UV 360 nm relative to the untreated control, demonstrating OH-Cbl association occurs in both regions (Fig. 6; Table 1).
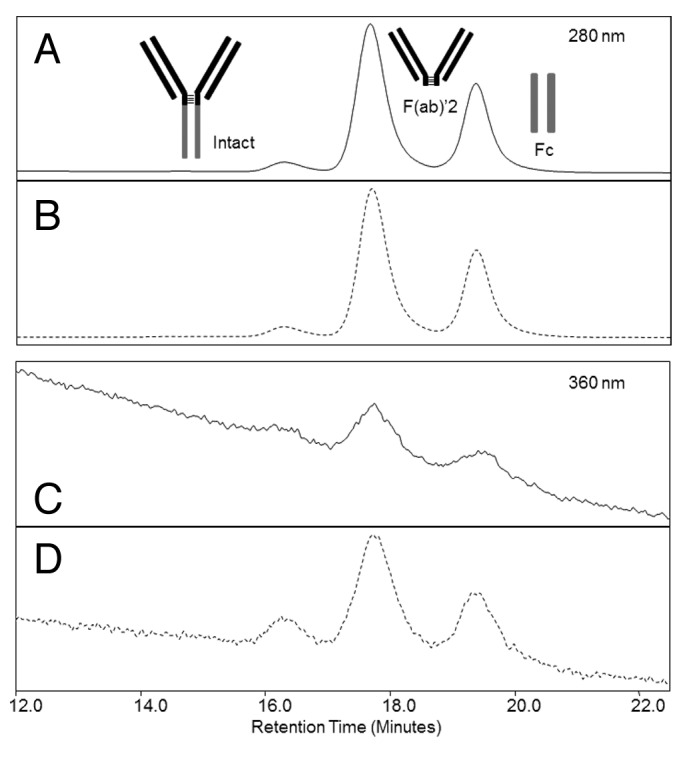
Figure 6. SEC separation of mAb control (solid) and mAb incubated in the presence of OH-Cbl (dashed) after digestion with the IdeS enzyme with detection at 280 nm (A, B) and 360 nm (C, D).
Table 1. Localization of OH-Cbl association to an IgG2 monoclonal antibody.
Conversion of CN-Cbl to OH-Cbl in cell culture media
Although OH-Cbl has been identified as the vitamin B12 form that binds to product, CN-Cbl is the form added to the cell culture media. CN-Cbl is the most stable B12 form, but can convert to OH-Cbl in the presence of light.12,15 Analysis of light-exposed and protected CN-Cbl confirms a small but distinct increase in OH-Cbl by RP-UPLC after only 30 min (Fig. 7A). Further investigation of CN-Cbl conversion to OH-Cbl was performed in Chinese hamster ovary (CHO) cell culture medium to determine whether light-induced conversion would occur in the complex matrix. Cell culture medium was initially prepared without CN-Cbl to prevent conversion to OH-Cbl during media preparation. After spiking medium samples with CN-Cbl, the effect of light exposure was evaluated over the course of 10 d at 37 °C. Formation of OH-Cbl was obscured due to co-elution with unidentified media components; however, CN-Cbl was well-resolved from other media components and easily quantified. CN-Cbl levels were significantly reduced upon light-exposure within a single day, and 50% or greater loss was seen over 10 d (Fig. 7B). In contrast, light-protected samples showed no significant loss of CN-Cbl, confirming that conversion to OH-Cbl occurs with light exposure. Approximately 90% of CN-Cbl was recovered after treatment of light-exposed samples with KCN, indicating CN-Cbl is converted to OH-Cbl as opposed to degraded in the presence of redox-active compounds (Fig. 7C).16 Combined, these data indicate that light-exposure of cell culture media can convert CN-Cbl to OH-Cbl on a timescale significantly shorter than a typical production cell culture.

Figure 7. (A) Analysis of freshly prepared CN-Cbl standard (solid) and CN-Cbl standard exposed to light at room temperature for 30 min (dashed). Inset is a zoom view of 7–9 min for visualization of OH-Cbl changes. (B) Stability plot of relative CN-Cbl levels in light exposed CN-Cbl standard (open diamond), and media at 37 °C either protected from light (filled squares) or light exposed (open squares). (C) RP-UPLC chromatogram of light protected media (top), light exposed media (middle), and light exposed then KCN incubated media (bottom) at 37°C after 10 d.
Vitamin B12 analysis of purified protein samples
A simple procedure to quantify OH-Cbl in relatively pure protein samples was developed by exploiting the differential association of OH-Cbl and CN-Cbl. Fc-fusion or mAb was incubated in the presence of KCN to release bound OH-Cbl into solution by conversion to CN-Cbl. CN-Cbl and protein were then separated using protein A chromatography, and the unbound fraction containing CN-Cbl was analyzed by RP-UPLC. CN-Cbl levels were quantified using a standard curve that displayed good linearity (r2 > 0.999) and a limit of quantification of 1 pg CN-Cbl. Sample recovery was determined by spiking to be ~80%. To verify the KCN treatment converted all OH-Cbl to unbound CN-Cbl, subsets of samples were also analyzed by elemental analysis of cobalt concentration using inductively coupled plasma mass spectrometry (ICP-MS) (Table 2). CN-Cbl is the main source of cobalt in the manufacturing process, with other minor contributors totaling less than 1%. Very good agreement was observed between the orthogonal analyses, indicating conversion of OH-Cbl to CN-Cbl is complete and CN-Cbl fully disassociates from the protein. The simple conversion, good recovery, and high throughput sample handling allows for analysis OH-Cbl levels in mAbs and Fc-fusion protein samples of varying degrees of color and purity.
Table 2. Comparison of RP-UPLC quantification of purified protein with elemental analysis for cobalt.
| Sample | Reported color | Conc. (g/L) | ICP-MS B12 (mg/kg)1 | RP-UPLC B12 (mg/kg) | ng B12/mg protein2 |
|---|---|---|---|---|---|
| Fc-Fusion: Protein A Pool | Very pink | 12.8 | N/T3 | 44.53 | 3478.77 |
| mAb1: Lot 1 DS | Pink | 70 | 4.4 | 3.40 | 48.54 |
| mAb1: Lot 2 DS | Non-pink | 70 | 0.2 | < LOQ4 | < LOQ4 |
| mAb2: Protein A Pool | Pink | 12.6 | 0.7 | 0.70 | 55.82 |
| mAb3: Protein A Pool | Pink | 16.5 | 0.7 | 0.67 | 40.42 |
| mAb3: Column 2 Pool | Non-pink | 25 | 0.3 | 0.29 | 11.47 |
| mAb3: DS | Peach | 72.9 | 0.8 | 0.68 | 9.35 |
The levels of OH-Cbl were protein normalized in three different lots of an IgG1 mAb that exhibited pink intensities from colorless to strongly pink to determine the affect of purification (Table 3). After protein A purification, Lot 1 was a strong pink color with 116 ng B12/mg mAb, while Lot 2 was less pink and contained 67 ng B12/mg protein. The amount of OH-Cbl in Lot 2 was reduced by almost 50% across the second chromatography step, suggesting that there may be conditions that dissociate OH-Cbl from the product, but no clearance was observed across the third chromatography step or in the drug substance (DS). Lot 3 was visually colorless during downstream processing, although a small amount of OH-Cbl was detected in each in-process pool. Overall, the OH-Cbl concentration in the samples correlated well with the intensity of pink color. The most intensely pink sample had an OH-Cbl concentration of 1.5 mg/kg while samples below 0.5 mg/kg appeared visually colorless. The data also suggest that OH-Cbl associates with the protein during the cell culture, harvest, or protein A chromatography because the OH-Cbl levels do not increase during further downstream processing.
Table 3. Quantification of purified lots of mAb2 by RP-UPLC.
| Lot | Process intermediate | Reported color | Conc. (g/L) | RP HPLC B12 (ppm) | ng B12/mg Protein1 |
|---|---|---|---|---|---|
| Lot 1 | Protein A Pool | Strongly pink | 12.8 | 1.49 | 116.4 |
| Lot 2 | Protein A Pool | Pink | 12.2 | 0.81 | 66.7 |
| Column 2 Pool | Colorless | 6.5 | 0.25 | 39.2 | |
| Column 3 Pool | Pink | 12.6 | 0.49 | 39.2 | |
| DS | Pink | 21.9 | 0.88 | 40.4 | |
| Lot 3 | Protein A Pool | Colorless | 21.6 | 0.18 | 8.3 |
| Column 2 Pool | Colorless | 11.9 | 0.06 | 5.1 | |
| Column 3 Pool | Colorless | 9.4 | 0.05 | 5.8 |
1 Assuming a molecular weight of 150 kD for mAbs, a conversion factor of 11 070 may be used to obtain B12-Protein molar ratio.
Discussion
This work demonstrates that pink coloration of purified mAb and Fc-fusion proteins is a result of OH-Cbl association. We found that incubation of colorless protein with OH-, Me-, or Ado-Cbl causes protein to become pink, although the appearance of pink coloration with both Me- and Ado-Cbl was determined to be OH-Cbl impurities generated during routine sample preparation. CN-Cbl, the form of vitamin B12 used in cell culture media, did not bind to mAbs or Fc-fusion proteins; however, pure CN-Cbl and CN-Cbl in cell culture media converted to OH-Cbl in the presence of light. This finding is consistent with previous studies on the conversion of CN-Cbl to OH-Cbl upon light exposure, and suggests a mechanism for OH-Cbl generation in media. It is likely that a combination of light exposure and other unknown factors facilitate conversion during media preparation, cell culture or harvest operations.15
In eukaryotes, vitamin B12 is an essential vitamin that is used as a cofactor for the enzymes methionine synthase and methyl-malonyl CoA mutase. The different forms of B12 interact with multiple proteins.9,10 To enter cells, vitamin B12 is shuttled through a series of chaperone complexes: haptocorrin (HC), intrinsic factor (IF), and transcobalamin II (TC). HC, the first of the transport proteins, has the broadest specificity, binding vitamin B12 and its analogs.17 IF, the second transport protein, has the narrowest specificity for vitamin B12 and can be thought of as a gatekeeper for the B12 pathway because it binds only B12.18 The third and final vitamin B12 transport, TC, is the only protein in the pathway that is non-glycosylated and has an intermediate specificity to the vitamin. TC has been found to bind vitamin B12 in a base-on configuration (nucleotide tail complexed to cobalt), and can change conformation to shield the weakly-bound OH- ligand in OH-Cbl.17 Once endocytosed into the cell, vitamin B12 is converted to the biologically relevant Me- or Ado-Cbl forms. Me-Cbl is a cofactor for methionine synthase in the cytoplasm, while Ado-Cbl is used by methyl-malonyl CoA mutase in the mitochondria.19-21 In both cases, the histidine in the Asp-X-His-X-X-Gly motif of the coenzyme displaces the nucleotide tail in a base-off/His-on configuration.19
OH-Cbl has been suggested to associate with proteins other than B12 transport proteins or enzymes.22 Additionally, OH-Cbl is used to treat B12 deficient patients because it has a longer half-life due to its ability to bind serum proteins.23 We show OH-Cbl binds IgG1, IgG2 mAbs, and Fc-fusion proteins and is not localized to mAb F(ab’)2 or Fc regions, suggesting it does not bind a specific motif. The lack of CN-Cbl binding to product also suggests the mechanism of association is different than that used for normal B12 uptake by trafficking proteins (HC, IF, and TC) since in that case both CN-Cbl and OH-Cbl are expected to bind.24 Furthermore, the Fc-fusion and mAbs lack the classic vitamin B12 binding motif (Asp-X-His-X-X-Gly), therefore it is unlikely that the binding mechanism is similar to base-off (released nucleotide tail) vitamin B12 binding exhibited by Me- and Ado-Cbl as enzyme cofactors.20,21
IgG-vitamin B12 complexes have been found in humans, but the techniques used to quantify vitamin B12 in these studies are not sensitive to a specific form.25,26 Considering the variety of Fc-containing molecules tested here and the documented IgG-B12 complex in human subjects, this may suggest OH-Cbl association is universal to IgGs. The increased half-life of OH-Cbl utilized for vitamin B12 deficiency treatment, the observation of IgG-vitamin B12 complexes in serum and the association of OH-Cbl to proteins may all be linked to the unique binding properties of OH-Cbl. It is known that the H2O of aquocobalamin (H2O-Cbl), which is in equilibrium with OH-Cbl at neutral pH, is easily displaced by many ligands, including histidine.10 In the presence of electron-rich amino acids in proteins, OH- may also be easily displaced from OH-Cbl, similar to base-on/His-on binding found in TC.27 The difficulty in removing OH-Cbl during routine downstream processing may then be due to a complex between the cobalt and protein. Additionally, the relative abundance of solvent-exposed, electron-rich amino acids across mAb sequences would explain the seemingly non-specific localization of OH-Cbl on IgG2. Transport proteins have been found to decyanate CN-Cbl during cellular trafficking; however, TC changes conformation when bound to OH-Cbl to protect the weakly bound OH- ligand.17,28 The conformation change of TC upon binding OH-Cbl likely prevents non-specific binding with proteins similar to those seen in this study.
To quantify OH-Cbl in our purified proteins, we developed a rapid and specific technique using KCN to release OH-Cbl from recombinant therapeutic proteins by converting it to CN-Cbl. The released CN-Cbl is easily separated from bulk protein either by protein A chromatography or sized-based sieving. The separation of OH-Cbl from protein has allowed us to use a relatively simple UV-based RP-UPLC technique to measure and monitor OH-Cbl levels in purified protein during process development. Although we have solely focused on recombinant proteins in our study, the release procedure described could easily be adapted to an LC-MS/MS assay to ensure that multi-vitamin measurements in complex mixtures (i.e., serum) accurately quantify total vitamin B12 levels.29
Analysis of OH-Cbl levels in samples from different stages of downstream processing indicates that OH-Cbl is present even in samples that lack pink color. This suggests that the variability of pink color in different development lots is due to varying OH-Cbl levels that occur during processing. Results from multiple recombinant mAbs and Fc-fusion proteins suggest a threshold between 0.3 and 0.5 mg/kg OH-Cbl for visible pink color, and highlights the inherent subjectivity in describing color. For example, mAb3 is alternately described as pink and peach at different stages (Table 2). Protein normalized results from analysis of in-process samples shows that there is never an increase in associated OH-Cbl after protein A purification, strongly suggesting that all OH-Cbl is generated during media compounding, cell culture, harvest, or protein A chromatography loading.
Further analysis of in-process samples demonstrates that polishing chromatography steps may remove some associated OH-Cbl, although in either case there was insufficient clearance to remove the pink color (Table 3). It does suggest the potential for optimizing downstream processes to improve OH-Cbl clearance. If the OH- ligand is displaced by electron-rich elements in the protein and the Cbl-protein complex is not further stabilized by both nucleotide tail interactions and OH-ligand protection as seen in TC-OH-Cbl, it is likely that process conditions can be optimized to discourage prolific OH-Cbl binding. Orthogonal verification of the RP-HPLC quantification with elemental analysis of cobalt ensures that relatively mild conditions are needed to return OH-Cbl to CN-Cbl. CN-Cbl is then readily released from the product and easily separated from protein solutions. Although addition of KCN during manufacturing is not possible due to safety concerns, other compounds can be explored to disassociate OH-Cbl. Simply adding wash steps with electron-rich donors such as histidine or imidazole may be sufficient to clear OH-Cbl during bind and elute chromatography.
In conclusion, the variability of pink color in the proteins discussed here is caused by the conversion of CN-Cbl in cell culture media to OH-Cbl. Since CN-Cbl is the only form of vitamin B12 added to cell culture media, it may be that protection from light during manufacturing would prevent conversion to OH-Cbl and pink coloration of product. It is possible, however, that other conversion pathways exist. OH-Cbl readily associates with both mAb and Fc fusion proteins and binding was not localized in any specific portion of the molecule. These studies suggest that routine downstream processing may not provide sufficient clearance to eliminate the risk of color variability. As the source of OH-Cbl is introduced prior to downstream processing, and begins as CN-Cbl, the level of vitamin B12 required in the media could be evaluated. Ultimately, a combined effort of upstream and downstream process development will be necessary to completely eliminate the risk of pink color variability.
Materials and Methods
Protein stocks
The mAbs and Fc-fusion protein used in these studies were produced in CHO cells and captured using protein A chromatography. Additional purification was performed using multiple polishing chromatography steps to a high purity. Protein types used in this study include Fc-fusion, IgG2 (mAb 1, mAb 3), and IgG1 (mAb 2) subclasses.
Release of pink color from protein
Samples were incubated in the presence of 0.01% (w/v) KCN (Ricca Chemical Co, 60-10-16) at 37°C for 30 min. To easily visualize color changes, excess KCN and unbound material was removed by filtering the solution and washing three times with phosphate buffered saline (PBS) through a 50 kD filter (Millipore, UFC505096). The retentate was transferred to a 3 cc glass vial and visually examined for color.
Association of Vitamin B12 with protein
Binding of B12 forms (Sigma-Aldrich, OH-Cbl: H7126; CN-Cbl: C3607; Me-Cbl: M9756; Ado-Cbl: C0884) to recombinant protein was evaluated by incubating 5 mg/mL protein with 1 mg/mL of each form. Samples were protected from light, and held at room temperature overnight. After incubation, excess B12 was removed as above and examined for color.
RP-UPLC separation of B12 forms with MS/MS identification
Vitamin B12 standards were separated by RP-UPLC with UV detection at 360 nm (Waters Acuity UPLC, Waters) on a Waters HSS T3 C18 2.1 × 150 mm, 1.8 µm particle column (Waters Corp) An in-line Thermo LTQ XL (Thermo Scientific) ion-trap mass spectrometer was used to positively identify the B12 forms.
Quantification of OH-Cbl associated with protein
Protein-associated OH-Cbl was quantified, by incubating samples with 0.01% (w/v) KCN (37°C, 30 min) to convert protein-bound OH-Cbl to free CN-Cbl. To simplify the solution for quantification, the now free CN-Cbl was separated from product by using small amounts of protein A chromatography resin in batch uptake mode automated on a Tecan Freedom Evo robotic liquid handling system (Tecan US). Protein A flow through was analyzed by RP-UPLC. Released CN-Cbl was quantified against a standard curve ranging from 0.03–10 mg/kg and recovery was confirmed through spiking. Vitamin B12 results are reported both in concentration (mg/kg) and protein normalized (ng B12/mg protein).
Elemental analysis of samples for cobalt
ICP-MS was utilized to analyze elemental cobalt concentrations in protein solutions. An Elan DRC II ICP-MS (Perkin Elmer) was set to measure cobalt (Co) at 59 m/z and internal standard cadmium (Cd) at 112 m/z. Co standard curve ranging between 0.2‒200 ng/mL was run with Cd at 50 ng/mL. Each sample was diluted 30-fold with 0.5% nitric acid, spiked with 50 ppb Cd, and analyzed. The acquired response against the standard curve gave the observed Co concentration. Observed Co concentration was converted to CN-Cbl levels by ratio conversion of Co:CN-Cbl molar masses.
Cell culture media photo-stability study
A CHO cell culture medium containing CN-Cbl was freshly prepared and sterile filtered. Medium samples were held for 10 d at 37 °C, either exposed to or protected from light. Time points were analyzed on different days by RP-UPLC for CN-Cbl. Loss of CN-Cbl by conversion to OH-Cbl was confirmed by incubation of light exposed medium with 0.01% KCN (37 °C, 30 min) and RP-UPLC analysis.
Localization of hydroxocobalamin binding to a monoclonal antibody
A colorless IgG2 recombinant antibody (5 mg/mL in 10 mM acetate pH 5.2) was incubated (RT, 17 h) in the dark with 0.05 mg/mL OH-Cbl, then buffer exchanged to remove unassociated vitamin. The pink-colored antibody was digested using fabRICATOR™ enzyme (IdeS; Genovis AB, A0-FR1–020) into F(ab’)2 and Fc fragments. The digest was separated by non-denaturing SEC using a Tosoh TSKgel G3000swxl 4.6 × 300 mm column (Tosoh Bioscience LLC, Japan). Detection of antibody fragments was performed at 280 nm for protein quantification and 360 nm for B12 quantification.
Acknowledgments
The authors thank Amy Guo, Steve Trimble, and Arvia Morris for discussion and input, and Alison Wallace, Ganesh Vedantham, and Randal Bass for their guidance and support during this research.
Glossary
Abbreviations:
- mAb
monoclonal antibody
- OH-Cbl
Hydroxocobalamin
- CN-Cbl
cyanocobalamin
- Me-Cbl
methylcobalamin
- Ado-Cbl
adenosylcobalamin
- H2O-Cbl
aquocobalamin
- CHO
Chinese hamster ovary
- IgG
immunoglobulin
- PBS
phosphate buffered saline
- RP-UPLC
reversed phase ultra performance liquid chromatography
- SEC
size exclusion chromatography
- UV
ultraviolet
- MS
mass spectrometer
- ICP-MS
inductively coupled plasma mass spectrometry
- LOQ
limit of quantification
- DS
drug substance
- HC
Haptocorrin
- IF
intrinsic factor
- TC
transcobalamin II
Submitted
06/18/2013
Revised
07/24/2013
Accepted
07/26/2013
Disclosure of Potential Conflicts of Interest
No potential conflict of interest was disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/mabs/article/25921
References
- 1.ICH. Q6B Specifications: Test Procedures and Acceptance Criteria for Biotechnological/Biological Products. 1999.
- 2.Galeazzi L, Groppa G, Giunta S. Mueller-Hinton broth undergoes visible oxidative color changes in the presence of peroxidase and hydrogen peroxide. J Clin Microbiol. 1990;28:2145–7. doi: 10.1128/jcm.28.9.2145-2147.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vijayasankaran N, Varma S, Yang Y, Mun M, Arevalo S, Gawlitzek M, et al. Effect of cell culture medium components on color of formulated monoclonal antibody drug substance. Biotechnol Prog. 2013 doi: 10.1002/btpr.1772. [DOI] [PubMed] [Google Scholar]
- 4.Liu HF, Ma J, Winter C, Bayer R. Recovery and purification process development for monoclonal antibody production. MAbs. 2010;2:480–99. doi: 10.4161/mabs.2.5.12645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shukla AA, Hinckley P. Host cell protein clearance during protein A chromatography: development of an improved column wash step. Biotechnol Prog. 2008;24:1115–21. doi: 10.1002/btpr.50. [DOI] [PubMed] [Google Scholar]
- 6.Farhangi M, Osserman EF. Myeloma with xanthoderma due to an IgG lambdamonoclonal anti-flavin antibody. N Engl J Med. 1976;294:177–83. doi: 10.1056/NEJM197601222940401. [DOI] [PubMed] [Google Scholar]
- 7.Zhu X, Wentworth P, Jr., Kyle RA, Lerner RA, Wilson IA. Cofactor-containing antibodies: crystal structure of the original yellow antibody. Proc Natl Acad Sci U S A. 2006;103:3581–5. doi: 10.1073/pnas.0600251103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.CRC handbook of chemistry and physics. Boca Raton, FL: Chapman and Hall/CRCnetBASE,, 1999:CD-ROMs.
- 9.Markle HV. Cobalamin. Crit Rev Clin Lab Sci. 1996;33:247–356. doi: 10.3109/10408369609081009. [DOI] [PubMed] [Google Scholar]
- 10.Randaccio L, Geremia S, Demitri N, Wuerges J. Vitamin B12: unique metalorganic compounds and the most complex vitamins. Molecules. 2010;15:3228–59. doi: 10.3390/molecules15053228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dilley KJ. Loss of tryptophan associated with photo-polymerization and yellowing of proteins exposed to light over 300nm. Biochem J. 1973;133:821–6. doi: 10.1042/bj1330821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kumar SS, Chouhan RS, Thakur MS. Trends in analysis of vitamin B12. Anal Biochem. 2010;398:139–49. doi: 10.1016/j.ab.2009.06.041. [DOI] [PubMed] [Google Scholar]
- 13.Drennan CL, Huang S, Drummond JT, Matthews RG, Lidwig ML. How a protein binds B12: A 3.0 A X-ray structure of B12-binding domains of methionine synthase. Science. 1994;266:1669–74. doi: 10.1126/science.7992050. [DOI] [PubMed] [Google Scholar]
- 14.Farquharson J, Adams JF. Conversion of hydroxo(aquo) cobalamin to sulfitocobalamin in the absence of light: a reaction of importance in the identification of the forms of vitamin B12, with possible clinical significance. Am J Clin Nutr. 1977;30:1617–22. doi: 10.1093/ajcn/30.10.1617. [DOI] [PubMed] [Google Scholar]
- 15.Ahmad I, Hussain W, Fareedi AA. Photolysis of cyanocobalamin in aqueous solution. J Pharm Biomed Anal. 1992;10:9–15. doi: 10.1016/0731-7085(92)80004-7. [DOI] [PubMed] [Google Scholar]
- 16.Abu-Soud HM, Maitra D, Byun J, Souza CE, Banerjee J, Saed GM, et al. The reaction of HOCl and cyanocobalamin: corrin destruction and the liberation of cyanogen chloride. Free Radic Biol Med. 2012;52:616–25. doi: 10.1016/j.freeradbiomed.2011.10.496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fedosov SN, Fedosova NU, Nexø E, Petersen TE. Conformational changes of transcobalamin induced by aquocobalamin binding. Mechanism of substitution of the cobalt-coordinated group in the bound ligand. J Biol Chem. 2000;275:11791–8. doi: 10.1074/jbc.275.16.11791. [DOI] [PubMed] [Google Scholar]
- 18.Kolhouse JF, Allen RH. Absorption, plasma transport, and cellular retention of cobalamin analogues in the rabbit. Evidence for the existence of multiple mechanisms that prevent the absorption and tissue dissemination of naturally occurring cobalamin analogues. J Clin Invest. 1977;60:1381–92. doi: 10.1172/JCI108899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Banerjee R, Gherasim C, Padovani D. The tinker, tailor, soldier in intracellular B12 trafficking. Curr Opin Chem Biol. 2009;13:484–91. doi: 10.1016/j.cbpa.2009.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Banerjee RV, Matthews RG. Cobalamin-dependent methionine synthase. FASEB J. 1990;4:1450–9. doi: 10.1096/fasebj.4.5.2407589. [DOI] [PubMed] [Google Scholar]
- 21.Padovani D, Labunska T, Palfey BA, Ballou DP, Banerjee R. Adenosyltransferase tailors and delivers coenzyme B12. Nat Chem Biol. 2008;4:194–6. doi: 10.1038/nchembio.67. [DOI] [PubMed] [Google Scholar]
- 22.Lien EL, Wood JM. The specificity of aquocobalamin-binding to bovine serum albumin. Biochim Biophys Acta. 1972;264:530–7. doi: 10.1016/0304-4165(72)90016-5. [DOI] [PubMed] [Google Scholar]
- 23.Hall CA, Begley JA, Green-Colligan PD. The availability of therapeutic hydroxocobalamin to cells. Blood. 1984;63:335–41. [PubMed] [Google Scholar]
- 24.Banerjee R. B12 trafficking in mammals: A for coenzyme escort service. ACS Chem Biol. 2006;1:149–59. doi: 10.1021/cb6001174. [DOI] [PubMed] [Google Scholar]
- 25.Jeffery J, Millar H, Mackenzie P, Fahie-Wilson M, Hamilton M, Ayling RM. An IgG complexed form of vitamin B12 is a common cause of elevated serum concentrations. Clin Biochem. 2010;43:82–8. doi: 10.1016/j.clinbiochem.2009.08.022. [DOI] [PubMed] [Google Scholar]
- 26.Bowen RA, Drake SK, Vanjani R, Huey ED, Grafman J, Horne MK., 3rd Markedly increased vitamin B12 concentrations attributable to IgG-IgM-vitamin B12 immune complexes. Clin Chem. 2006;52:2107–14. doi: 10.1373/clinchem.2006.073882. [DOI] [PubMed] [Google Scholar]
- 27.Wuerges J, Garau G, Geremia S, Fedosov SN, Petersen TE, Randaccio L. Structural basis for mammalian vitamin B12 transport by transcobalamin. Proc Natl Acad Sci U S A. 2006;103:4386–91. doi: 10.1073/pnas.0509099103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim J, Gherasim C, Banerjee R. Decyanation of vitamin B12 by a trafficking chaperone. Proc Natl Acad Sci U S A. 2008;105:14551–4. doi: 10.1073/pnas.0805989105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lu B, Ren Y, Huang B, Liao W, Cai Z, Tie X. Simultaneous determination of four water-soluble vitamins in fortified infant foods by ultra-performance liquid chromatography coupled with triple quadrupole mass spectrometry. J Chromatogr Sci. 2008;46:225–32. doi: 10.1093/chromsci/46.3.225. [DOI] [PubMed] [Google Scholar]


