Abstract
Accumulation of hepatic lipid droplet (HLD) is the hallmark pathology of non-alcoholic fatty liver disease (NAFLD). This study examined the effects of soy isoflavones (ISF) and different amounts of soy proteins on the accumulation of HLD, lipid metabolism and related gene expression in rats. Weanling Sprague–Dawley rats were fed diets containing either 20 % casein protein without (D1) or with (D2) supplemental ISF (50 mg/kg diet) or substitution of casein with increasing amounts of alcohol-washed soy protein isolate (SPI, 5, 10, and 20 %; D3, D4, D5) for 90 days. Dietary casein (20 %) induced accumulation of HLD in female, but not in male rats. Both soy proteins and ISF remarkably prevented the formation of HLD. Soy proteins lowered hepatic total cholesterol and triglyceride in a dose-dependent manner. Interestingly, soy proteins but not ISF significantly increased free fatty acids in the liver of the female rats compared to D1. Proteomic analysis showed that at least 3 enzymes involved in lipogenesis were down-regulated and 7 proteins related to fatty acid β-oxidation or lipolysis were up-regulated by soy protein over D1. Additionally, 9 differentially expressed proteins identified were related to amino acid metabolism, 5 to glycolysis and 2 to cholesterol metabolism. Dietary ISF and SPI markedly reduced hepatic-peroxisome-proliferator-activated receptor γ2 (PPARγ2) and fat-specific protein 27 (FSP27) in female rats. Overall, this study has shown that partial or full replacement of dietary casein by soy protein or supplementation with soy ISF can effectively prevent the accumulation of HLD. The potential molecular mechanism(s) involved might be due to suppression of lipogenesis and stimulation of lipolysis and down-regulation of PPARγ2 and FSP27. This suggests that consumption of soy foods or supplements might be a useful strategy for the prevention or treatment of fatty liver diseases.
Electronic supplementary material
The online version of this article (doi:10.1007/s12263-013-0373-3) contains supplementary material, which is available to authorized users.
Keywords: Soy proteins, Isoflavones, Fatty liver, Lipid metabolism, Gene expression
Introduction
Fatty liver is the most common chronic liver disease in the Western world and has a prevalence of up to 34 % in the USA. This rate increases to over 50 % in the obese population and 70 % in type II diabetic patients (Browning et al. 2004; Ong and Younossi 2007). Accumulation of triglyceride (TG) in hepatocytes and formation of excessive hepatic lipid droplets (HLD) are typical features of non-alcoholic fatty liver disease (NAFLD) (Angulo 2002). NAFLD involves a variety of histological changes including simple steatosis progressing to steatohepatitis, fibrosis and cirrhosis (Hakkak et al. 2012).
Soy consumption has been shown to improve blood lipid profiles, reduce hepatic lipid accumulation, suppress gene expression for lipogenic enzymes and up-regulate gene expression for lipid degradation (Badger et al. 2008; Frigolet et al. 2011) (Kitawaki et al. 2009) (Torre-Villalvazo et al. 2008). Feeding soy diets attenuated the formation and accumulation of liver lipid droplets and reduced TG content in both obese (Tovar et al. 2005; Gudbrandsen et al. 2006; Davis et al. 2007) and non-obese rats (Simmen et al. 2010). However, the molecular events involved in the hypolipidemic effects and NAFLD prevention of soy are different in obese and non-obese animals. In non-obese rats, soy proteins modulated the lipid metabolism through suppression of the expression of hepatic lipogenic genes such as sterol regulatory element-binding protein-1, malic enzyme and fatty acid synthase and increased 3-hydroxy-3-methyl-glutaryl-CoA reductase, 3-hydroxy-3-methyl-glutaryl-CoA synthase and low density lipoprotein receptor (Tovar et al. 2002). Conversely, in the obese rats, soy proteins (20 %) increased the hepatic lipogenesis, which might be attributed to increased serum insulin levels (Hakkak et al. 2012). Attenuation of the hepatic fat accumulation and liver damage as well as hepatocellular vacuolation by soy proteins was shown to be through restoration of the β-catenin signaling which is suppressed in obese rats compared to their lean mates (Zhou et al. 2013).
Soy contains two major storage proteins, β-conglycinin and glycinin which account for about 90 % of total protein (Torres et al. 2006). The protein-associated isoflavones are the major soy phytoestrogens, and mainly consist of genistein, daidzein and glycitein (Anthony et al. 1996; Miniello et al. 2003). Although many studies on the potential physiological functions of soy proteins and isoflavones have been conducted, the components involved in the hypolipidemic functions of soy remain controversial. For example, soy protein significantly lowered serum TG and cholesterol levels and altered the expression of genes involved in fatty acid and/or steroid synthesis in the liver of the rats compared to casein. Isoflavone supplementation had little effect on these parameters. This led to the conclusion that soy proteins, but not isoflavones, reduced liver lipogenesis (Takahashi and Konishi 2011). Moreover, prevention of fat droplet formation and accumulation in the liver of non-obese rats fed soy diets was independent of genistein (Simmen et al. 2010). On the other hand, isoflavones have been shown to regulate hepatic lipogenesis, insulin resistance or adiposity as well as adipocytokines involved in hepatic steatosis (Kim and Kang 2012). Daidzein, one of the major soy isoflavones, down-regulated carbohydrate-responsive element-binding protein, liver X receptor β and its target genes for lipogenic enzymes in the mice (Kim et al. 2011) (Crespillo et al. 2011). Genistein remarkably ameliorated development of NAFLD in insulin-resistant rats (Mohamed et al. 2009) and high-fat-fed mice (Kim et al. 2010).
The molecular event(s) of soy proteins and isoflavones involved in the modulation of HLD accumulation and prevention of NAFLD are still not fully understood. Particularly, the amount of soy intake needed to be effective has not been established. In most of the previous studies published, soy proteins were used to completely replace animal proteins (mainly casein). However, this appears to be unlikely in human consumption except in the infants fed soy-based formulas. This raises the question as to how much soy proteins or isoflavones should be consumed to have beneficial effects such as prevention of NAFLD. The objective of this study was to determine the effect of feeding soy-derived isoflavones or various amounts of soy proteins on blood lipid levels and HLD formation as well as hepatic gene expression in rats.
Materials and methods
Chemicals and reagents
Alcohol-washed soy protein isolate (SPI, Pro Fam 930) and Novasoy soy isoflavone concentrate were purchased from Archer Daniels Midland Company (Decatur, IL). Casein protein (90 % purity) was from Harlan Laboratories (Madison, WI). Tris, glycine and phenylmethylsulfonyl fluoride (PMSF) were from Sigma Chemical Co. (St. Louis, MO). ECL Western blotting detection kits and CL-X PosureTM Film were obtained from Thermo Scientific (Rockford, IL). Nitrocellulose membranes, Criterion precast gels, goat anti-rabbit IgG (H + L)-horseradish peroxidase (HRP)-conjugated antibody, goat anti-mouse IgG (H + L)-HRP-conjugated antibody and Bio-Rad protein assay kits were purchased from Bio-Rad Laboratories (Hercules, CA). Rabbit polyclonal antibodies against fat-specific protein 27 (FSP27) and peroxisome-proliferator-activated receptor (PPAR) γ2, mouse monoclonal antibodies against FSP27 and β-tubulin were from Abcam Inc (Cambridge, MA). Rabbit polyclonal antibody against patatin-like phospholipase domain containing 3 (PNPLA3) was purchased from LifeSpan BioSciences, Inc. (Seattle, WA). Reagents for cholesterol, triglyceride and non-esterified fatty acid (NEFA) were from Wako Chemicals (Richmond, VA).
Animals and diets
Animal experimental protocols were approved by the Health Canada-Ottawa Animal Care Committee, and all animal handling and care followed the guidelines of the Canadian Council for Animal Care. Weanling Sprague–Dawley rats were randomly divided into 5 groups (8 females and 8 males/group) and fed diets containing either 20 % casein without (D1) or with (D2) supplemental ISF (50 mg/kg diet) to provide the same amount of ISF as contained in 20 % SPI diet, or increasing amounts (5, 10, or 20 %; D3, D4, D5) of alcohol-washed SPI for 90 days. All diets were formulated according to the specifications for the AIN93G diet (Reeves et al. 1993) except that L-cystine was replaced by l-methionine, and in D3-5, casein by equal amounts of alcohol-washed SPI (5, 10, and 20 %) (Table 1). At the end of the feeding, all rats were necropsied for collection of blood and tissue samples. One portion of the liver from the same lobe of each rat was fixed in 4 % formaldehyde for immunohistological analysis.
Table 1.
Composition of experimental diets
| Ingredient (g/kg diet) | D1 | D2 | D3 | D4 | D5 |
|---|---|---|---|---|---|
| Casein1 | 222.2 | 222.2 | 166.5 | 111.1 | – |
| Soy protein2 | – | – | 55.6 | 111.1 | 222.2 |
| Sucrose | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 |
| Cornstarch | 375.3 | 375.1 | 375.3 | 375.3 | 375.3 |
| Dextronized cornstarch | 132.0 | 132.0 | 132.0 | 132.0 | 132.0 |
| Soybean oil | 70.0 | 70.0 | 70.0 | 70.0 | 70.0 |
| Cellulose | 50.0 | 50.0 | 50.0 | 50.0 | 50.0 |
| Mineral mix3 | 35.0 | 35.0 | 35.0 | 35.0 | 35.0 |
| Vitamin mix3 | 10.0 | 10.0 | 10.0 | 10.0 | 10.0 |
| Choline Bitartrate | 2.5 | 2.5 | 2.5 | 2.52.5 | |
| l-Methionine | 3.0 | 3.0 | 3.0 | 3.0 | 3.0 |
| Tert-Butylhydroquinone | 0.014 | 0.014 | 0.014 | 0.014 | 0.014 |
| Novasoy2 | – | 0.167 | – | – | – |
| Actual content of isoflavones4 (mg/kg diet) | 0.0 | 50.1 | 12.7 | 25.4 | 50.1 |
1Casein from Harlan Laboratories (Madison, WI) contains 90 % crude protein
2Alcohol-washed soy protein isolate containing 90 % crude protein and Novasoy isoflavone concentrate containing 30 % total isoflavones were purchased from Archer Daniels Midland Company (Decatur, IL)
3AIN-93G Mineral mix (Reeves et al. 1993) and AIN-93G Vitamin mix (Reeves et al. 1993) were from Harlan Laboratories
4The actual content of isoflavones was determined by Waters HPLC linear gradient with UV detection monitored at 254 nm (Wang and Murphy 1994)
Measurement of liver and serum lipids
Total lipids were extracted from liver samples using chloroform–methanol method (FOLCH et al. 1957). Briefly, homogenized tissues were mixed with chloroform–methanol mixture (2:1) in a final dilution of 20-fold the volume of the tissue sample. The crude extract was collected after centrifugation. After rinsing and washing, the lipid phase was evaporated to dryness under nitrogen. Lipids were resuspended in isopropanol and 10 % Triton 100 by sonication and stored at −84 °C until further analysis. Total cholesterol (TC), TG and free fatty acid (FFA) levels in liver extracts, total, free, HDL and LDL cholesterol, and TG in serum were measured by a microplate enzymatic method (Wako Chemicals).
Measurement of HLD and immunohistological analysis of FSP27
The fixed liver tissues were embedded and sectioned. For the assessment of HLD formation and accumulation, sections were stained with hematoxylin and eosin. The circumference of 100 randomly selected fat droplets in five fields of each section at 20× was measured under microscope using the software Northern eclipse version 7.0 (Empix Imaging, Inc., Mississauga, ON), and the total HLD areas were calculated. Liver sections were immunostained with mouse anti-FSP27 monoclonal antibody (1:100).
Total protein extraction and Western blotting
For the total protein extraction, rat liver samples were homogenized in lysis buffer using an Ultra Turrax T8 homogenizer (VWR). The samples were centrifuged (15,000×g, 20 min), and the supernatant was retained. Protein content of the extracts was determined with the Bio-Rad DC Protein Assay Reagent. Total protein samples (120 μg) were mixed with loading buffer, resolved by 8–16 % gradient SDS-PAGE and electrotransferred onto nitrocellulose membranes. After blocking for 1 h with nonfat milk powder (5 %) in Tris-buffered saline (10 mmol/L Tris, 150 mmol/L NaCl; TBS) and Tween-20 (0.05 %; TBS-T), membranes were incubated overnight with FSP27, PPARγ2 or PNPLA3 antibodies in TBS-T containing nonfat milk powder, and subsequently with HRP-conjugated secondary antibody (1:5,000) in TBS-T with milk powder at room temperature for 45 min. Immunoreactivity was detected by chemiluminescence autoradiography in accordance with the manufacturer’s instructions, and the images were scanned. All membranes were stripped and reprobed with β-tubulin antibody (1:5000). The intensities of protein bands of interest and the β-tubulin band were determined densitometrically using Scion Image (Scion Corporation, Frederick, MA). The intensities of the target proteins were normalized to the respective β-tubulin intensity.
Proteomic analysis of liver proteins
Liver tissue samples (3/group) randomly chosen from the rats fed diets containing either 20 % casein (D1) or 20 % SPI (D5) were sonicated in 2-D cell lysis buffer, and then centrifuged. The supernatant was collected and measured for protein concentration using the Bradford assay (Bio-Rad). An equal amount of protein from each sample was mixed as the internal standard and labeled with diluted Cyanine (Cy) 2. For each pair, 30 μg of protein was mixed with 1.0 μl of diluted Cy3 (casein) and Cy5 (soy), respectively, and kept in the dark on ice for 30 min. Each Cy-dye-labeled soy and casein pair was mixed together, and added with an equal volume of labeled internal standard, and loaded to pH 3–10 linear IPG strips (GE Healthcare). The IEF was run for 12-h rehydration at 20 °C, followed by 500 V for 1,000 VHr, 1,000 V for 2,000 VHr and 8,000 V for 24,000 VHr. Upon completion of the IEF, the IPG strips were transferred into 12 % SDS-PAGE gels and run at 15 °C.
Gel images were scanned immediately using Typhoon TRIO (Amersham BioSciences) and analyzed by Image Quant software (version 6.0, Amersham BioSciences). Biological Variation Analysis was conducted using DeCyder 2D software Version 6.5 (Amersham BioSciences). Protein expression fold changes and p-values were obtained from DeCyder analysis. The spots of interest were picked up by Ettan Spot Picker (Amersham BioSciences) based on the inter-gel analysis and spot picking designed by DeCyder software. Protein in-gel digestion was performed and the peptides resulting from the digestion were further cleaned up using C18 packed tips before injection onto the nano LC/MS/MS system. Data acquisition was performed using the Water’s Masslynx software in data-directed analysis mode to obtain a list of MS and their corresponding MS/MS spectra. The data were analyzed using a bioinformatic software package: Mascot (from Matrix Science) and Proteinlynx global server (PLGS from Water’s) for the characterization and identification of peptides and proteins.
Statistical analysis
One-way or two-way ANOVA were used to determine the significance of overall effect. Differences between individual group means were determined by Fisher’s least significant difference test. A probability of p < 0.05 was considered to be significant. Data are presented as the mean ± SEM. Means with different letters differ, p < 0.05.
Results
Food consumption, liver and body weights
Food consumption and body weights did not differ among the dietary groups in both male and female rats (p > 0.05, data not shown). The relative liver weights (% body weight) were not different among the dietary groups except that the male rats fed 10 % (2.49 ± 0.05) (D4) or 20 % SPI (2.48 ± 0.06) (D5) had significantly lower relative liver weights compared to those fed Casein + ISF (2.74 ± 0.09, p < 0.05) (D2).
Hepatic lipid droplets
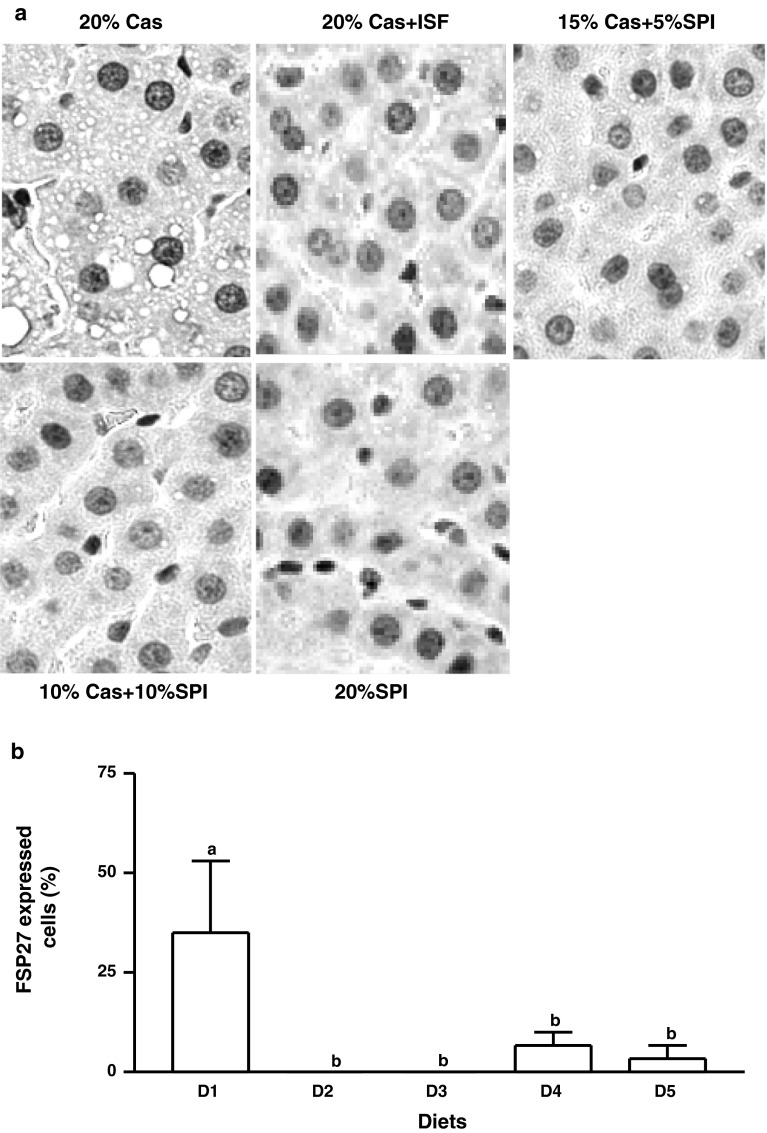
The size and number of hepatic lipid droplets in the livers of the female rats fed D1 (Fig. 1a) were remarkably larger than those of the male rats fed the same diet (image not shown) and were significantly reduced by addition of supplemental isoflavones (D2, Fig. 1b). Partial (Fig. 1c, d) or full (Fig. 1e) replacement of casein by increasing amounts of soy proteins completely prevented the formation of lipid droplets (p < 0.01). The total areas of lipid droplets randomly measured in each liver section of the rats fed isoflavones or soy proteins were remarkably smaller than those of the rats fed D1 (p < 0.001, Fig. 1f).
Fig. 1.
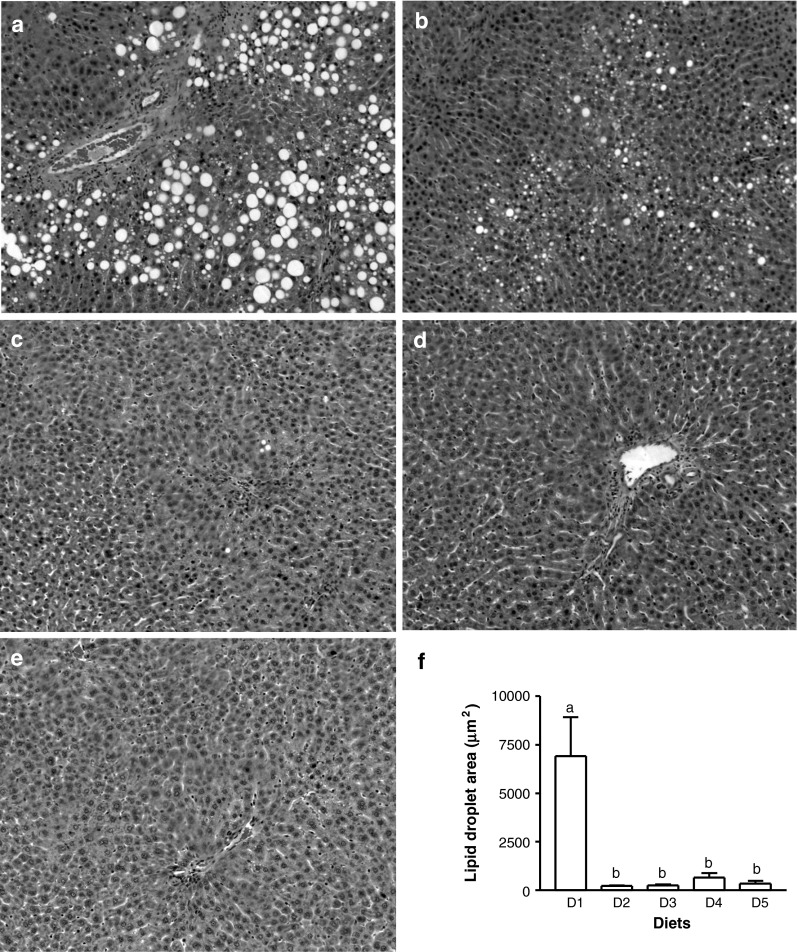
Liver histology of the female rats fed diets containing 20 % casein in the absence (a) or presence (b) of supplemental ISF (50 mg/kg diet) or increasing amounts of alcohol-washed SPI, 5 % (c), 10 % (d) or 20 % (e) for 90 days. Hematoxylin and eosin staining shows accumulation of intracellular lipid droplets, original magnification ×100 and the total areas of hepatic lipid droplets (f) were measured. The images shown are representatives of 4 replicates of each
Liver total cholesterol (TC), triglycerides (TG) and free fatty acid (FFA) contents
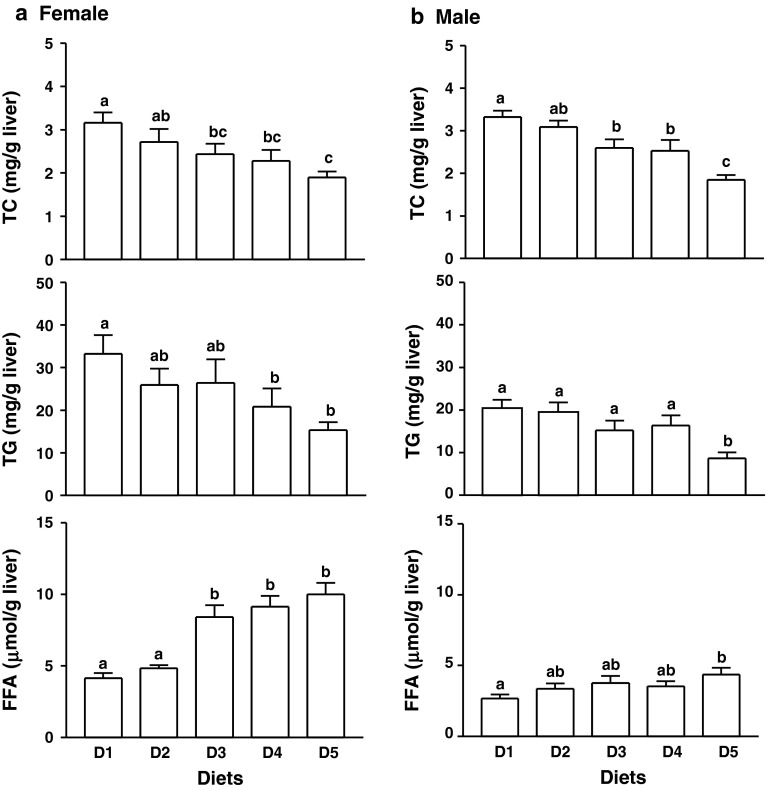
Hepatic total cholesterol contents were not different in the rats fed casein with (D2) or without (D1) supplemental isoflavones (p > 0.05), however, were remarkably lowered by increasing amounts of alcohol-washed soy proteins (p < 0.05, Fig. 2). Hepatic TG and FFA contents in the female rats fed D1 were markedly higher than those of the male rats fed the same diet (p < 0.01). Intake of higher amounts of SPI (10 and 20 % in females, and 20 % in males) significantly reduced liver TG compared to D1. Interestingly, the hepatic FFA contents in the female rats fed all SPI diets (D3-5) and males fed 20 % SPI (D5) were markedly higher than in those fed D1 (p < 0.05, Fig. 2).
Fig. 2.
Hepatic total cholesterol (TC), triglyceride (TG) and free fatty acid (FFA) contents in the female (a) and male (b) rats fed diets containing 20 % casein in the absence (D1) or presence (D2) of supplemental ISF (50 mg/kg diet) or increasing amounts of alcohol-washed SPI (D3: 5 %, D4: 10 %, or D5: 20 %) for 90 days. Values are mean ± SEM, n = 8. Means with different letter differ, p < 0.05
Serum lipid profiles
Levels of serum lipids including free, total, HDL and LDL cholesterol, and TG were unchanged among dietary groups except that free, total, HDL and LDL cholesterol, and TG were significantly lower in the male rats fed diet containing 10 % Casein + 10 % SPI (D4) than in those fed D1 (Table 2).
Table 2.
Serum lipid content in rats fed diets containing either casein or increasing amounts of alcohol-washed SPI
| mg/ml | |||||
|---|---|---|---|---|---|
| D11 | D21 | D31 | D41 | D51 | |
| Male | |||||
| Cholesterol | |||||
| Total | 1.05 ± 0.10a | 0.95 ± 0.08a | 0.90 ± 0.09ab | 0.74 ± 0.06b | 0.94 ± 0.10a |
| Free | 0.23 ± 0.02a | 0.21 ± 0.02ab | 0.20 ± 0.02ab | 0.17 ± 0.01b | 0.24 ± 0.03a |
| LDL2 | 0.78 ± 0.08a | 0.70 ± 0.06ab | 0.68 ± 0.07ab | 0.57 ± 0.04b | 0.69 ± 0.06ab |
| HDL2 | 0.57 ± 0.05a | 0.51 ± 0.04ab | 0.49 ± 0.04ab | 0.43 ± 0.03b | 0.55 ± 0.05a |
| Triglyceride | 0.68 ± 0.11 | 0.85 ± 0.17 | 0.98 ± 0.22 | 0.67 ± 0.12 | 0.90 ± 0.16 |
| FFA2 (mmol/L) | 0.61 ± 0.02 | 0.58 ± 0.03 | 0.59 ± 0.06 | 0.49 ± 0.04 | 0.54 ± 0.03 |
| Female | |||||
| Cholesterol | |||||
| Total | 0.92 ± 0.07 | 0.89 ± 0.07 | 0.93 ± 0.04 | 0.93 ± 0.10 | 0.91 ± 0.07 |
| Free | 0.24 ± 0.02 | 0.24 ± 0.02 | 0.25 ± 0.02 | 0.24 ± 0.03 | 0.23 ± 0.01 |
| LDL | 0.64 ± 0.05 | 0.61 ± 0.05 | 0.67 ± 0.04 | 0.71 ± 0.07 | 0.69 ± 0.05 |
| HDL | 0.53 ± 0.04 | 0.51 ± 0.03 | 0.50 ± 0.02 | 0.56 ± 0.06 | 0.54 ± 0.03 |
| Triglyceride | 0.58 ± 0.16 | 0.48 ± 0.10 | 0.42 ± 0.06 | 0.59 ± 0.09 | 0.41 ± 0.11 |
| FFA (mmol/L) | 0.73 ± 0.07a | 0.60 ± 0.04b | 0.66 ± 0.06ab | 0.62 ± 0.04ab | 0.61 ± 0.02b |
Values are means ± SEM, n = 8. Means in the same row with different superscripts differ, p < 0.05
1D1: 20 % casein; D2: 20 % casein + 50 mg isoflavones/kg diet; D3: 15 % casein + 5 % SPI; D4: 10 % casein + 10 % SPI; D5: 20 % SPI
2 LDL low density lipoprotein, HDL high density lipoprotein, FFA free fatty acids
Hepatic proteins differentially expressed or modified by dietary proteins in female rats
Fifty-eight protein spots were detected to be different between the rats fed 20 % casein (D1) and 20 % SPI (D5) (Suppl. Fig. 1) at a cut off of p < 0.10 and were picked up for identification. They were identified to be 35 proteins. Among them, seven proteins involved in either FA β-oxidation (peroxisomal acyl-CoA oxidase 1, long-chain enoyl-CoA hydratase and peroxisomal multifunctional enzyme type 2) or lipolysis (liver carboxyl- esterase 3, carboxyl-esterase, acyl-CoA synthetase and ATP synthase γ) were up-regulated and 3 involved in lipogenesis (fatty acid synthase, cytoplasmic aconitate hydratase and glucose-6-phosphate dehydrogenase) were down-regulated by soy proteins; Other differentially expressed or modified proteins identified were 5 involved in glycolysis, 2 related to cholesterol metabolism, 9 to amino acid metabolism, 4 oxidative stress and 3 to immune responses (Table 3).
Table 3.
Differentially expressed liver proteins in the female rats fed diets containing either 20 % casein or SPI
| Protein | Ratio ofSPI/Casein | p value |
|---|---|---|
| FA β-oxidation | ||
| Peroxisomal acyl-coenzyme A oxidase 1 | 1.34 | 0.002 |
| Long-chain enoyl-CoA hydratase/3-hydroxycyl-CoA dehydrogenase α | 1.42 | 0.002 |
| Peroxisomal multifunctional enzyme type 2 | 1.89 | 0.026 |
| Lipolysis | ||
| Liver carboxylesterase 3 | 1.49 | 0.037 |
| Carboxylesterase | 1.86 | 0.058 |
| Acyl-CoA synthetase | 2.11 | 0.070 |
| ATP synthase γ | 1.56 | 0.015 |
| FA synthesis | ||
| Fatty acid synthase | −1.63 | 0.019 |
| Cytoplasmic aconitate hydratase | −1.87 | 0.094 |
| Glucose-6-phosphate dehydrogenase | −2.01 | 0.091 |
| Glycolysis | ||
| Glyceraldehyde 3-phosphate-dehydrogenase | 1.84 | 0.003 |
| Cytochrome b-c1 complex subunit 2 | 1.33 | 0.034 |
| Fructose-biphosphatase | 1.60 | 0.097 |
| Biliverdin reductase A precursor | −1.38 | 0.06 |
| Pyruvate dehydrogenase E1 α1 | −1.36 | 0.00091 |
| Cholesterol metabolism | ||
| Non-specific lipid-transfer protein | 2.45 | 0.0027 |
| SEC14-like protein 2 | 1.46 | 0.00027 |
| Amino acid metabolism | ||
| Sarcosine dehydrogenase | −2.62 | 0.0063 |
| Carbamoyl-phosphate synthase | −1.53 | 0.0180 |
| Ω-amidase NIT2 | 1.31 | 0.0072 |
| S-adenosylmethionine synthase isoform type-1 | 1.35 | 0.0017 |
| Cystathionine γ-lyase | 1.77 | 0.0360 |
| Alanine–glyoxylate aminotransferase 2 | 2.04 | 0.0013 |
| Dihydrodipicolinate synthase-like | 2.14 | 0.0055 |
| 4-trimethylaminobutyraldehyde dehydrogenase | 2.19 | 3.9E − 05 |
| Kynurenine aminotransferase III, isoform CRA_a | 1.78 | 0.02 |
| Oxidative stress | ||
| Glutathione S-transferase Ω-1 | 1.37 | 0.046 |
| Glutathione S-transferase α-2 | 1.32 | 0.038 |
| Hydroxyacid oxidase 1 | 1.56 | 0.001 |
| Catalase | 1.98 | 0.0002 |
| Others | ||
| Low M(r) phosphotyrosine protein phosphatase isoenzyme AcP1 | 1.32 | 0.012 |
| Rhodanese | 1.90 | 0.0002 |
| Ubfd1 protein | 1.35 | 0.022 |
| rCG50422, isoform CRA_a | −1.83 | −0.051 |
| Cytokeratin 8 polypeptide | −1.67 | 0.011 |
| F alloantigen | −1.48 | 0.005 |
| Complement component C8 α | −1.43 | 0.015 |
| Early endosome antigen 1 | −1.40 | 0.032 |
| Hypothetical protein LOC499136 | −1.39 | 0.001 |
| PREDICTED: ribosomal protein L9-like | 1.30 | 0.006 |
| Keratin, type I cytoskeletal 10 | 1.37 | 0.002 |
| Alcohol sulfotransferase | 1.71 | 0.009 |
| PREDICTED: hypothetical protein | 1.73 | 0.013 |
| rCG55900 | 1.73 | 0.001 |
| Uricase | 1.83 | 0.0005 |
Hepatic FSP27, PNPLA3 and PPARγ2 expressions
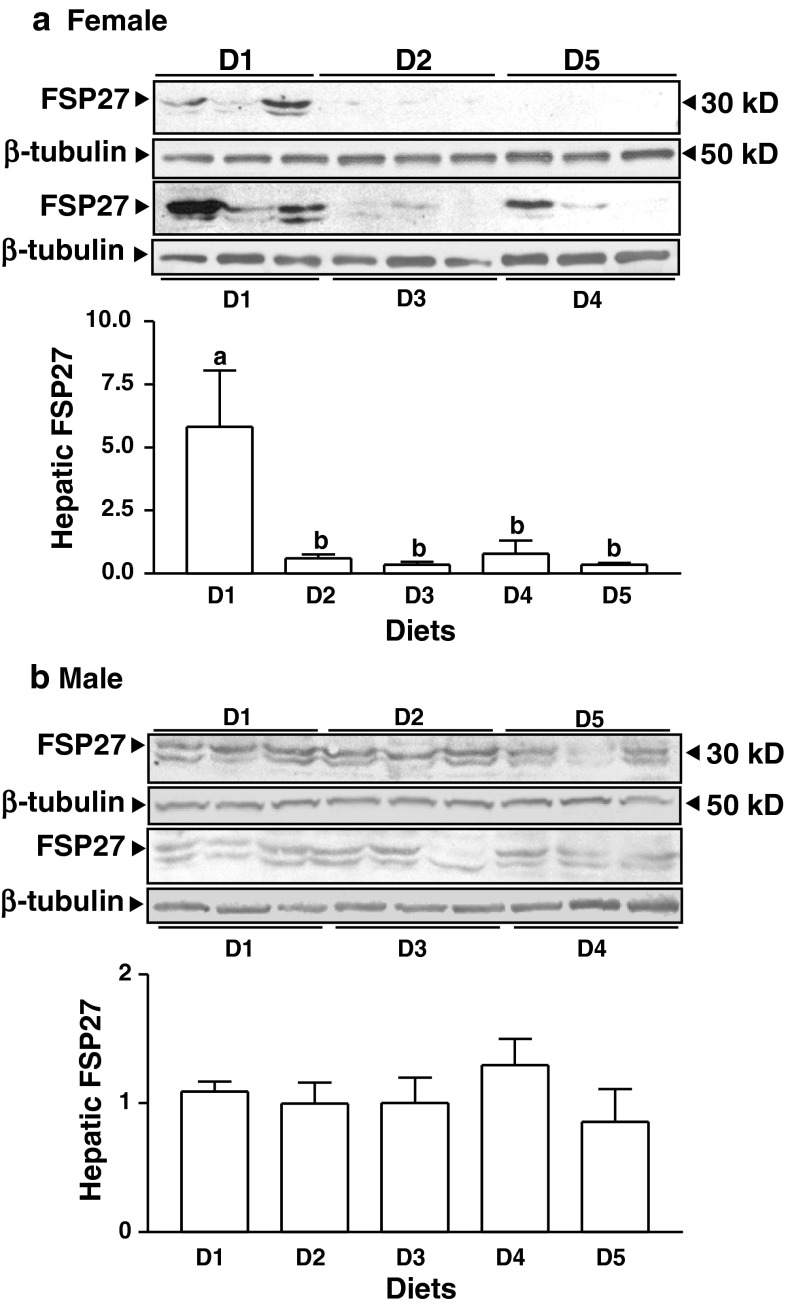
Dietary SPI (D3-5) and ISF (D2) reduced hepatic FSP27 protein content compared to casein diet (D1, p < 0.01) in female (Fig. 3a), but not in male rats (Fig. 3b). Immunohistological results showed that FSP27 is heavily localized in the cytoplasm of the liver cells in the female rats fed 20 % casein diet (D1, Fig. 4a). FSP27 labeling indexes (percentage of the cells expressed FSP27 and abundance of FSP27) in the rats fed ISF (D2) and soy proteins (D3-5) were remarkably lower than in those fed D1 (p < 0.01, Fig. 4b).
Fig. 3.
Hepatic-fat-specific protein 27 (FSP27) contents in the female (a) and male (b) rats fed diets containing either 20 % casein in the absence (D1) or presence (D2) of supplemental ISF (50 mg/kg diet) or increasing amounts of alcohol-washed SPI (D3: 5 %, D4: 10 %, D5: 20 %) for 90 days. Values are mean ± SEM, n = 8. Means with different letter differ, p < 0.05
Fig. 4.
Immunohistological images of FSP27 expression in the liver cells (a) and percentage of liver cells expressing FSP27 (b) in the female rats fed diets containing either 20 % casein or 20 % casein + ISF for 90 days. Values are mean ± SEM, n = 5. Means with different letter differ, p < 0.05
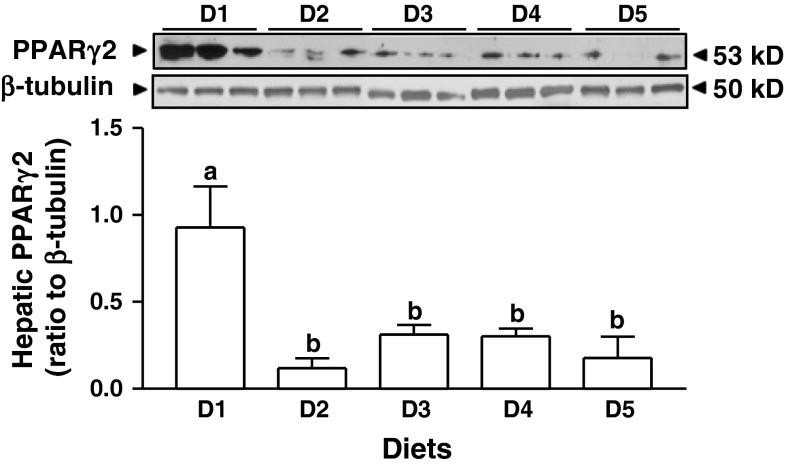
The protein contents of the liver PPARγ2, a direct regulator of FSP27, in the female rats fed diets containing supplemental ISF (D2) or increasing amounts of SPI (D3-5) were markedly lower than that in the rats fed casein diet (D1, p < 0.01, Fig. 5). The abundances of hepatic PNPLA3 protein in the female rats were not changed by different diets (Suppl. Fig. 2).
Fig. 5.
Hepatic PPARγ2 protein in the female rats fed diets containing either 20 % casein in the absence (D1) or presence (D2) of supplemental ISF (50 mg/kg diet) or increasing amounts of alcohol-washed SPI (D3: 5 %, D4: 10 %, D5: 20 %) for 90 days. Values are mean ± SEM, n = 8. Means with different letter differ, p < 0.05
Discussion
The present study showed that female rats fed casein diet had much more and larger lipid droplets accumulated in the livers than the male rats fed the same diet. Both dietary soy proteins (D3-5) and ISF (D2) remarkably reduced the formation of hepatic lipid droplets in the female rats. The hepatic TC levels in both females and males fed 20 % casein diet were similar, however, and were significantly lowered by a replacement of as low as 5 % casein with the same amount of SPI. Addition of soy ISF similar to the amount contained in 20 % SPI diet (D5) into the casein diet (D2) did not significantly reduce the hepatic TC compared to casein control (D1). This indicates that soy protein may play a major role in the hypocholesterolemic actions of the soy observed in this study.
Interestingly, the hepatic TG content in the female rats fed casein diet was 65 % higher than in the male rats fed the same diet. This difference may explain why the HLD was not formed and accumulated in the males. Intake of increasing amounts of soy protein dose-dependently reduced the hepatic levels of TG compared to the casein diet in the female rats, whereas the ISF added in the casein diet had no significant effect. These results were consistent with previous studies showing that soy protein plays a major role in the lipid-lowering action and hypocholesterolemic properties of soy (Forsythe 1995; Adams et al. 2002; Jenkins et al. 2002). It has also been shown that intact dietary soy protein, but not an ISF-rich soy extract improved plasma lipids in ovariectomized cynomolgus monkeys (Greaves et al. 1999). Additionally, 20 % SPI significantly reduced abdominal fat, liver TG and accumulation of lipid droplets in SD rats compared to 20 % casein diet containing the similar amount of genistein to the SPI diet (Simmen et al. 2010). Our results are also supported by a study showing hypolipidemic effects of β-conglycinin, one of the major soy storage proteins, in the hypercholesterolemic rats, which are comparable to the hypolipidemic drugs, fenofibrate and rosuvastatin (Ferreira et al. 2012). It has been suggested that the lipid-lowering actions of β-conglycinin might be a result of increased insulin sensitivity of the rat liver (Tachibana et al. 2010). However, short peptides derived from β-conglycinin were able to suppress the synthesis of TG and cholesterol in the cultured human liver cell line, HepG2 cells (Mochizuki et al. 2009), with absence of insulin. This indicates that other mechanisms may be involved in the action of soy proteins. Glycinin, another storage protein, was also shown to decrease liver TG and improve the atherogenic index in the rats exposed to hypercholesterolemic diet (Fassini et al. 2011).
Soy proteins and ISF had minimal or no effect on serum lipids in the present study. This might be attributable to the low fat content in the diets used, which failed to remarkably increase the base levels of the blood lipids in the control rats. Interestingly, all soy protein diets significantly increased FFA contents in the liver of the female rats compared to the casein diets, and added ISF had no such effect. The serum levels of FFA were significantly lower in the female rats fed casein + ISF (D2) and 20 % SPI (D5) compared to the rats fed casein diet (D1). This suggests that dietary soy proteins may be responsible for the high levels of FFA in the liver, whereas ISF is associated with decreased FFA levels in the blood. Increased liver FFA by soy protein was probably derived from increased lipolysis in the liver and/or adipose tissues (Bradbury 2006; Tovar and Torres 2010) rather than de novo synthesis since the content of the enzymes involved in FA synthesis were suppressed while the abundances of lipolytic enzymes were increased as demonstrated by the proteomic analysis in the present study.
Casein is a major protein (80 %) in bovine milk, a basic food for most infants and children and a common food for adults in most western societies, while soy proteins are the most consumed plant proteins worldwide. Our results showed that dietary soy proteins compared to casein up-regulated the expression of genes involved in FA β-oxidation, lipolysis, glycolysis and oxidative stress, and down-regulated the gene expression related to FA synthesis. This indicates that the hypotriglyceridemic actions of soy proteins may be mediated through suppressed synthesis and enhanced catabolism of TG and FA in the liver. Interestingly, it appears that the formation and accumulation of HLD is not only determined by the amount of hepatic TG. For example, although the hepatic TG content in the female rats fed casein + ISF (D2) and 5 % SPI (D3) were not significantly different from that of the rats fed casein (D1), their HLD accumulation was remarkably lowered. These results suggest that soy protein and ISF may impact the formation of lipid droplets through different mechanism(s).
To further understand the potential cellular events involved in the modulation of HLD formation and accumulation by soy protein and ISF, we measured the expression of FSP27 and its upstream regulator PPARγ2 (Uno et al. 2012) as well as PNPLA3 in the liver. FSP27, originally identified as a cell-death-inducing DFF45-like effector C, was shown to be lipid-droplet-associated protein and promote the formation of lipid droplet by enhancing TG accumulation within lipid droplets and regulating fat storage (Puri et al. 2007, 2008; Keller et al. 2008). Overexpression of FSP27 in the liver of the leptin-deficient (ob/ob) mouse increased hepatic TG content (Matsusue et al. 2008). The expression of liver PPARγ2 was up-regulated in response to overnutrition and genetic obesity (Vidal-Puig et al. 1996; Medina-Gomez et al. 2005) and plays an important role in mediating the preventive effect of β-conglycinin on high-fat-diet-induced fatty liver in mouse (Yamazaki et al. 2012). PNPLA3 gene polymorphisms (Romeo et al. 2008) and expression have been recently shown to be associated with NAFLD. Hepatic PNPLA3 expression was strongly correlated with hepatic TG content in humans (Pirazzi et al. 2012), and reduced PNPLA3 expression prevented NAFLD in rats (Kumashiro et al. 2013).
Our results showed that the casein diet with addition of ISF and all SPI diets markedly reduced the hepatic content of FSP27 protein compared to the casein diet. This was also supported by the immunohistological analysis showing that the percentage of FSP27-expressed cells in the liver of the female rats fed casein diet was markedly higher than those fed ISF and SPI diets. This indicates that the reduced accumulation of HLD by added ISF may be mainly mediated through suppression of FSP27 in the liver and that soy proteins were able to attenuate the abundance of both FSP27 and hepatic TG, thereby preventing fatty liver. We further demonstrated that suppression of FSP27 by dietary ISF and SPI was probably through dow-regulation of its upstream transcription factor, PPARγ2. This is consistent with that soy-β-conglycinin-decreased FSP27 mRNA expression and suppressed PPARγ2 in mouse (Yamazaki et al. 2012). To the best of our knowledge, the present study is the first to show the inhibitory effects of soy isoflavones on hepatic FSP27 protein and accumulation of HLD. PNPLA3 protein level was not affected by diets in this study, indicating that it did not play a major role in mediating the effect of dietary ISF and soy proteins on the prevention of HLD accumulation.
In summary, this study demonstrated that feeding a very low level (50 mg/kg diet) of ISF and partial or full replacement of dietary casein by soy proteins can effectively prevent the formation and accumulation of HLD in female rats. Dietary ISF and soy proteins markedly reduced the liver FSP27 and PPARγ2 proteins in female rats. The potential underlying molecular mechanism(s) might be through suppression of FA synthesis or stimulation of lipolysis and down-regulation of PPARγ2 and FSP27. The results suggest that consumption of soy foods or supplements might be a useful strategy in prevention or treatment of fatty liver diseases. However, the gender difference in diet-induced HLD accumulation and lipid profile observed in this study remains to be elucidated.
Electronic supplementary material
Supplementary material 2 (PPTX 1,163 kb)
Acknowledgments
This study was funded by Health Canada. There are no any financial and other contractual agreements that may cause conflicts of interests involved in this study.
References
- Adams MR, Golden DL, Anthony MS, Register TC, Williams JK. The inhibitory effect of soy protein isolate on atherosclerosis in mice does not require the presence of LDL receptors or alteration of plasma lipoproteins. J Nutr. 2002;132:43–49. doi: 10.1093/jn/132.1.43. [DOI] [PubMed] [Google Scholar]
- Angulo P. Nonalcoholic fatty liver disease. N Engl J Med. 2002;346:1221–1231. doi: 10.1056/NEJMra011775. [DOI] [PubMed] [Google Scholar]
- Anthony MS, Clarkson TB, Hughes CL, Morgan TM, Burke GL. Soybean isoflavones improve cardiovascular risk factors without affecting the reproductive system of peripubertal rhesus monkeys. J Nutr. 1996;126:43–50. doi: 10.1093/jn/126.1.43. [DOI] [PubMed] [Google Scholar]
- Badger TM, Ronis MJ, Wolff G, Stanley S, Ferguson M, Shankar K, Simpson P, Jo CH. Soy protein isolate reduces hepatosteatosis in yellow Avy/a mice without altering coat color phenotype. Exp Biol Med (Maywood) 2008;233:1242–1254. doi: 10.3181/0802-RM-60. [DOI] [PubMed] [Google Scholar]
- Bradbury MW. Lipid metabolism and liver inflammation. I. Hepatic fatty acid uptake: possible role in steatosis. Am J Physiol Gastrointest Liver Physiol. 2006;290:G194–G198. doi: 10.1152/ajpgi.00413.2005. [DOI] [PubMed] [Google Scholar]
- Browning JD, Szczepaniak LS, Dobbins R, Nuremberg P, Horton JD, Cohen JC, Grundy SM, Hobbs HH. Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology. 2004;40:1387–1395. doi: 10.1002/hep.20466. [DOI] [PubMed] [Google Scholar]
- Crespillo A, Alonso M, Vida M, Pavon FJ, Serrano A, Rivera P, Romero-Zerbo Y, Fernandez-Llebrez P, Martinez A, Perez-Valero V, Bermudez-Silva FJ, Suarez J, de Fonseca FR. Reduction of body weight, liver steatosis and expression of stearoyl-CoA desaturase 1 by the isoflavone daidzein in diet-induced obesity. Br J Pharmacol. 2011;164:1899–1915. doi: 10.1111/j.1476-5381.2011.01477.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis J, Higginbotham A, O’Connor T, Moustaid-Moussa N, Tebbe A, Kim YC, Cho KW, Shay N, Adler S, Peterson R, Banz W. Soy protein and isoflavones influence adiposity and development of metabolic syndrome in the obese male ZDF rat. Ann Nutr Metab. 2007;51:42–52. doi: 10.1159/000100820. [DOI] [PubMed] [Google Scholar]
- Fassini PG, Noda RW, Ferreira ES, Silva MA, Neves VA, Demonte A. Soybean glycinin improves HDL-C and suppresses the effects of rosuvastatin on hypercholesterolemic rats. Lipids Health Dis. 2011;10:165. doi: 10.1186/1476-511X-10-165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira ES, Silva MA, Demonte A, Neves VA. Beta-conglycinin combined with fenofibrate or rosuvastatin have exerted distinct hypocholesterolemic effects in rats. Lipids Health Dis. 2012;11:11. doi: 10.1186/1476-511X-11-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- Forsythe WA. Soy protein, thyroid regulation and cholesterol metabolism. J Nutr. 1995;125:619S–623S. doi: 10.1093/jn/125.3_Suppl.619S. [DOI] [PubMed] [Google Scholar]
- Frigolet ME, Torres N, Uribe-Figueroa L, Rangel C, Jimenez-Sanchez G, Tovar AR. White adipose tissue genome wide-expression profiling and adipocyte metabolic functions after soy protein consumption in rats. J Nutr Biochem. 2011;22:118–129. doi: 10.1016/j.jnutbio.2009.12.006. [DOI] [PubMed] [Google Scholar]
- Greaves KA, Parks JS, Williams JK, Wagner JD. Intact dietary soy protein, but not adding an isoflavone-rich soy extract to casein, improves plasma lipids in ovariectomized cynomolgus monkeys. J Nutr. 1999;129:1585–1592. doi: 10.1093/jn/129.8.1585. [DOI] [PubMed] [Google Scholar]
- Gudbrandsen OA, Wergedahl H, Mork S, Liaset B, Espe M, Berge RK. Dietary soya protein concentrate enriched with isoflavones reduced fatty liver, increased hepatic fatty acid oxidation and decreased the hepatic mRNA level of VLDL receptor in obese Zucker rats. Br J Nutr. 2006;96:249–257. doi: 10.1079/BJN20061837. [DOI] [PubMed] [Google Scholar]
- Hakkak R, Al Dwairi A, Fuchs GJ, Korourian S, Simmen FA. Dietary soy protein induces hepatic lipogenic enzyme gene expression while suppressing hepatosteatosis in obese female Zucker rats bearing DMBA-initiated mammary tumors. Genes Nutr. 2012;7:549–558. doi: 10.1007/s12263-012-0294-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins DJ, Kendall CW, Jackson CJ, Connelly PW, Parker T, Faulkner D, Vidgen E, Cunnane SC, Leiter LA, Josse RG. Effects of high- and low-isoflavone soyfoods on blood lipids, oxidized LDL, homocysteine, and blood pressure in hyperlipidemic men and women. Am J Clin Nutr. 2002;76:365–372. doi: 10.1093/ajcn/76.2.365. [DOI] [PubMed] [Google Scholar]
- Keller P, Petrie JT, De Rose P, Gerin I, Wright WS, Chiang SH, Nielsen AR, Fischer CP, Pedersen BK, MacDougald OA. Fat-specific protein 27 regulates storage of triacylglycerol. J Biol Chem. 2008;283:14355–14365. doi: 10.1074/jbc.M708323200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MH, Kang KS. Isoflavones as a smart curer for non-alcoholic fatty liver disease and pathological adiposity via ChREBP and Wnt signaling. Prev Med. 2012;54:S57–S63. doi: 10.1016/j.ypmed.2011.12.018. [DOI] [PubMed] [Google Scholar]
- Kim MH, Kang KS, Lee YS. The inhibitory effect of genistein on hepatic steatosis is linked to visceral adipocyte metabolism in mice with diet-induced non-alcoholic fatty liver disease. Br J Nutr. 2010;104:1333–1342. doi: 10.1017/S0007114510002266. [DOI] [PubMed] [Google Scholar]
- Kim MH, Park JS, Jung JW, Byun KW, Kang KS, Lee YS. Daidzein supplementation prevents non-alcoholic fatty liver disease through alternation of hepatic gene expression profiles and adipocyte metabolism. Int J Obes. 2011;35:1019–1030. doi: 10.1038/ijo.2010.256. [DOI] [PubMed] [Google Scholar]
- Kitawaki R, Nishimura Y, Takagi N, Iwasaki M, Tsuzuki K, Fukuda M. Effects of Lactobacillus fermented soymilk and soy yogurt on hepatic lipid accumulation in rats fed a cholesterol-free diet. Biosci Biotechnol Biochem. 2009;73:1484–1488. doi: 10.1271/bbb.80753. [DOI] [PubMed] [Google Scholar]
- Kumashiro N, Yoshimura T, Cantley JL, Majumdar SK, Guebre-Egziabher F, Kursawe R, Vatner DF, Fat I, Kahn M, Erion DM, Zhang XM, Zhang D, Manchem VP, Bhanot S, Gerhard GS, Petersen KF, Cline GW, Samuel VT, Shulman GI. Role of patatin-like phospholipase domain-containing 3 on lipid-induced hepatic steatosis and insulin resistance in rats. Hepatology. 2013;57:1763–1772. doi: 10.1002/hep.26170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsusue K, Kusakabe T, Noguchi T, Takiguchi S, Suzuki T, Yamano S, Gonzalez FJ. Hepatic steatosis in leptin-deficient mice is promoted by the PPARgamma target gene Fsp27. Cell Metab. 2008;7:302–311. doi: 10.1016/j.cmet.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina-Gomez G, Virtue S, Lelliott C, Boiani R, Campbell M, Christodoulides C, Perrin C, Jimenez-Linan M, Blount M, Dixon J, Zahn D, Thresher RR, Aparicio S, Carlton M, Colledge WH, Kettunen MI, Seppanen-Laakso T, Sethi JK, O’Rahilly S, Brindle K, Cinti S, Oresic M, Burcelin R, Vidal-Puig A. The link between nutritional status and insulin sensitivity is dependent on the adipocyte-specific peroxisome proliferator-activated receptor-gamma2 isoform. Diabetes. 2005;54:1706–1716. doi: 10.2337/diabetes.54.6.1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miniello VL, Moro GE, Tarantino M, Natile M, Granieri L, Armenio L. Soy-based formulas and phyto-oestrogens: a safety profile. Acta Paediatr Suppl. 2003;91:93–100. doi: 10.1111/j.1651-2227.2003.tb00655.x. [DOI] [PubMed] [Google Scholar]
- Mochizuki Y, Maebuchi M, Kohno M, Hirotsuka M, Wadahama H, Moriyama T, Kawada T, Urade R. Changes in lipid metabolism by soy beta-conglycinin-derived peptides in HepG2 cells. J Agric Food Chem. 2009;57:1473–1480. doi: 10.1021/jf8031793. [DOI] [PubMed] [Google Scholar]
- Mohamed SS, Nallasamy P, Muniyandi P, Periyasami V, Carani VA. Genistein improves liver function and attenuates non-alcoholic fatty liver disease in a rat model of insulin resistance. J Diabetes. 2009;1:278–287. doi: 10.1111/j.1753-0407.2009.00045.x. [DOI] [PubMed] [Google Scholar]
- Ong JP, Younossi ZM (2007) Epidemiology and natural history of NAFLD and NASH. Clin Liver Dis 11:1–16, vii [DOI] [PubMed]
- Pirazzi C, Adiels M, Burza MA, Mancina RM, Levin M, Ståhlman M, Taskinen MR, Orho-Melander M, Perman J, Pujia A, Andersson L, Maglio C, Montalcini T, Wiklund O, Boérn J, Romeo S. Patatin-like phospholipase domain-containing 3 (PNPLA3) I148 M (rs738409) affects hepatic VLDL secretion in humans and in vitro. J Hepatol. 2012;57:1276–1282. doi: 10.1016/j.jhep.2012.07.030. [DOI] [PubMed] [Google Scholar]
- Puri V, Konda S, Ranjit S, Aouadi M, Chawla A, Chouinard M, Chakladar A, Czech MP. Fat-specific protein 27, a novel lipid droplet protein that enhances triglyceride storage. J Biol Chem. 2007;282:34213–34218. doi: 10.1074/jbc.M707404200. [DOI] [PubMed] [Google Scholar]
- Puri V, Virbasius JV, Guilherme A, Czech MP. RNAi screens reveal novel metabolic regulators: RIP140, MAP4k4 and the lipid droplet associated fat specific protein (FSP) 27. Acta Physiol (Oxf) 2008;192:103–115. doi: 10.1111/j.1748-1716.2007.01786.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves PG, Nielsen FH, Fahey GC., Jr AIN-93 purified diets for laboratory rodents: final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J Nutr. 1993;123:1939–1951. doi: 10.1093/jn/123.11.1939. [DOI] [PubMed] [Google Scholar]
- Romeo S, Kozlitina J, Xing C, Pertsemlidis A, Cox D, Pennacchio LA, Boerwinkle E, Cohen JC, Hobbs HH. Genetic variation in PNPLA3 confers susceptibility to nonalcoholic fatty liver disease. Nat Genet. 2008;40:1461–1465. doi: 10.1038/ng.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmen FA, Mercado CP, Zavacki AM, Huang SA, Greenway AD, Kang P, Bowman MT, Prior RL. Soy protein diet alters expression of hepatic genes regulating fatty acid and thyroid hormone metabolism in the male rat. J Nutr Biochem. 2010;21:1106–1113. doi: 10.1016/j.jnutbio.2009.09.008. [DOI] [PubMed] [Google Scholar]
- Tachibana N, Iwaoka Y, Hirotsuka M, Horio F, Kohno M. Beta-conglycinin lowers very-low-density lipoprotein-triglyceride levels by increasing adiponectin and insulin sensitivity in rats. Biosci Biotechnol Biochem. 2010;74:1250–1255. doi: 10.1271/bbb.100088. [DOI] [PubMed] [Google Scholar]
- Takahashi Y, Konishi T. Tofu (soybean curd) lowers serum lipid levels and modulates hepatic gene expression involved in lipogenesis primarily through its protein, not isoflavone, component in rats. J Agric Food Chem. 2011;59:8976–8984. doi: 10.1021/jf201403u. [DOI] [PubMed] [Google Scholar]
- Torres N, Torre-Villalvazo I, Tovar AR. Regulation of lipid metabolism by soy protein and its implication in diseases mediated by lipid disorders. J Nutr Biochem. 2006;17:365–373. doi: 10.1016/j.jnutbio.2005.11.005. [DOI] [PubMed] [Google Scholar]
- Torre-Villalvazo I, Tovar AR, Ramos-Barragan VE, Cerbon-Cervantes MA, Torres N. Soy protein ameliorates metabolic abnormalities in liver and adipose tissue of rats fed a high fat diet. J Nutr. 2008;138:462–468. doi: 10.1093/jn/138.3.462. [DOI] [PubMed] [Google Scholar]
- Tovar AR, Torres N. The role of dietary protein on lipotoxicity. Biochim Biophys Acta. 2010;1801:367–371. doi: 10.1016/j.bbalip.2009.09.007. [DOI] [PubMed] [Google Scholar]
- Tovar AR, Murguia F, Cruz C, Hernandez-Pando R, Aguilar-Salinas CA, Pedraza-Chaverri J, Correa-Rotter R, Torres N. A soy protein diet alters hepatic lipid metabolism gene expression and reduces serum lipids and renal fibrogenic cytokines in rats with chronic nephrotic syndrome. J Nutr. 2002;132:2562–2569. doi: 10.1093/jn/132.9.2562. [DOI] [PubMed] [Google Scholar]
- Tovar AR, Torre-Villalvazo I, Ochoa M, Elias AL, Ortiz V, Aguilar-Salinas CA, Torres N. Soy protein reduces hepatic lipotoxicity in hyperinsulinemic obese Zucker fa/fa rats. J Lipid Res. 2005;46:1823–1832. doi: 10.1194/jlr.M500067-JLR200. [DOI] [PubMed] [Google Scholar]
- Uno K, Yamada T, Ishigaki Y, Imai J, Hasegawa Y, Gao J, Kaneko K, Matsusue K, Yamazaki T, Oka Y, Katagiri H. Hepatic peroxisome proliferator-activated receptor-gamma-fat-specific protein 27 pathway contributes to obesity-related hypertension via afferent vagal signals. Eur Heart J. 2012;33:1279–1289. doi: 10.1093/eurheartj/ehr265. [DOI] [PubMed] [Google Scholar]
- Vidal-Puig A, Jimenez-Linan M, Lowell BB, Hamann A, Hu E, Spiegelman B, Flier JS, Moller DE. Regulation of PPAR gamma gene expression by nutrition and obesity in rodents. J Clin Invest. 1996;97:2553–2561. doi: 10.1172/JCI118703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HJ, Murphy P. Isoflavone content in commercial soybean foods. J Agric Food Chem. 1994;42:1666–1673. doi: 10.1021/jf00044a016. [DOI] [Google Scholar]
- Yamazaki T, Kishimoto K, Miura S, Ezaki O. Dietary beta-conglycinin prevents fatty liver induced by a high-fat diet by a decrease in peroxisome proliferator-activated receptor gamma2 protein. J Nutr Biochem. 2012;23:123–132. doi: 10.1016/j.jnutbio.2010.11.006. [DOI] [PubMed] [Google Scholar]
- Zhou D, Lezmi S, Wang H, Davis J, Banz W, Chen H. Fat accumulation in the liver of obese rats is alleviated by soy protein isolate through beta-catenin signaling. Obesity (Silver Spring) 2013 doi: 10.1002/oby.20421. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material 2 (PPTX 1,163 kb)