Abstract
The sulfenic acid form of glyceraldehyde-3-phosphate dehydrogenase (EC 1.2.1.12), which is an acyl phosphatase, will catalyze an acetyl phosphate-Pi exchange reaction. This exchange reaction is reversibly inhibited by the uncouplers of oxidative phosphorylation, 2,4-dinitrophenol, m-Cl carbonylcyanide-phenylhydrazone, pentachlorophenol, and 5-chloro-3-tert-butyl-2′-chloro-4′-nitrosalicylanalide, and is irreversibly inhibited by cyanide and dicumarol. An ATP-Pi exchange reaction similar to that catalyzed by mitochondria can be simulated by a system composed of oxidized glyceraldehyde-3-phosphate dehydrogenase, phosphoglycerate kinase (EC 2.7.1.28), 3-phosphoglycerate, ATP, 32Pi, and appropriate cofactors. The ATP-Pi exchange is inhibited by uncouplers of oxidative phosphorylation. Higher concentrations of uncouplers will also inhibit the ATPase reaction catalyzed by the coupled enzyme system. The exchange reactions catalyzed by the sulfenic acid form of glyceraldehyde-3-phosphate are consistent with a sulfenyl carboxylate intermediate. On the basis of these observations, a reaction scheme has been postulated for covalent coupling in oxidative phosphorylation that includes a sulfenyl carboxylate as a nonphosphorylated, high energy intermediate and an acyl phosphate as a phosphorylated, high energy intermediate.
Keywords: acyl-phosphatase, sulfenyl carboxylate, high-energy intermediate, energy conservation
Full text
PDF
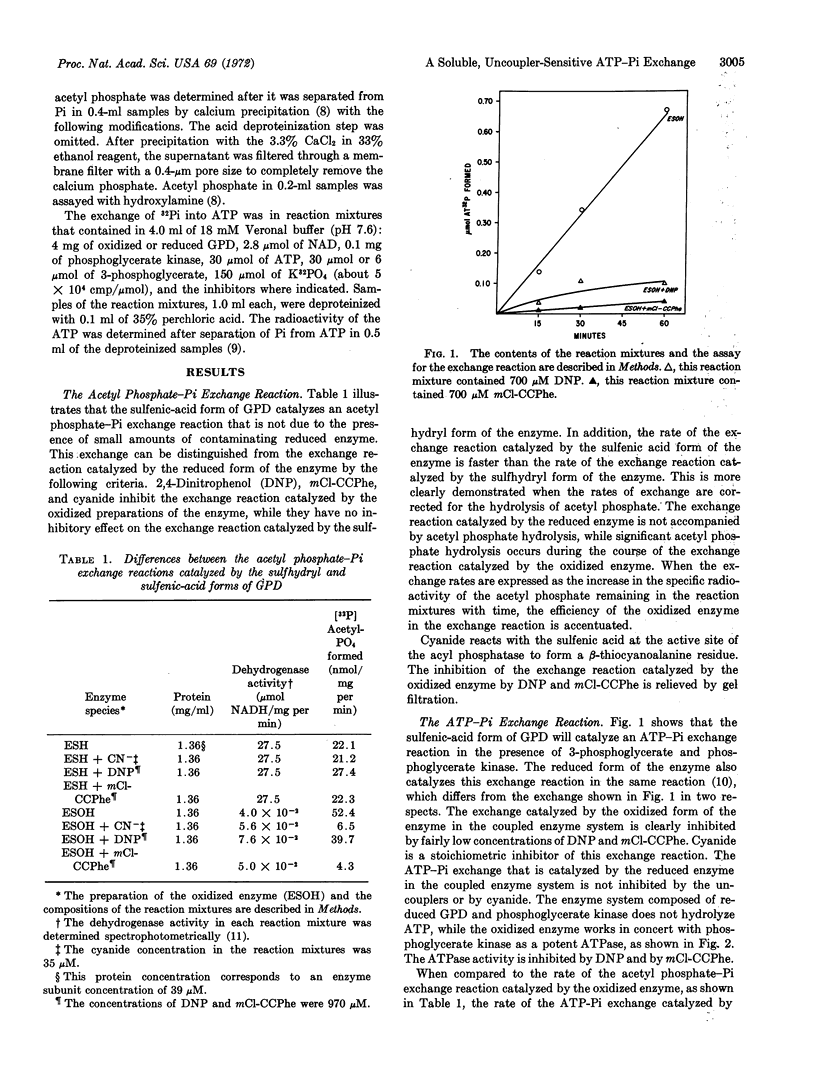

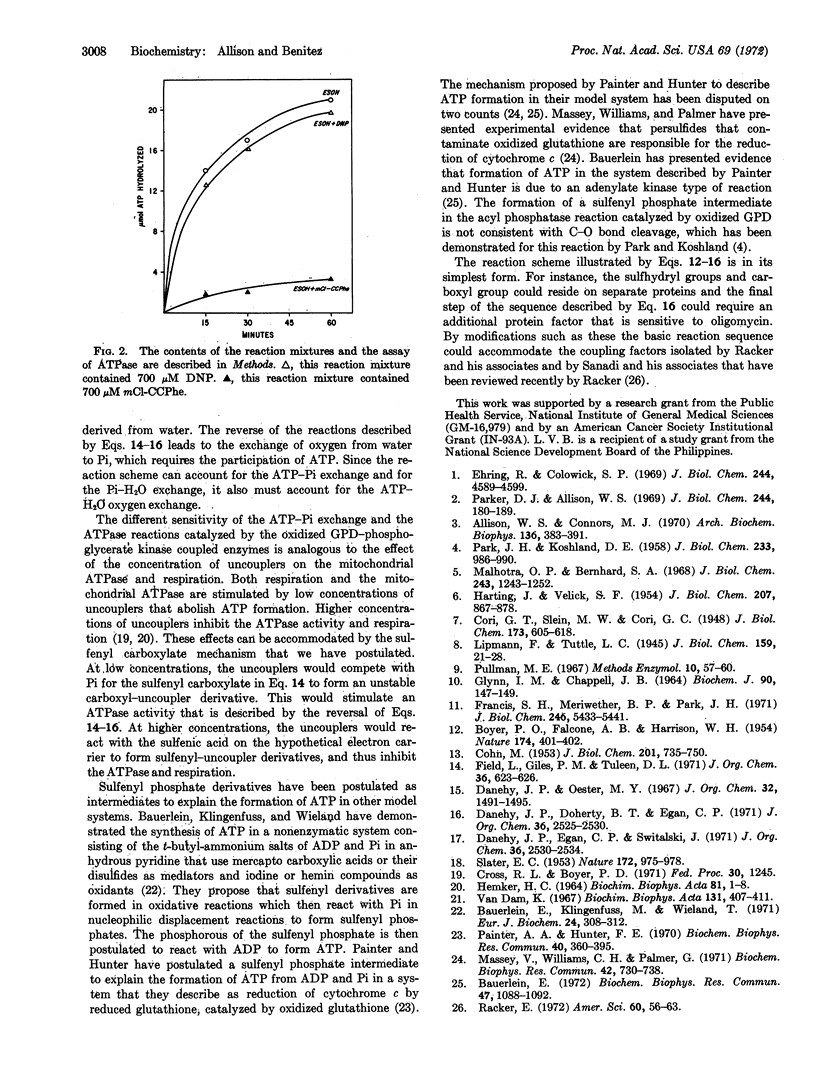
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allison W. S., Connors M. J. The activation and inactivation of the acyl phosphatase activity of glyceraldehyde-3-phosphate dehydrogenase. Arch Biochem Biophys. 1970 Feb;136(2):383–391. doi: 10.1016/0003-9861(70)90209-2. [DOI] [PubMed] [Google Scholar]
- BOYER P. D., FALCONE A. B., HARRISON W. H. Reversal and mechanism of oxidative phosphorylation. Nature. 1954 Aug 28;174(4426):401–402. doi: 10.1038/174401b0. [DOI] [PubMed] [Google Scholar]
- Bäuerlein E. A reinvestigation of Hunter's model system for oxidative phosphorylation. Biochem Biophys Res Commun. 1972 Jun 9;47(5):1088–1092. doi: 10.1016/0006-291x(72)90945-x. [DOI] [PubMed] [Google Scholar]
- Bäuerlein E., Klingenfuss M., Wieland T. Iodine, hemin and heminester as oxidants in a synthesis of ATP from ADP and P i mediated by thiols and disulfides. Eur J Biochem. 1971 Dec;24(2):308–312. doi: 10.1111/j.1432-1033.1971.tb19687.x. [DOI] [PubMed] [Google Scholar]
- COHN M. A study of oxidative phosphorylation with O18-labeled inorganic phosphate. J Biol Chem. 1953 Apr;201(2):735–750. [PubMed] [Google Scholar]
- Ehring R., Colowick S. P. The two-step formation and inactivation of acylphosphatase by agents acting on glyceraldehyde phosphate dehydrogenase. J Biol Chem. 1969 Sep 10;244(17):4589–4599. [PubMed] [Google Scholar]
- Francis S. H., Meriwether B. P., Park J. H. Interaction between adenine nucleotides and 3-phosphoglyceraldehyde dehydrogenase. II. A study of the mechanism of catalysis and metabolic control of the multi-functional enzyme. J Biol Chem. 1971 Sep 10;246(17):5433–5441. [PubMed] [Google Scholar]
- Glynn I. M., Chappell J. B. A simple method for the preparation of 32-P-labelled adenosine triphosphate of high specific activity. Biochem J. 1964 Jan;90(1):147–149. doi: 10.1042/bj0900147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HARTING J., VELICK S. F. Transfer reactions of acetyl phosphate catalyzed by glyceraldehyde-3-phosphate dehydrogenase. J Biol Chem. 1954 Apr;207(2):867–878. [PubMed] [Google Scholar]
- Malhotra O. P., Bernhard S. A. Spectrophotometric identification of an active site-specific acyl glyceraldehyde 3-phosphate dehydrogenase. The regulation of its kinetic and equilibrium properties by coenzyme. J Biol Chem. 1968 Mar 25;243(6):1243–1252. [PubMed] [Google Scholar]
- Massey V., Williams C. H., Jr, Palmer G. The presence of S degrees-containing impurities in commercial samples of oxidized glutathione and their catalytic effect on the reduction of cytochrome c. Biochem Biophys Res Commun. 1971 Feb 19;42(4):730–738. doi: 10.1016/0006-291x(71)90548-1. [DOI] [PubMed] [Google Scholar]
- PARK J. H., KOSHLAND D. E., Jr The hydrolytic activity of glyceraldehyde-3-phosphate dehydrogenase. J Biol Chem. 1958 Oct;233(4):986–990. [PubMed] [Google Scholar]
- Parker D. J., Allison W. S. The mechanism of inactivation of glyceraldehyde 3-phosphate dehydrogenase by tetrathionate, o-iodosobenzoate, and iodine monochloride. J Biol Chem. 1969 Jan 10;244(1):180–189. [PubMed] [Google Scholar]
- Racker E. Bioenergetics and the problem of tumor growth. Am Sci. 1972 Jan-Feb;60(1):56–63. [PubMed] [Google Scholar]
- SLATER E. C. Mechanism of phosphorylation in the respiratory chain. Nature. 1953 Nov 28;172(4387):975–978. doi: 10.1038/172975a0. [DOI] [PubMed] [Google Scholar]
- Van Dam K. The inhibitory effect of uncouplers of oxidative phosphorylation on mitochondrial respiration. Biochim Biophys Acta. 1967 Mar 8;131(2):407–411. doi: 10.1016/0005-2728(67)90157-0. [DOI] [PubMed] [Google Scholar]


