Abstract
G protein coupled receptors play crucial roles in mediating cellular responses to external stimuli, and increasing evidence suggests that they function as multiple units comprising homo/heterodimers and hetero-oligomers. Adenosine and β-adrenergic receptors are co-expressed in numerous tissues and mediate important cellular responses to the autocoid adenosine and sympathetic stimulation, respectively. The present study was undertaken to examine whether adenosine A1ARs heterodimerize with β1-and/or β2-adrenergic receptors (β1R and β2R), and whether such interactions lead to functional consequences. Co-immunoprecipitation and co-localization studies with differentially epitope-tagged A1, β1, and β2 receptors transiently co-expressed in HEK-293 cells indicate that A1AR forms constitutive heterodimers with both β1R and β2R. This heterodimerization significantly influenced orthosteric ligand binding affinity of both β1R and β2R without altering ligand binding properties of A1AR. Receptor-mediated ERK1/2 phosphorylation significantly increased in cells expressing A1AR/β1R and A1AR/β2R heteromers. β-receptor-mediated cAMP production was not altered in A1AR/β1R expressing cells, but was significantly reduced in the A1AR/β2R cells. The inhibitory effect of the A1AR on cAMP production was abrogated in both A1AR/β1R and A1AR/β2R expressing cells in response to the A1AR agonist CCPA. Co-immunoprecipitation studies conducted with human heart tissue lysates indicate that endogenous A1AR, β1R, and β2R also form heterodimers. Taken together, our data suggest that heterodimerization between A1 and β receptors leads to altered receptor pharmacology, functional coupling, and intracellular signaling pathways. Unique and differential receptor cross-talk between these two important receptor families may offer the opportunity to fine-tune crucial signaling responses and development of more specific therapeutic interventions.
Keywords: G protein coupled receptors, heterodimerization, adenosine receptor, β-adrenergic receptors
1. INTRODUCTION
G protein-coupled receptors (GPCR), which comprise the largest family of cell surface receptors, play crucial roles in regulating intracellular signaling. The conventional dogma is that GPCRs function as monomers at the cell surface and couple to G proteins in a one-to-one ratio. However, extensive research over the past decade has clearly established that GPCRs can function as multiple units ranging from dimers (homo and hetero) as well as higher order oligomers, even among members from distinct receptor families [1–3]. The functional consequences of GPCR heterodimerization include altered receptor pharmacology, signaling, trafficking, subcellular localization and receptor desensitization [1–3]. In the present study, we examined heterodimeric interactions between adenosine and β-adrenergic receptor families, which play important physiological roles in various tissues.
Adenosine and β-adrenergic receptors are co-expressed in numerous cell types including the mammalian heart. There are four distinct adenosine receptor (AR) subtypes (A1AR, A2AAR, A2BAR, and A3AR), which exert differential effects in response to the autocoid adenosine. There are three mammalian β-adrenergic receptors subtypes–β1R, β2R, and β3R. The β1-and β2R have distinct pharmacological and functional properties with the former being the primary mediator of the β-adrenergic-mediated increase in cardiac contractility [4, 5]. Like most other GPCRs, adenosine and β-adrenergic receptors couple to specific G proteins with A1AR coupling to inhibitory G protein (Gαi) while β1R and β2R predominantly couple to stimulatory G protein (Gαs). Signaling through their cognate G proteins, both adenosine and β-receptors stimulate various effector molecules including the mitogen activated protein kinase (MAPK) kinase pathway, leading to the activation of extracellular signal-regulated kinase (ERK).
There is significant evidence that A1AR and both β1 and β2Rs function as homo-and heterodimers. It has been reported that the A1AR heterodimerizes within the adenosine receptor family (A2aAR), and also with D1-dopamine and mGlu1α-glutamate receptors leading to various functional ramifications [6–8]. β1-and β2Rs have also been reported to form functional homodimers and heterodimers with receptors such α2A-adrenergic, δ-opioid, and somatostain receptors [9–12]. The β1R can heterodimerize with β2Rs increasing β2R internalization and signaling efficacy as well as switching signaling via Gαs to Gαi [13, 14]. In addition, there is evidence for functional interaction between these two receptor families whereby A1AR inhibits the contractile and biochemical responses of β-adrenergic receptor activation, referred to as the A1AR anti-adrenergic effect [15–17]. However, the possibility of receptor heterodimerization between these two receptor types has not been evaluated previously.
Given that adenosine and β-adrenergic receptors are co-expressed in numerous cell types, and that A1AR antagonizes the β-adrenergic receptor effects in the heart, we investigated whether the A1AR can form functional heterodimers with β1 and β2Rs. In this study, the formation of heterodimers was assessed using co-immunoprecipitation and co-localization approaches with co-transfected HEK-293 cells and normal adult human heart tissue lysates. The functional impact of co-expression was assessed by measuring binding affinity of orthosteric radioligands as well as by examining agonist-induced activation of intracellular signaling pathways (cAMP production and ERK phosphorylation).
2. MATERIALS AND METHODS
2.1. Materials
Receptor agonists, antagonists, and other inhibitors were purchased from Tocris Bioscience (Ellisville, MO). All cell culture reagents and reagents for immunofluorescence were from Invitrogen (Carlsbad, CA). The Tropix cAMP Screen System was obtained from Applied Biosystems (Carlsbad, CA). Full length, N-terminally FLAG-tagged human β1-adrenergic receptor in pcDNA3.1+ vector was a generous gift from Dr. Suleiman Bahouth (Department of Pharmacology, University of Tennessee Health Sciences Center). Full length, N-terminal hemagglutinin (HA) epitope-tagged human A1AR, β2-adrenergic receptor, adenosine A3AR, and M2 muscarinic receptor constructs in pcDNA3.1+ vector were purchased from Missouri S&T cDNA Resource Center (www.cdna.org). In order to have differential epitope tags, the HA-tag present on the A1AR construct was replaced by inserting a Myc epitope tag (EQKLISEEDL) by polymerase chain reaction (PCR). High quality plasmid DNA was isolated using plasmid isolation kits available from QIAGEN (Valencia, CA). Epitope tag antibodies include THE anti-HA from GenScript Inc. (Piscataway, NJ), anti-FLAG-M2 and anti-Myc from Sigma-Aldrich (St. Louis, MO), HRP-conjugated antibodies anti-HA rat monoclonal-HRP antibody (Roche Applied Science, Indianapolis, IN), anti-FLAG-M2-HRP antibody, and anti-Myc-HRP antibody (Sigma-Aldrich, St. Louis, MO). Phosphorylated ERK (pERK 42/44) and HRP-conjugated GAPDH antibodies were obtained from Cell Signaling (Danvers, MA) and Sigma-Aldrich (St. Louis, MO), respectively. Protein G Dynabeads and Novex Tris-Glycine polyacrylamide gels were purchased from Invitrogen (Carlsbad, CA). Normal adult human heart tissue lysate was purchased from Novus Biologicals (Littleton, CO).
2.2. Cell culture and transfection
Human embryonic kidney (HEK-293) cells were maintained in Dulbecco’s modified Eagle Medium (DMEM) supplemented with 10% fetal bovine serum, 2 mM L-glutamine, 0.1 mM non-essential amino acids, and 1% penicillin/streptomycin in a 37°C/5% CO2/humidified incubator. Cells were transfected using a standard calcium phosphate method with 2 μg of DNA per receptor and harvested 48 hours post-transfection for various assays, as described below. In single receptor transfections, transfected plasmid DNA levels were kept constant by using empty vector DNA (pcDNA 3.1+).
2.3. Co-immunoprecipitation and Western blotting
Forty eight hours following transfection, cells were harvested and lysed in a modified RIPA buffer (1% Triton X-100, 50 mM Tris-pH 7.5, 150 mM NaCl, 5 mM EDTA, 100 mM iodoacetamide, and protease inhibitors). Solubilized lysates were incubated overnight in the presence of appropriate epitope tag antibodies. The antibody-bound protein complexes were pulled down with Protein G-Dynabeads and eluted with Laemmli sample buffer (without DTT or β-mercaptoethanol). Samples were resolved by denaturing PAGE and protein complexes were visualized by Western blotting using primary antibodies conjugated to HRP: anti-HA rat monoclonal-HRP antibody, anti-FLAG-HRP antibody, and anti-Myc-HRP antibody. For human heart lysates, 200 μg of total protein was solubilized in RIPA buffer and incubated with antibodies against A1AR and βR overnight followed by immunoprecipitation as described above. Total heart lysates (25 μg) and A1AR-antibody bound complexes were detected by immunoblotting using antibodies against β1-and β2R. Converse immunoprecipitation reactions were carried out using anti-βR antibodies and visualized with anti-A1AR antibody.
2.4. Co-localization via immunofluorescence microscopy
HEK-293 cells were plated on poly-D-lysine coated coverslips and transfected singly with A1AR, β1-and β2-adrenergic receptors or co-transfected with A1AR/β1-and A1AR/β2-adrenergic receptors. Forty-eight hours post-transfection, cells were washed with ice-cold PBS and fixed with 4% paraformaldehyde. Cells were then used without permeabilization or with permeabilization (0.2% Triton-X 100), blocked with normal goat serum, and incubated with various primary antibodies in combination: rat monoclonal anti-HA, mouse monoclonal anti-FLAG-M2, and rabbit polyclonal anti-cMyc. Cells were washed and incubated with AlexaFluor-conjugated secondary antibodies: AF-488, AF-594, or AF-350, as indicated in the figures. Cover slips were mounted with ProLong Gold antifade reagent and visualized with a Leica SD6000 confocal microscope.
2.5. Receptor-mediated activation of ERK
Cells grown in 6-well cell culture plates were transiently transfected in various combinations as described above. Forty-four hours post-transfection, cells were starved in serum-free DMEM for 4 hours in the presence of adenosine deaminase (2U/mL). Cells were then treated for 5 min with the A1AR agonist CCPA (250 nM) or βR agonist isoproterenol (ISO, 500 nM) and lysed with RIPA buffer containing protease and phosphatase inhibitor cocktails. Receptor-mediated activation of the mitogen-activated protein kinase (MAPK) pathway was assessed by Western blotting using anti-phospho extracellular regulated kinase (pERK 42/44) antibody. The pERK signal was normalized to total protein present in the samples by probing with an HRP-conjugated anti-GAPDH antibody. All drug treated samples were also normalized against vehicle-treated samples to determine basal activation of pERK. Densitometric quantification was carried out using the NIH Image J software and groups compared using two-tailed unpaired t-test.
2.6. cAMP assay
Following serum starvation in the presence of adenosine deaminase as described above, cells were treated for 20 min with the phosphodiesterase inhibitor rolipram (50 μM) prior to 10 min exposure to ISO or CCPA. Cells were then lysed and intracellular cAMP levels were determined by a competitive immunoassay kit, Tropix cAMP Screen System, according to the manufacturer’s instructions. All samples were normalized to the total protein amount, as determined by the method of Bradford. Vehicle treated samples were used to determine basal cAMP levels. cAMP levels normalized to the total protein concentration were further normalized against the maximal receptor stimulation obtained from single receptor transfections, as described in the figure legends.
2.7. Radioligand binding assay
Radioligand binding assays were conducted with membranes prepared from HEK 293 cells transiently expressing the various combinations of adenosine and β-adrenergic receptors using the agonist radioligand N6-(4-amino-3-[125I]iodobenzyl) adenosine-5′-N-methyl-carboxamide ([125I]I-AB-MECA) in assays with the A1R and the antagonist radioligand [125I]iodo-(-)-cyanopindolol (125I-CYP) in assays with β1 or β2Rs. [125I]I-AB-MECA and 125I-CYP were prepared by radioiodination of the precursors AB-MECA and CYP, respectively, using the chloramine-T method [18]. Receptor transfected HEK-293 cell membranes were prepared as described previously [18]. For saturation equilibrium radioligand binding studies, cell membranes (2 μg protein/tube for β receptor assays and 50 μg/tube for A1R assays) were tested against a range of 6–8 increasing concentrations of either radioligand. After incubating at room temperature for 3 h, the membranes were collected by filtration over Whatman GF/C filters and the radioactivity trapped in the filters was quantified using a gamma counter. Non-specific binding was determined in the presence of alprenolol (10 μM for β receptors) or adenosine-5′-N-ethylcarboxamide (100 μM for A1Rs). In all assays, specific binding of the radioligands fit optimally to a single-site binding model: y = (Bmax x [L])/(Kd+[L]), from which Bmax and Kd values were obtained.
3. RESULTS
3.1. A1ARs co-immunoprecipiate with both β1 and β2 receptors
The ability of the A1AR to heterodimerize with β1 or β2Rs was first assessed using a co-immunoprecipitation approach in HEK-293 cells. Using appropriate antibody pairs, each of the receptors was immunoprecipitated and the presence of additional receptor subtypes in the precipitates was assessed by Western blotting. In order to show that heterodimerization only occurs in co-transfected cells, cells singly transfected with each receptor were first mixed and then lysed. As shown in Figure 1a, both β1 and β2Rs co-immunoprecipitated with the A1AR in lysates prepared from cells co-expressing A1AR/β1R and A1AR/β2R, but not in lysates prepared from a mixture of cells expressing each of the receptors alone. The doublet present in immunoblots for the A1AR likely represents the glycosylated (higher band) and unglycosylated (lower band) forms of the receptor, as reported previously [19].
Figure 1.
Heterodimerization of A1AR with β1R and β2R assessed by co-immunoprecipitation. (A) Epitope-tagged recombinant human A1AR/β1R and A1AR/β2R were transiently co-expressed in HEK-293 cells and the receptor heterodimers were captured and detected using co-immunoprecipitation and Western blotting, as described in Materials and Methods. Cell lysates (L) were immunoprecipitated (I) using antibody pairs as indicated. Control immunoprecipitations were also carried out using cell lysates from a mixture (M) of HEK-293 cells individually expressing each receptor type; (B) Epitope-tagged recombinant human A3AR/β1R and M2MR/β1R were co-transfected, co-immunoprecipitated, and probed with the epitope tag antibody pairs as shown. IP, immunoprecipitation; IB, immunoblot; MW, molecular weight. Data are representative of 2–3 independent experiments.
In order to ensure specificity of interaction between A1AR and βRs, co-immunoprecipitation experiments were conducted in which A1AR was substituted with the only other Gi-coupled adenosine receptor subtype, the A3AR, and a Gi-coupled receptor from a completely different family, muscarinic M2 receptor (M2MR). HEK-293 cells were co-transfected with A3AR/β1R or M2MR/β1R and co-immunoprecipitation was performed as described above. As shown in Figure 1b, heterodimerization was not detectable with either A3AR/β1R or M2MR/β1R. These immunoprecipitates were also probed with the respective epitope tag antibody for each receptor type to ensure that receptors were properly expressed (1b, right panel). Taken together, these results suggest that the A1AR is capable of forming specific stable heterodimeric complexes with either β1 or β2Rs.
3.2 A1ARs co-localize with β1 and β2 receptors at the cell plasma membrane
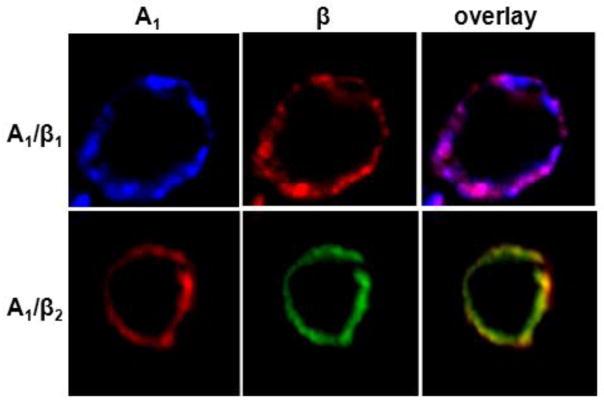
The potential for co-localization of A1ARs and β receptors in HEK-293 cells was assessed using immunofluorescence microscopy. As shown in Figure 2, confocal images of HEK-293 cells co-expressing A1ARs and either β receptor subtype revealed a similar cellular expression pattern for the A1AR and both β receptor isoforms in co-transfected cells. In these non-permeabilized cells, it is clear that all three of the receptors were localized at the plasma membrane, and the merged images revealed almost complete overlap in the cellular expression pattern between the A1AR and β-receptors. These results indicate that A1AR and βR are capable of co-localizing at the plasma membrane, providing further support for their propensity for dimerization.
Figure 2.
Heterodimerization of A1AR with β1R and β2R assessed by immunofluorescence co-localization. Cell surface co-localization was confirmed by double-label immunofluorescence studies conducted in fixed, non-permeabilized HEK-293 cells co-expressing epitope-tagged recombinant human HA-A1AR/FLAG-β1R and myc-A1AR/HA-β2R. Note that receptors remain co-localized at the cell surface as depicted by the overlay in purple (for A1AR/β1R) or yellow (A1AR/β2R). Data are representative of 2–3 independent experiments.
3.3 Ligand binding properties of A1ARs and βRs during co-expression
Saturation radioligand binding was performed with membranes isolated from cells expressing various combinations of A1, β1, and β2 receptors to determine whether co-expression influences orthosteric ligand binding affinity. As shown in Table 1, affinity of the A1AR for the agonist radioligand [125I]I-AB-MECA did not change when co-expressed with either β1 or β2 receptors. In contrast, affinity of both the β1R and the β2R for the antagonist radioligand 125I-CYP increased 1.6-fold and 3.7-fold when co-expressed with the A1AR. It is notable that the calculated Bmax for both of the radioligands was significantly reduced by ~50% in assays using membranes from cells co-expressing receptors. These data suggest that dimerization may alter characteristics of the orthosteric binding site of β-receptors, but not the A1AR.
Table 1.
Equilibrium, saturation radioligand binding data
| [125I]I-AB-MECA binding | 125I-CYP binding | |||
|---|---|---|---|---|
|
| ||||
| Kd (nM) | Bmax (fmol/mg) | Kd (nM) | Bmax (fmol/mg) | |
| A1AR | 7.33 ± 0.89 | 2011 ± 85 | - | - |
| β1R | - | - | 188 ± 9 | 15,502 ± 1212 |
| β2R | - | - | 48 ± 8 | 3320 ± 635 |
| A1AR/β1R | 7.72 ± 0.24 | 1020 ± 59* | 120 ± 15* | 7714 ± 533* |
| A1AR/β2R | 9.87 ± 2.28 | 1250 ± 202* | 13 ± 2* | 1120 ± 50* |
Data represent means ± SEM (n = 3–4). −, no specific binding.
P< 0.05, single receptor transfection vs. co-expression.
3.4 G protein-coupled signaling properties of A1AR and βRs during co-expression
The functional consequences of co-expressing adenosine and β-adrenergic receptors were first evaluated at the level of MAPK activation, since both adenosine and β-adrenergic receptors increase ERK phosphorylation. As shown in Figure 3, when cells singly expressing A1AR, β1R and β2R were treated with their selective agonists there was an increase in pERK1/2 compared to the basal levels in vehicle-treated cells. There were no differences among basal pERK1/2 levels or total ERK protein expression levels under all receptor transfection combinations (data not shown). As depicted in the representative western blot in Figure 3a and summarized in Figure 3c, the βR agonist isoproterenol (ISO, 500 nM) exerted similar effects on ERK phosphorylation in the β1R singly and A1AR/β1R dually transfected systems. In contrast, β2R receptor stimulation with ISO in A1AR/β2R cells was associated with significantly increased (2.29 ± 0.16-fold) pERK1/2 levels compared to that of the β2R singly transfected cells.
Figure 3.
Agonist-mediated activation of MAPK pathway – phosphorylation of ERK1/2. Transiently transfected HEK-293 cells individually expressing epitope-tagged recombinant A1AR, β1R, and β2R or cells co-expressing A1AR/β1R and A1AR/β2R receptors were stimulated with their respective agonists and the activation of MAPK was assessed by immunoblotting using an anti-phospho ERK1/2 antibody and quantified by densitometry. Representative immunoblots for each category are shown here: (A) cells individually expressing β1R and β2R or co-expressing A1AR/β1R and A1AR/β2R were stimulated with βR-agonist isoproterenol (ISO); (B) cells individually expressing A1AR or co-expressing A1AR/β1R and A1AR/β2R stimulated with A1AR agonist CCPA; (C) summary of all densitometry data representative of three independent experiments conducted in duplicate or triplicate (mean ± SEM). * p < 0.05, single receptor expression vs. co-expression.
In the next set of experiments, the activation of ERK by A1AR was tested using the A1AR selective agonist CCPA (250 nM). When cells singly expressing A1AR were treated with CCPA, there was a significant increase in ERK phosphorylation compared to vehicle, confirming previous observations that A1AR can activate the MAPK pathway (Figure 3b). When A1AR/β1R dually transfected cells were treated with CCPA, there was a significant, yet relatively small increase in pERK of only 1.46±0.14-fold compared to the dramatic 9.61±0.59-fold increase observed in ERK phosphorylation in A1AR/β2R dually transfected cells (Figure 3c). Taken together, these data suggest that dimerization with A1AR alters the ability of β2R to promote MAPK activation but not that of β1R, whereas activation of MAPK by A1AR is significantly altered when dimerization occurs with both βR subtypes.
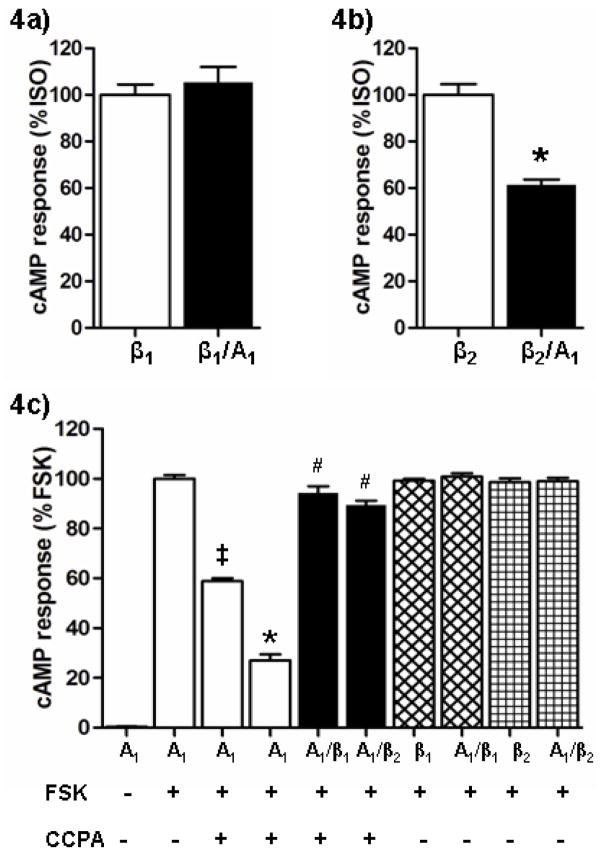
The effect of receptor co-expression on Gs protein-coupled activation of adenylyl cyclase and cAMP production was also examined. As expected, a significant increase in cAMP production was observed upon ISO (5 nM) stimulation in cells expressing β1 or β2Rs alone. Basal cAMP levels in these cells ranged from 4.8–6.1 pmol/mg protein and increased to 2000–3000 pmol/mg protein in response to ISO; data were normalized to maximal ISO response in cells singly transfected with each receptor subtype. As shown in Figure 4a, there was no significant increase in cAMP levels in response to ISO in cells dually expressing A1AR and β1R. However, the level of cAMP production in response to ISO was markedly less in cells co-expressing A1AR and β2Rs. In these cells, the cAMP concentration that was achieved following ISO stimulation was decreased by ~40% in comparison to the response seen in cells expressing the β2R alone (Figure 4b). These data suggest that A1AR/β2R heterodimer formation appears to modulate G protein coupling leading to cAMP formation, but this appears not to be the case with the A1AR/β1R heterodimer.
Figure 4.
Agonist-mediated generation of cAMP. HEK-293 cells individually expressing A1AR, β1R, and β2R or cells co-expressing A1AR/β1R and A1AR/β2R receptors were stimulated with their respective agonists and the intracellular cAMP levels were measured using a competitive cAMP ELISA kit. (A) β1R stimulated with 5 nM isoproterenol (ISO) and data are represented as % change from maximal stimulation resulting from single β1R expression. (B) β2R stimulated with 5 nM ISO and data are represented as % change from maximal stimulation resulting from single β2R expression. *P<0.05, β2R vs. A1AR/β2R expression. (C) For A1AR, the agonist CCPA (‡ 0.1 nM CCPA and 1 nM CCPA for all other experiments) was used in conjunction with forskolin (FSK) to determine the concentration-dependent reduction of maximal FSK (10 μM)-stimulated cAMP. Data are normalized to maximal cAMP response generated by FSK treatment in cells singly expressing A1AR. ‡P<0.0001, A1AR (FSK only) vs. A1AR (FSK + 0.1 nM CCPA); *P<0.0001, A1AR (FSK only) vs. A1AR (FSK + 1 nM CCPA); # P < 0.0001 for A1AR (FSK+1 nM CCPA) vs. A1AR/β1R and A1AR/β2R (FSK + 1 nM CCPA). For all cAMP experiments, data shown represent average results (mean ± SEM) from 3 independent experiments performed in duplicate or triplicate.
The A1AR couples to the inhibitory G-protein (Gi) to inhibit the production of cAMP, thus the effect of co-expressing this receptor with the β-receptors was assessed by determining the effects of the A1 agonist CCPA on forskolin (FSK)-induced increases in cAMP production. There were no differences in basal cAMP levels in the different groups of transfected cells (range: 4.9–6.4 pmol/mg protein), nor were there differences in responses to FSK (10 μM), which increased cAMP to ~1200 pmol/mg protein in all groups. As shown in Figure 4c, when cells singly expressing the A1AR were simultaneously treated with CCPA and FSK, cAMP production was significantly decreased in a concentration-dependent manner (59 ± 1% of control when co-stimulated with 0.1 nM CCPA and 27 ± 2% of control when stimulated with 1 nM CCPA). However the inhibitory effect of CCPA (1 nM) was completely absent in cells co-expressing A1ARs with either β1 or β2Rs. Therefore, it appears that heterodimer formation with either β receptor subtype may impede coupling of the A1AR to inhibition of adenylyl cyclase and subsequent reduction in cAMP.
3.5. Endogenous A1AR co-immunoprecipiates with endogenous β1 and β2 receptors
Since it was clear that heterologously expressed A1AR heterodimerizes with β1R and β2R, the ability of endogenously expressed A1AR to heterodimerize with βRs was also assessed using a co-immunoprecipitation approach. Using normal adult human heart tissue lysates and appropriate antibody pairs, each of the receptors was co-immunoprecipitated and the presence of additional receptor subtypes in these immunoprecipitates was assessed by Western blotting. As shown in Figure 5, both β1 and β2Rs co-immunoprecipitated with the A1AR in human heart tissue lysates. These data suggest that endogenously expressed A1AR is also capable of forming stable heterodimeric complexes with β1 and β2Rs.
Figure 5.

Heterodimerization of endogenously expressed A1AR with β1R and β2R Co-immunoprecipitation of endogenous A1AR, β1R, and β2R was conducted with normal adult human heart tissue lysate using antibodies specific to each receptor. Receptor heterodimers were captured and detected as described in Materials and Methods. L, lysate; I, immunoprecipitate; IP, immunoprecipitation; IB, immunoblot; MW, molecular weight.
4. DISCUSSION
G protein-coupled receptors from distinct families are ubiquitously co-expressed in numerous tissue types and have been shown to interact with one another at various levels including receptor, G protein, and downstream signal transduction pathways [1–3]. There is extensive evidence that the A1AR and both β1-and β2-adrenergic receptors can form heterodimers with multiple different GPCRs. The present findings describe the first report that the A1AR can heterodimerize with members of the β-adrenergic receptor family. Using a heterologous transient transfection system with HEK-293 cells, our results suggest that co-expression of A1ARs with β1- or β2-adrenergic receptors resulted in the formation of functional heterodimers with altered pharmacological and signaling properties. Furthermore, our data also suggest that these receptors form constitutive heterodimers in the human heart.
Our observations indicate that A1AR can form heterodimers with β1-and β2-adrenergic receptors. Co-immunoprecipitation studies revealed that the A1AR forms stable heteromeric complexes only when the different receptors were co-expressed in the same cell. The inability to co-immunoprecipitate such receptor complexes from mixtures of cells singly expressing these receptors indicate that the observed heterodimerization is not due to aggregation artifacts induced during detergent solubilization. In fact, preliminary data obtained with lysates prepared from different detergents (such as NP-40, β-maltoside, digitonin, C12E8, Brij-35, and CHAPS) yielded the same observations, confirming that these heteromers are not detergent-induced aggregates. Furthermore, our observations are not due to spurious disulfide bond formation since these complexes were stable in the presence of high concentrations of iodoacetamide during cell lysis and co-immunoprecipitation procedures. These dimers were detected under basal conditions in the absence of exogenous receptor ligands. Since all cells, including HEK-293 cells, release some adenosine into the extracellular space, as a byproduct of ATP catabolism, it is possible that dimer formation was dependent on the presence of endogenous adenosine which activates the A1AR. However, preliminary studies indicated that the inclusion of adenosine deaminase (which catabolizes adenosine to inosine, which is not an agonist for the A1AR) had no effect on co-immunoprecipitation patterns. Thus, the ability to co-immunoprecipitate heterodimers in the presence and absence of adenosine deaminase suggests that this dimerization is constitutive.
In order to further validate the specificity of interaction between A1AR and the βR subtypes, co-immunoprecipitation was conducted using cells in which Gi-coupled A1AR was substituted with the only other Gi-coupled AR, the adenosine A3 receptor (A3AR), or another Gi-coupled receptor from a completely different family, muscarinic M2 receptor (M2MR). M2MR was chosen as a suitable candidate because is receptor attenuates the cardiac β-adrenergic contractile response in much the same way A1AR does [20]. No heterodimerization was observed between A3AR/β1R or M2MR/β1R, and these results further confirm that the observed heterodimeric interactions are specific to A1AR and the βR subtypes and not the result of possible overexpression or aggregation artifacts.
Double-label immunofluorescence data indicated that these receptors co-localize at the plasma membrane. Although co-localization does not confirm heterodimerization, assays designed to evaluate receptor-mediated cellular signaling confirmed that these complexes likely exist as dimeric cell surface receptors (see results below). For each receptor combination, radioligand binding data indicated that a 50% reduction in receptor density was observed when the receptors were co-expressed. It is possible that dimer formation may influence surface expression or the turnover rate of the receptors, but a more likely explanation is that these cells are operating at maximal capacity in this overexpression system, and cannot maintain expression of both receptor subtypes when they are co-transfected with two cDNA constructs. It has been widely reported that most GPCR dimerization occurs at the ER, during receptor biosynthesis. The decreased receptor expressions in the co-transfected cells did not seem to interfere with receptor activity since significant functional alterations (including increased functional activity) occurred in co-transfected cells even with lower receptor density compared to the singly transfected cells.
Formation of these heterodimers appeared to influence A1AR signaling. A1AR-mediated ERK activation was not significantly altered when it was co-expressed with β1Rs, but a prominent change occurred when it was expressed with the β2R where pERK levels increased 9-fold. Thus, these data suggest that dimer formation increased the efficacy of the A1AR to couple to ERK signaling. In contrast, the ability of the A1AR to inhibit forskolin-induced cAMP production was blocked in both A1AR/β1R and A1AR/β2R co-transfected cells. These differences do not appear to be due to altered A1AR binding properties (Table 1), nor do they appear to be an artifact of co-transfection since the responses to forskolin were comparable in the single and co-transfection groups. Formation of GPCR heterodimers, including those with both A1AR and βR subtypes, has been reported to alter normal G protein coupling exhibited by the monomeric form of the receptor [21–23]. It is possible that the inter-and intra-molecular interactions that occur in the formation of the A1AR/β1R and A1AR/β2R dimers hinder proper coupling of the A1AR to Gi proteins, as has been suggested for other heterodimers [24–26]. Alternatively, it is possible that the A1AR transactivates the β1R and β2R receptors in the heterodimers rendering the heterodimers Gs protein-coupled during A1AR agonism. The lack of correlation between changes in MAPK activation and cAMP production may be explained by G protein independent MAPK activation mechanisms such as β-arrestin mediated MAPK activation, which has been observed with β2 and A1ARs [27, 28], and it is possible that this pathway may also be altered due to dimerization.
Co-expression of the A1AR exerted subtype specific effects on β1R and β2R ligand binding affinities and function. The binding affinity (Kd) of the β1R was increased 1.6-fold when co-expressed with the A1AR; this modest change in binding affinity was associated with only minor changes in ERK phosphorylation, and no change in the cAMP response. Thus, it appears that the β1R undergoes limited pharmacological and functional alterations when it dimerizes with the A1AR. This could be due to the fact that β1R expression was ~ 7-fold greater than that achieved with the A1AR in co-transfected cells. In contrast the β2R, which showed a similar level of expression as compared to the A1AR, exhibited almost a 4-fold increase in agonist binding affinity when co-expressed with the A1AR. The functional data indicate a 2-fold increase in ERK phosphorylation, but a ~40% reduction in the β2R-mediated cAMP production in response to ISO when the β2R was co-expressed with the A1AR. It is possible that the β2R operates primarily via a Gs-coupled mechanism when expressed alone (consistent with large increases in cAMP) and switches to a Gi-coupled system when it constitutively dimerizes with the A1AR (consistent with a decrease in cAMP production). As described above, GPCR heterodimerization can be associated with changes in preferences for specific G proteins, and there is evidence that the β2R may switch G protein coupling from Gs to Gi [28]. Furthermore, it is possible that the Gs-coupling efficiency for the β2R is altered when it is co-expressed with A1AR due to changes that occur in the βγ-subunit composition, which has been shown to contribute directly to the coupling specificity of β2R to Gs [30].
Our present observations may have physiological relevance for cell types in which these two receptor types are co-expressed, particularly the cardiovascular system. All three of these receptors are expressed in ventricular cardiomyocytes, where the A1AR is known to exert profound anti-adrenergic effects. Stimulation of the A1AR in these cells exerts essentially no direct effects on intracellular calcium, cAMP, or contractility, but blunts βR induced increases in these parameters [15–17]. Although it is generally thought that the A1AR anti-adrenergic effect is due to reductions in cAMP, previous reports indicated that high affinity agonist binding of the βR and Gs-protein cycling are significantly reduced in rat myocardial membranes treated with an A1AR agonist [31, 32]. Our findings suggest that A1AR dimerization with β1 and/or β2Rs may play a role in the anti-adrenergic effect mediated by adenosine and A1AR agonists. In this regard, our co-immunoprecipitation data using human heart lysates suggest that endogenous β1-and β2R can form constitutive heterodimers with endogenous A1AR, implicating possible functional ramifications in vivo in endogenous tissue. Although there is no direct evidence to support this hypothesis in cardiac tissue, there are several reports suggesting that receptor heterodimerization does modulate βR-induced increases in contractility. There is evidence that β1R/β2R heterodimerization in cardiac ventricular myocytes modulates βR-induced positive inotropy [5]. In addition, pharmacological blockade of the A1AR and gene deletion of the A1AR may both potentiate βR-induced increases in cardiac contractility [33, 34].
Given the importance of both adenosine and β-adrenergic receptor systems in the modulation of cardiac contractility and response to stress, receptor heterodimerization is likely to have significant functional ramifications, particularly under pathophysiological conditions in which receptor expression levels are altered. The level of expression and/or function of A1AR and βR receptor subtypes have been reported to be altered in heart failure [4, 35, 36]. Changes in activity even in only one of these receptor subtypes and the subsequent heteromeric interactions may alter the physiological effects of adenosine and sympathetic system activation. These studies may have significant potential therapeutic impact as well. It is estimated that ~40% of drugs on the market today target GPCRs, and the results of receptor heterodimerization studies, such as our present findings, suggest that targeting only one receptor system may have significant effects on another GPCR. The development of more selective drugs that can exert dual interactions with two different GPCRs that dimerize may prove to be more beneficial than those drugs that target only one receptor at a time. For example, the cardiac angiotensin II receptor and β1 adrenergic receptor form functional heterodimers and a single antagonist can dually inhibit both receptor functions [37]. The development of agonists capable of modulating the properties of heterodimer units would contribute to more efficient and selective control of signaling cascades. Our observations may elucidate a novel signaling pathway that could potentially be targeted and manipulated for therapeutic use.
5. CONCLUSION
We report here for the first time that human A1AR forms functional heterodimers with human β1R and β2R where heterodimerization leads to altered receptor pharmacology, functional coupling, and receptor-mediated intracellular signaling. Unique and differential cross talk between such receptors may significantly modulate the function in tissues where these are co-expressed. Such novel and differential molecular interactions between important receptor families also offer the opportunity to fine-tune crucial signaling responses and development of more specific therapeutic interventions.
HIGHLIGHTS.
Human adenosine A1 receptors heterodimerize with β1 and β2 adrenergic receptors.
This causes alterations in receptor pharmacology, coupling, and downstream signaling.
A1, β1, β2 heterodimerization creates novel and differential molecular interactions.
Such cross-talk leads to physiological consequences and represent new drug targets.
Acknowledgments
This study was supported by grants from NIH/NHLBI to R.D. Lasley (R01HL-066132) and J.A. Auchampach (R01HL-077707).
ABBREVIATIONS
- GPCR
G protein-coupled receptors
- A1AR
A1 adenosine receptor
- β1R & β2R
β-adrenergic receptor subtypes
- HEK-293
human embryonic kidney cells
- IP
immunoprecipitation
- IB
immunoblotting
- IF
immunofluorescence
- cAMP
cyclic adenosine monophosphate
- pERK
phosphorylated extracellular signal-regulated kinase
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Milligan G. British Journal of Pharmacology. 2009;158:5. doi: 10.1111/j.1476-5381.2009.00169.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Minneman KP. Biochemical Pharmacology. 2007;73:1043. doi: 10.1016/j.bcp.2006.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lakshmi AD. Trends in Pharmacological Sciences. 2001;22:532. doi: 10.1016/s0165-6147(00)01799-5. [DOI] [PubMed] [Google Scholar]
- 4.Brodde OE. Pharmacological Reviews. 1991;43:203. [PubMed] [Google Scholar]
- 5.Zhu WZ, Chakir K, Zhang S, Yang D, Lavoie C, Bouvier M, Hébert TE, Lakatta EG, Cheng H, Xiao RP. Circulation Research. 2005;97:244. doi: 10.1161/01.RES.0000176764.38934.86. [DOI] [PubMed] [Google Scholar]
- 6.Ciruela F, Casadó V, Rodrigues RJ, Luján R, Burgueño J, Canals M, Borycz J, Rebola N, Goldberg SR, Mallol J, Cortes A, Canela EI, Loepz-Gimenez JF, Milligan G, Lluis C, Cunha RA, Ferre S, Franco R. The Journal of Neuroscience. 2006;26:2080. doi: 10.1523/JNEUROSCI.3574-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ciruela F, Escriche M, Burgueño J, Angulo E, Casadó V, Soloviev MM, Canela EI, Mallol J, Chan WY, Lluis C, McIlhinney RA, Franco R. Journal of Biological Chemistry. 2001;276:18345. doi: 10.1074/jbc.M006960200. [DOI] [PubMed] [Google Scholar]
- 8.Ginés S, Hillion J, Torvinen M, Le Crom S, Casadó V, Canela EI, Rondin S, Lew JY, Watson S, Zoli M, Agnati LF, Verniera P, Lluis C, Ferre S, Fuxe K, Franco R. Proceedings of the National Academy of Sciences. 2000;97:8606. doi: 10.1073/pnas.150241097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xu J, He J, Castleberry AM, Balasubramanian S, Lau AG, Hall RA. Journal of Biological Chemistry. 2003;278:10770. doi: 10.1074/jbc.M207968200. [DOI] [PubMed] [Google Scholar]
- 10.Jordan BA, Gomes I, Nivarthi R, Devi LA. Proceedings of the National Academy of Sciences. 2001;98:343. doi: 10.1073/pnas.011384898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Somvanshi RK, War SA, Chaudhari N, Qiu X, Kumar U. Cellular Signalling. 2011;23:794. doi: 10.1016/j.cellsig.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 12.Somvanshi RK, Chaudhari N, Qiu X, Kumar U. Journal of Molecular Signaling. 2011;6:9. doi: 10.1186/1750-2187-6-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lavoie C, Mercier JF, Salahpour A, Umapathy D, Breit A, Villeneuve LR, Zhu WZ, Xiao RP, Lakatta EG, Bouvier M, Hébert TE. Journal of Biological Chemistry. 2002;277:35402. doi: 10.1074/jbc.M204163200. [DOI] [PubMed] [Google Scholar]
- 14.Zhu WZ, Chakir K, Zhang S, Yang D, Lavoie C, Bouvier M, Hébert TE, Lakatta EG, Cheng H, Xiao RP. Circulation research. 2005;97:244. doi: 10.1161/01.RES.0000176764.38934.86. [DOI] [PubMed] [Google Scholar]
- 15.Song Y, Belardinelli L. The American Journal of Physiology. 1996;271:C1233. doi: 10.1152/ajpcell.1996.271.4.C1233. [DOI] [PubMed] [Google Scholar]
- 16.Fenton RA, Moore ED, Fay FS, Dobson JG., Jr American Journal of Physiology. 1991;261:C1107. doi: 10.1152/ajpcell.1991.261.6.C1107. [DOI] [PubMed] [Google Scholar]
- 17.Narayan P, Mentzer RM, Jr, Lasley RD. American Journal of Physiology - Heart and Circulatory Physiology. 2000;278:H1. doi: 10.1152/ajpheart.2000.278.1.H1. [DOI] [PubMed] [Google Scholar]
- 18.Auchampach JA, Jin X, Wan TC, Caughey GH, Linden J. Molecular Pharmacology. 1997;52:846. doi: 10.1124/mol.52.5.846. [DOI] [PubMed] [Google Scholar]
- 19.Yoshioka K, Saitoh O, Nakata H. Proceedings of the National Academy of Sciences. 2001;98:7617. doi: 10.1073/pnas.121587098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Inui J, Brodde OE, Schumann HJ. Naunyn-Schmiedeberg’s Archives of Pharmacology. 1982;320:152. doi: 10.1007/BF00506315. [DOI] [PubMed] [Google Scholar]
- 21.Cristóvão-Ferreira S, Navarro G, Brugarolas M, Pérez-Capote K, Vaz SH, Fattorini G, Conti F, Lluis C, Ribeiro JA, McCormick PJ, Casadó V, Franco R, Sebastião AM. The Journal of Neuroscience. 2011;31:15629. doi: 10.1523/JNEUROSCI.2526-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 22.Breit A, Lagace M, Bouvier M. Journal of Biological Chemistry. 2004;279:28756. doi: 10.1074/jbc.M313310200. [DOI] [PubMed] [Google Scholar]
- 23.Suzuki T, Namba K, Tsuga H, Nakata H. Biochemical and Biophysical Research Communications. 2006;351:559. doi: 10.1016/j.bbrc.2006.10.075. [DOI] [PubMed] [Google Scholar]
- 24.Breit A, Lagacé M, Bouvier M. Journal of Biological Chemistry. 2004;279:28756. doi: 10.1074/jbc.M313310200. [DOI] [PubMed] [Google Scholar]
- 25.Gines S, Hillion J, Torvinen M, Le Crom S, Casado V, Canela EI, Rondin S, Lew JY, Watson S, Zoli M, Agnati LF, Verniera P, Lluis C, Ferré S, Fuxe K, Franco R. Proceedings of the National Academy of Sciences. 2000;97:8606. doi: 10.1073/pnas.150241097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Smith NJ, Milligan G. Pharmacological Reviews. 2010;62:701. doi: 10.1124/pr.110.002667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jajoo S, Mukherjea D, Kumar S, Sheth S, Kaur T, Rybak LP, Ramkumar V. American Journal of Physiology - Cell Physiology. 2010;298:C56. doi: 10.1152/ajpcell.00190.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shenoy SK, Drake MT, Nelson CD, Houtz DA, Xiao K, Madabushi S, Reiter E, Premont RT, Lichtarge O, Lefkowitz RJ. Journal of Biological Chemistry. 2006;281:1261. doi: 10.1074/jbc.M506576200. [DOI] [PubMed] [Google Scholar]
- 29.Daaka Y, Luttrell LM, Lefkowitz RJ. Nature. 1997;88:88. doi: 10.1038/36362. [DOI] [PubMed] [Google Scholar]
- 30.Kühn B, Christel C, Wieland T, Schultz G, Gudermann T. Naunyn-Schmiedeberg’s Archives of Pharmacology. 2002;365:231. doi: 10.1007/s00210-001-0512-z. [DOI] [PubMed] [Google Scholar]
- 31.Fenton RA, Dobson JG. Journal of Cellular Physiology. 2007;213:785. doi: 10.1002/jcp.21149. [DOI] [PubMed] [Google Scholar]
- 32.Romano FD, Dobson JG., Jr Second Messngers Phosphoproteins. 12(988):29. [PubMed] [Google Scholar]
- 33.Dobson JG, Jr, Fenton RA, Sawmiller DR. Annals of the New York Academy of Sciences. 793(996):64. doi: 10.1111/j.1749-6632.1996.tb33505.x. [DOI] [PubMed] [Google Scholar]
- 34.McIntosh VJ, Chandrasekera PC, Lasley RD. American journal of physiology - Heart and Circulatory Physiology. 2011;301:H1127. doi: 10.1152/ajpheart.00711.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bristow MR, Ginsburg R, Umans V, Fowler M, Minobe W, Rasmussen R, Zera P, Menlove R, Shah P, Jamieson S. Circulation Research. 1986;59:297. doi: 10.1161/01.res.59.3.297. [DOI] [PubMed] [Google Scholar]
- 36.Hershberger RE, Feldman AM, Bristow MR. Circulation. 1991;83:1343. doi: 10.1161/01.cir.83.4.1343. [DOI] [PubMed] [Google Scholar]
- 37.Barki-Harrington L, Luttrell LM, Rockman HA. Circulation. 2003;108:1611. doi: 10.1161/01.CIR.0000092166.30360.78. [DOI] [PubMed] [Google Scholar]