Abstract
An fi- type of R factor for transferable chloramphenicol resistance carries the structural gene for a novel species of chloramphenicol acetyltransferase (EC 2.3.1.99). The enzyme associated with the fi- plasmid is distinct from that described for fi+ R factors. The fi+- and fi--related variants of chloramphenicol acetyltransferase were hybridized in vivo and in vitro. Both techniques yielded only a single symmetrical heteromeric species rather than the three (A3B1, A2B2, and A1B3) hybrids predicted from the tetrameric (identical monomers) structure of chloramphenicol acetyltransferase.
Keywords: episomes, enzyme evolution, antibiotic resistance, plasmids, protein subunits
Full text
PDF


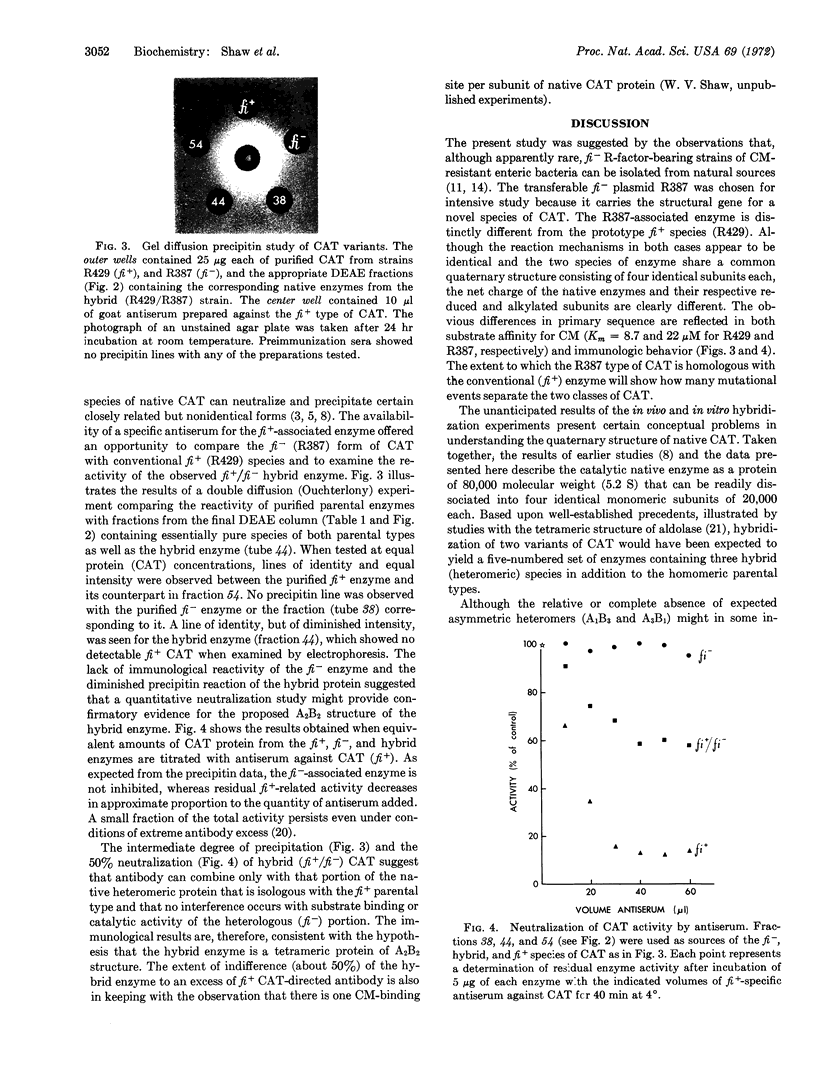

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aoki T., Egusa S., Ogata Y., Watanabe T. Detection of resistance factors in fish pathogen Aeromonas liquefaciens. J Gen Microbiol. 1971 Mar;65(3):343–349. doi: 10.1099/00221287-65-3-343. [DOI] [PubMed] [Google Scholar]
- CINADER B. Antibody to enzymes--a three-component system. Introduction: immunochemistry of enzymes. Ann N Y Acad Sci. 1963 May 8;103:495–548. doi: 10.1111/j.1749-6632.1963.tb53717.x. [DOI] [PubMed] [Google Scholar]
- Datta N., Hedges R. W. Compatibility groups among fi - R factors. Nature. 1971 Nov 26;234(5326):222–223. doi: 10.1038/234222a0. [DOI] [PubMed] [Google Scholar]
- Fish W. W., Mann K. G., Tanford C. The estimation of polypeptide chain molecular weights by gel filtration in 6 M guanidine hydrochloride. J Biol Chem. 1969 Sep 25;244(18):4989–4994. [PubMed] [Google Scholar]
- Foster T. J., Howe T. G. Recombination and complementation between R factors in Escheichia coli K 12. Genet Res. 1971 Dec;18(3):287–297. doi: 10.1017/s0016672300012696. [DOI] [PubMed] [Google Scholar]
- Hashimoto H., Hirota Y. Gene recombination and segregation of resistance factor R in Escherichia coli. J Bacteriol. 1966 Jan;91(1):51–62. doi: 10.1128/jb.91.1.51-62.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lew K. K., Roth J. R. Genetic approaches to determination of enzyme quaternary structure. Biochemistry. 1971 Jan 19;10(2):204–207. doi: 10.1021/bi00778a002. [DOI] [PubMed] [Google Scholar]
- Mise K., Suzuki Y. Temperature-sensitive chloramphenicol acetyltransferase from Escherichia coli carrying mutant R factors. J Bacteriol. 1968 Jun;95(6):2124–2130. doi: 10.1128/jb.95.6.2124-2130.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penhoet E. E., Rutter W. J. Catalytic and immunochemical properties of homomeric and heteromeric combinations of aldolase subunits. J Biol Chem. 1971 Jan 25;246(2):318–323. [PubMed] [Google Scholar]
- Shaw W. V., Bentley D. W., Sands L. Mechanism of Chloramphenicol Resistance in Staphylococcus epidermidis. J Bacteriol. 1970 Dec;104(3):1095–1105. doi: 10.1128/jb.104.3.1095-1105.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw W. V., Brodsky R. F. Characterization of chloramphenicol acetyltransferase from chloramphenicol-resistant Staphylococcus aureus. J Bacteriol. 1968 Jan;95(1):28–36. doi: 10.1128/jb.95.1.28-36.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw W. V. The enzymatic acetylation of chloramphenicol by extracts of R factor-resistant Escherichia coli. J Biol Chem. 1967 Feb 25;242(4):687–693. [PubMed] [Google Scholar]
- Shaw W. V. The problems of drug-resistant pathogenic bacteria. Comparative enzymology of chloramphenicol resistance. Ann N Y Acad Sci. 1971 Jun 11;182:234–242. doi: 10.1111/j.1749-6632.1971.tb30660.x. [DOI] [PubMed] [Google Scholar]
- Suzuki Y., Okamoto S. The enzymatic acetylation of chloramphenicol by the multiple drug-resistant Escherichia coli carrying R factor. J Biol Chem. 1967 Oct 25;242(20):4722–4730. [PubMed] [Google Scholar]
- Vesterberg O., Svensson H. Isoelectric fractionation, analysis, and characterization of ampholytes in natural pH gradients. IV. Further studies on the resolving power in connection with separation of myoglobins. Acta Chem Scand. 1966;20(3):820–834. doi: 10.3891/acta.chem.scand.20-0820. [DOI] [PubMed] [Google Scholar]
- WATANABE T., NISHIDA H., OGATA C., ARAI T., SATO S. EPISOME-MEDIATED TRANSFER OF DRUG RESISTANCE IN ENTEROBACTERIACEAE. VII. TWO TYPES OF NATURALLY OCCURRING R FACTORS. J Bacteriol. 1964 Sep;88:716–726. doi: 10.1128/jb.88.3.716-726.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe T., Furuse C., Sakaizumi S. Transduction of various R factors by phage P1 in Escherichia coli and by phage P22 in Salmonella typhimurium. J Bacteriol. 1968 Nov;96(5):1791–1795. doi: 10.1128/jb.96.5.1791-1795.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Winshell E., Shaw W. V. Kinetics of induction and purification of chloramphenicol acetyltransferase from chloramphenicol-resistant Staphylococcus aureus. J Bacteriol. 1969 Jun;98(3):1248–1257. doi: 10.1128/jb.98.3.1248-1257.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witchitz J. L., Chabbert Y. A. High level transferable resistance to gentamicin. J Antibiot (Tokyo) 1971 Feb;24(2):137–139. doi: 10.7164/antibiotics.24.137. [DOI] [PubMed] [Google Scholar]





