Abstract
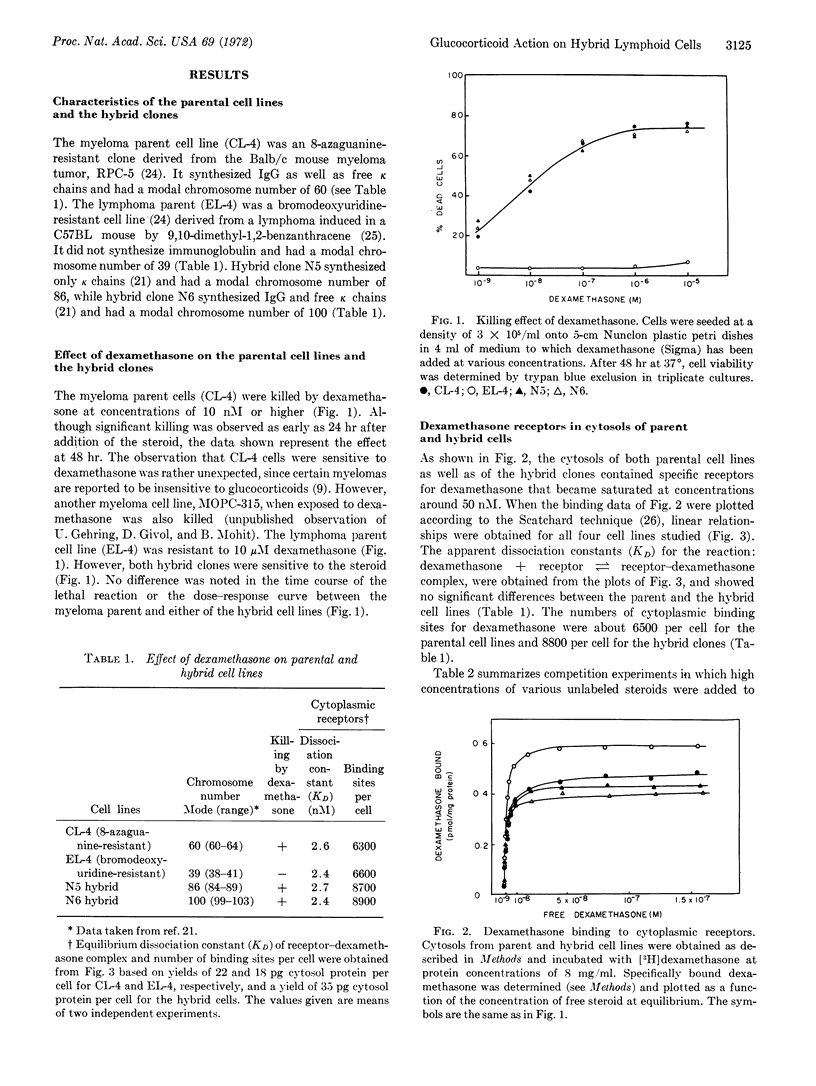
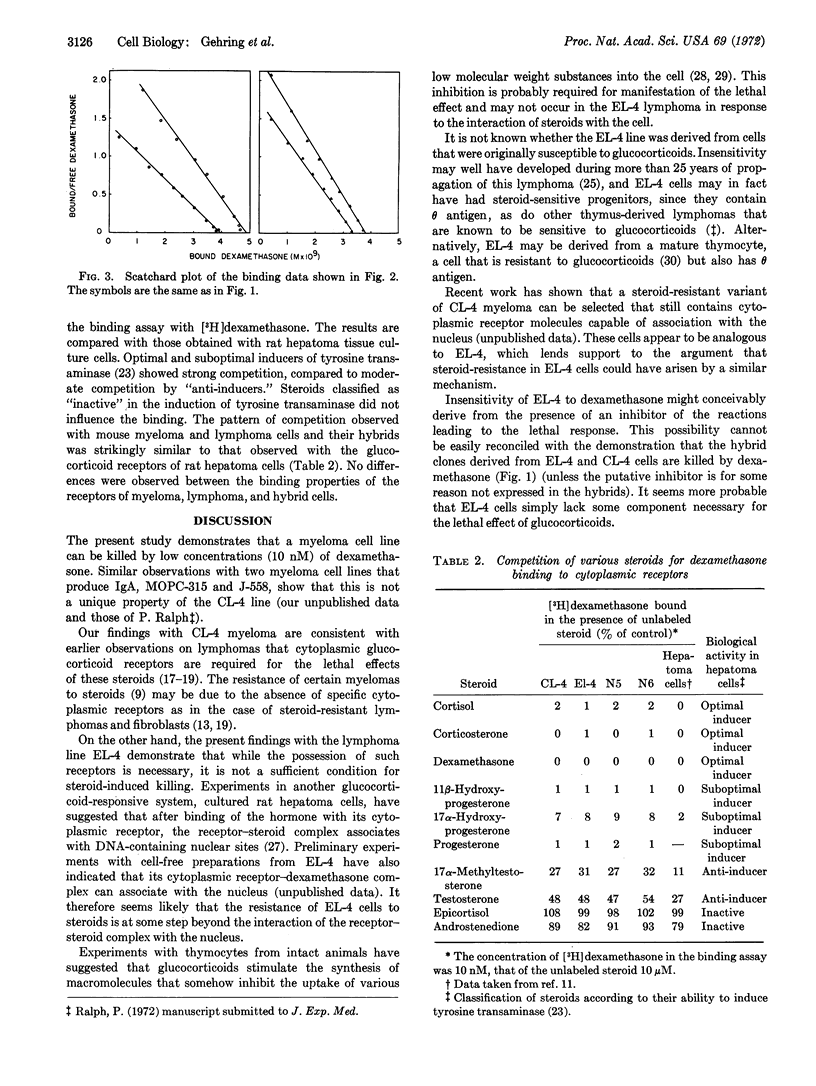
Cells of a cloned myeloma line from a Balb/c mouse contain specific cytoplasmic glucocorticoid receptors and are killed by dexamethasone. Cells of a lymphoma line (from mouse strain C57BL) also contain specific glucocorticoid receptors but are resistant to the steroid. Cells of two hybrid clones with widely differing chromosome numbers, derived by fusion between the resistant lymphoma and the sensitive myeloma, contain specific glucocorticoid receptors with similar binding properties as the parental receptors and are killed by dexamethasone. Since the lethal effect of the steroid is expressed in the hybrid cells, failure of the parent lymphoma line to be affected by dexamethasone is probably not due to an inhibitor of the lethal reaction.
Keywords: steroid-induced killing, dexamethasone, cytoplasmic receptors
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baxter J. D., Harris A. W., Tomkins G. M., Cohn M. Glucocorticoid receptors in lymphoma cells in culture: relationship to glucocorticoid killing activity. Science. 1971 Jan 15;171(3967):189–191. doi: 10.1126/science.171.3967.189. [DOI] [PubMed] [Google Scholar]
- Baxter J. D., Rousseau G. G., Benson M. C., Garcea R. L., Ito J., Tomkins G. M. Role of DNA and specific cytoplasmic receptors in glucocorticoid action. Proc Natl Acad Sci U S A. 1972 Jul;69(7):1892–1896. doi: 10.1073/pnas.69.7.1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter J. D., Tomkins G. M. Specific cytoplasmic glucocorticoid hormone receptors in hepatoma tissue culture cells. Proc Natl Acad Sci U S A. 1971 May;68(5):932–937. doi: 10.1073/pnas.68.5.932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton A. F., Storr J. M., Dunn W. L. Cytolytic action of corticosteroids on thymus and lymphoma cells in vitro. Can J Biochem. 1967 Feb;45(2):289–297. doi: 10.1139/o67-032. [DOI] [PubMed] [Google Scholar]
- Cohen J. J., Claman H. N. Thymus-marrow immunocompetence. V. Hydrocortisone-resistant cells and processes in the hemolytic antibody response of mice. J Exp Med. 1971 May 1;133(5):1026–1034. doi: 10.1084/jem.133.5.1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GORER P. A. Studies in antibody response of mice to tumour inoculation. Br J Cancer. 1950 Dec;4(4):372–379. doi: 10.1038/bjc.1950.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackney J. F., Gross S. R., Aronow L., Pratt W. B. Specific glucocorticoid-binding macromolecules from mouse fibroblasts growing in vitro. A possible steroid receptor for growth inhibition. Mol Pharmacol. 1970 Sep;6(5):500–512. [PubMed] [Google Scholar]
- Harris A. W. Differentiated functions expressed by cultured mouse lymphoma cells. I. Specificity and kinetics of cell responses to corticosteroids. Exp Cell Res. 1970 Jun;60(3):341–353. doi: 10.1016/0014-4827(70)90527-6. [DOI] [PubMed] [Google Scholar]
- Hollander N., Chiu Y. W. In vitro binding of cortisol-1,2-3H by a substance in the supernatant fraction of P1798 mouse lymphosarcoma. Biochem Biophys Res Commun. 1966 Nov 11;25(3):291–297. doi: 10.1016/0006-291x(66)90774-1. [DOI] [PubMed] [Google Scholar]
- Horibata K., Harris A. W. Mouse myelomas and lymphomas in culture. Exp Cell Res. 1970 Apr;60(1):61–77. doi: 10.1016/0014-4827(70)90489-1. [DOI] [PubMed] [Google Scholar]
- Kirkpatrick A. F., Milholland R. J., Rosen F. Stereospecific glucocorticoid binding to subcellular fractions of the sensitive and resistant lymphosarcoma P1798. Nat New Biol. 1971 Aug;232(33):216–218. doi: 10.1038/newbio232216a0. [DOI] [PubMed] [Google Scholar]
- LAMPKIN J. M., POTTER M. Response to cortisone and development of cortisone resistance in a cortisone-sensitive lymphosarcoma of the mouse. J Natl Cancer Inst. 1958 Jun;20(6):1091–1111. doi: 10.1093/jnci/20.6.1091. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Makman M. H., Dvorkin B., White A. Evidence for induction by cortisol in vitro of a protein inhibitor of transport and phosphorylation processes in rat thymocytes. Proc Natl Acad Sci U S A. 1971 Jun;68(6):1269–1273. doi: 10.1073/pnas.68.6.1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohit B., Fan K. Hybrid cell line from a cloned immunoglobulin-producing mouse myeloma and a nonproducing mouse lymphoma. Science. 1971 Jan 8;171(3966):75–77. doi: 10.1126/science.171.3966.75. [DOI] [PubMed] [Google Scholar]
- Mohit B. Immunoglobulin G and free kapa-chain synthesis in different clones of a hybrid cell line. Proc Natl Acad Sci U S A. 1971 Dec;68(12):3045–3048. doi: 10.1073/pnas.68.12.3045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munck A. Glucocorticoid inhibition of glucose uptake by peripheral tissues: old and new evidence, molecular mechanisms, and physiological significance. Perspect Biol Med. 1971 Winter;14(2):265–269. doi: 10.1353/pbm.1971.0002. [DOI] [PubMed] [Google Scholar]
- Pratt W. B., Aronow L. The effect of glucocorticoids on protein and nucleic acid synthesis in mouse fibroblasts growing in vitro. J Biol Chem. 1966 Nov 25;241(22):5244–5250. [PubMed] [Google Scholar]
- RUHMANN A. G., BERLINER D. L. EFFECT OF STEROIDS ON GROWTH OF MOUSE FIBROBLASTS IN VITRO. Endocrinology. 1965 May;76:916–927. doi: 10.1210/endo-76-5-916. [DOI] [PubMed] [Google Scholar]
- Rosenau W., Baxter J. D., Rousseau G. G., Tomkins G. M. Mechanism of resistance to steroids: glucocorticoid receptor defect in lymphoma cells. Nat New Biol. 1972 May 3;237(70):20–24. doi: 10.1038/newbio237020a0. [DOI] [PubMed] [Google Scholar]
- Rousseau G. G., Baxter J. D., Tomkins G. M. Glucocorticoid receptors: relations between steroid binding and biological effects. J Mol Biol. 1972 Jun 14;67(1):99–115. doi: 10.1016/0022-2836(72)90389-0. [DOI] [PubMed] [Google Scholar]
- Samuels H. H., Tomkins G. M. Relation of steroid structure to enzyme induction in hepatoma tissue culture cells. J Mol Biol. 1970 Aug 28;52(1):57–74. doi: 10.1016/0022-2836(70)90177-4. [DOI] [PubMed] [Google Scholar]
- Schaumburg B. P., Bojesen E. Specificity and thermodynamic properties of the corticosteroid binding to a receptor of rat thymocytes in vitro. Biochim Biophys Acta. 1968 Nov 12;170(1):172–188. doi: 10.1016/0304-4165(68)90171-2. [DOI] [PubMed] [Google Scholar]
- Thompson E. B., Tomkins G. M., Curran J. F. Induction of tyrosine alpha-ketoglutarate transaminase by steroid hormones in a newly established tissue culture cell line. Proc Natl Acad Sci U S A. 1966 Jul;56(1):296–303. doi: 10.1073/pnas.56.1.296. [DOI] [PMC free article] [PubMed] [Google Scholar]


