Abstract
The interaction of CD4+ T cells with major histocompatibility complex class II (MHCII)-expressing hematopoietic antigen presenting cells (APCs) plays a critical role in both the generation of protective immune responses and maintenance of tolerance in the lung. The functional significance of MHCII expression by non-hematopoietic stromal cells, however, has not been defined in vivo. Using a novel mouse model of orthotopic left lung transplantation we demonstrate that selective elimination of MHCII expression on non-hematopoietic cells leads to an inflammatory response due to reduced peripheral generation of regulatory CD4+ T cells. Absence of MHCII expression on non-hematopoietic cells also inhibits local growth of metastatic pulmonary tumor. These findings indicate that non-hematopoietic cells play a previously unrecognized role in downregulating inflammatory responses in non-lymphoid tissues.
Introduction
Antigen uptake by lung-resident hematopoietic cells with migratory capacity and antigen presentation in draining lymph nodes is a key mechanism for pulmonary immune homeostasis (1). Although non-hematopoietic cells are an integral component of the lung and can express MHCII in vitro (2), their role in CD4+ T lymphocyte-restricted antigen presentation is controversial. Some have reported that MHCII-expressing non-hematopoietic cells can initiate proliferation of CD4+ T cell clones (3), while others have suggested that CD4+ T cell-restricted antigen presentation by this cell population is a neutral encounter (4). We and others have provided in vitro data suggesting a tolerogenic role for this cell population (2, 5). Specifically, we demonstrated that CD4+Foxp3+ T cells can be generated from CD4+Foxp3− T cells after alloantigen presentation by endothelium in vitro (2). One study attempted to address the controversy of in vitro observations using an in vivo model of influenza strain hemagglutinin expression restricted to airway epithelial cells (6). The physiologic consequence of this approach is difficult to define due to multiple lung-resident hematopoietic cells that have the capacity to process and present exogenous antigen derived from epithelial cells. Here, we use an alternative and novel approach to selectively eliminate MHCII expression only on pulmonary non-hematopoietic cells while preserving MHCII expression on bone marrow-derived lung-resident APCs (7). The absence of non-hematopoietic MHCII resulted in a decrease in the peripheral generation of CD4+Foxp3+ regulatory T cells and a local inflammatory response. Furthermore, in a clinically relevant model of pulmonary metastases the elimination of MHCII on non-hematopoietic cells led to attenuation in tumor growth.
Materials and Methods
Animals
Male C57BL/6 (B6) and OT-II mice, TCR transgenic for ovalbumin323–339, were purchased from Jackson Laboratory (Bar Harbor, Maine) while B6/SJL CD45.1+ and B6/129 F1 mice were obtained from Taconic (Hudson, NY). MHC Class II-deficient mice on a B6 background(B6II−) were originally developed by introducing a loss of functional mutation into the Abβ gene in animals that harbor a natural deletion in their Ebα gene (8). B6Foxp3GFP mice were provided by A.S. Rudensky (Memorial Sloan Kettering Cancer Center, NY)(9). Lung and bone marrow transplants were performed as previously described (7, 10). Unless specifically identified all mice were sacrificed two weeks after transplantation. For tumor studies mice were injected intravenously with 2.5×105 B16 murine melanoma (ATCC, Manassas, VA) one week after transplantation and euthanized 21 days later.
Flow Cytometry
Tissues were processed as previously described (11) and stained with anti-I-A/I-E (clone M5/114.15.2), anti-CD90.2 (clone 30-H12), anti-CD4 (clone RM4–5), anti-CD8 (clone 53–6.7), anti-CD62L (clone MEL-14), anti-CD44 (clone IM7), anti-GITR (DTA-1), anti-CD45.1 (clone A20), anti-CD45.2 (clone 104) and isotype controls (BD Biosciences, Franklin Lakes, NJ). Human lung tissue was stained with anti-CD45 (clone HI30), anti-DR (clone LN3) and isotype controls (eBioscience, San Diego, CA). Intracellular staining was performed with anti-FoxP3 (clone FJK-16s) (eBioscience, San Diego, CA), anti-CD74 (In-1) and isotype controls as previously described (12). H-2Kb/SVYDFFVWL pentamer was used for tyrosinase-related protein-2 staining (ProImmune Inc., Bradenton, FL). Flow cytometric analysis was performed by gating on live events with strict doublet discrimination. For ex vivo analysis of DQ-ovalbumin processing lungs were digested and incubated with DQ-ovalbumin in vitro.
Adoptive Transfer Experiments
CD4+ T cells were purified either by magnetic bead separation (Miltenyi Biotec, Auburn, CA) or flow cytometric sorting. CD4+Foxp3− cells were sorted based on GFP expression in B6Foxp3GFP mice or CD25 and GITR in OT-II mice. Cells were injected one week after transplantation and analyzed a week later.
Statistical Analysis
Analyses of the experimental and control groups were performed by Student's t test, expressed as mean ± SEM and considered significantly different if p<0.05.
Results and Discussion
Mouse Lung Non-Hematopoietic Cells Express MHCII Constitutively
Similar to humans, a substantial portion of pulmonary non-hematopoietic cells, such as vascular endothelium and airway epithelium, express MHCII constitutively in the mouse (Fig. 1A,B). Furthermore, unlike the case for secondary lymphoid organs, up to 50% of all MHCII+ cells in the lung are non-hematopoietic in both mice and humans (Fig. 1C). Non-hematopoietic pulmonary cells also express the invariant chain (Fig. 1D) and are able to take up and process exogenous antigen, as evidenced by cleavage and fluorescence of DQ ovalbumin (Fig. 1E). Taken together with recent reports by our group and others that the lung provides a suitable environment for T cell activation (11, 13) these findings support the notion that CD4+ T cell-restricted antigen presentation by pulmonary non-hematopoietic cells may have functional consequences.
Figure 1.
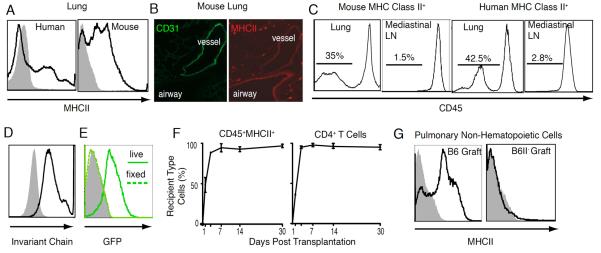
MHC Class II expression in the lung. (A) MHCII on pulmonary CD45− non-hematopoietic cells. (B) Immunostaining for CD31 (FITC) and MHC II (Texas Red) in lungs of B6 chimeras after reconstitution with B6II− hematopoietic cells. (C) CD45 expression on MHCII-positive cells in lungs and mediastinal lymph nodes. (D) Invariant chain expression by pulmonary non-hematopoietic cells (black line-antibody; shaded grey isotype). (E) DQ-ovalbumin processing and cleavage as identified by green fluorescence in live CD45−MHCII+ cells (thick green line) compared to cells fixed in 5% paraformaldehyde prior to incubation (dotted green line) or unlabeled cells (shaded grey plot). (F) Substitution of donor with recipient-derived hematopoietic APCs and CD4+ T cells in left lung grafts. (G) MHC class II expression on non-hematopoietic cells in transplanted B6 and B6II− lungs. Analysis is representative of at least four separate experiments.
While we and others have used donor organs derived from bone marrow chimeras to study CD4+ T cell allorecognition in transplant rejection (10), the use of bone marrow chimeras as hosts to study MHCII expression on non-hematopoietic cells is limited by altered thymic CD4+ T cell development (14). To evaluate lung transplantation as a model for the study of CD4+ T cell-restricted antigen presentation we engrafted left lungs of B6CD45.1 mice into B6 recipients and documented near complete substitution of donor with recipient CD4+ T cells and professional hematopoietic APCs (defined as CD45+MHCII+) within a few days of engraftment (Fig. 1F). Non-hematopoietic cells of the graft, however, remained of the donor genotype (Fig. 1G). Thus, transplantation of a B6II− donor into a wild-type B6 recipient can be used to create a “chimeric lung” consisting of MHCII-deficient non-hematopoietic cells repopulated by wild-type CD4+ T cells and MHCII-expressing hematopoietic professional APCs. Such an experimental system would result in the local disruption of CD4+ T cell-restricted antigen presentation solely by non-hematopoietic cells in the lung.
Elimination of MHCII on Pulmonary Non-Hematopoietic Cells Results in Local Inflammation
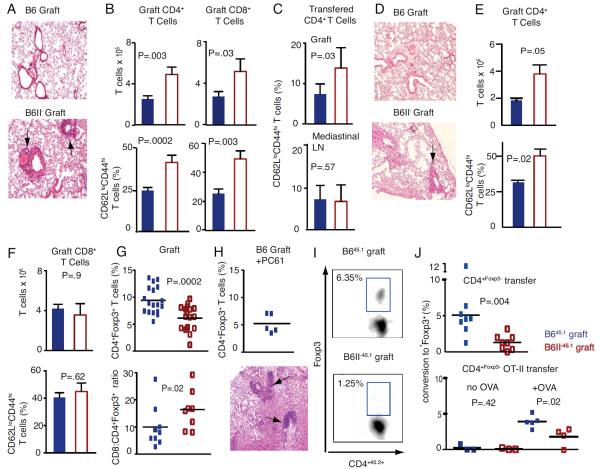
Two weeks after transplantation B6 to B6 lung grafts remain free of inflammation, but B6II− grafts demonstrate inflammatory changes characterized by perivascular and peribronchial infiltrates (Fig. 2A) with an increase in the number of CD4+ T cells and a higher proportion of CD62LloCD44hi cells consistent with an effector memory phenotype (Fig. 2B). Based on the known heterogeneity of perivascular T cell infiltrates in pulmonary inflammation, we also analyzed CD8+ T cells and demonstrated increased accumulation and activation of this cell population as well (Fig. 2B)(12). No qualitative or quantitative differences are detectable in the CD4+ T cells in the mediastinal lymph nodes or native right lungs suggesting that differences between T cells in B6 and B6II− grafts are due to their local activation (Supp. Fig. 1). Adoptive transfer of naïve B645.1 CD62LhiCD44lo CD4+ T cells demonstrates increased generation of effector memory CD62LloCD44hi CD4+ T cells in the B6II− compared to B6 grafts with no detectable differences in the mediastinal lymph nodes (Fig. 2C) or native right lungs (data not shown). To rule out the possibility that the inflammatory changes were due to an allogeneic immune response to a minor antigen in the B6II− mouse that may not be present in the B6 recipient, we performed single nucleotide polymorphism analysis of B6II− mice and determined that this mutant was 98.5% B6 origin (Supp. Fig. 2A). To further rule out the possibility that minor antigens that have co-segregated with this deletion were contributing to the inflammation we transplanted B6 and B6II− lungs into B6/B6II− F1 recipients and demonstrated inflammatory changes as well as increased T cell activation and accumulation only in B6II− grafts (Fig. 2D, E). Similar differences in T cell activation and accumulation were evident if B6/129 f1 mice were used as graft recipients (Supp. Fig. 2B). Furthermore, B6/B6II− F1 grafts did not elicit any inflammatory response when used as donors (Supp. Fig. 2C). These data provided compelling evidence that inflammatory changes evident in B6II− lung grafts were a result of MHC Class II deficiency rather than a minor antigen mismatch. To determine if the transient initial presence of hematopoietic MHCII− professional APCs in B6II− grafts contributes to the inflammatory response we used donor organs derived from bone marrow chimeras, where MHCII expression was restricted to either the hematopoietic or non-hematopoietic cells. We found increased CD4+ T cell accumulation and activation only in mice lacking MHCII on non-hematopoietic cells (Supp. Fig. 2D).
Figure 2.
Analysis of B6 and B6II− lung grafts. (A) Hematoxylin and eosin staining of perivascular and peribronchial infiltrates (arrows) in a B6II− or B6 graft (100x). (B) Absolute T cell numbers (top) and percentage of effector memory T cells (bottom) in grafts (B6=blue; B6II−=red). (C) Percentage of congenic CD62LloCD44hi CD4+ effector memory T cells generated from adoptively transferred CD62LhiCD44lo CD4+ naïve T cells in lung grafts and mediastinal lymph nodes of B6 and B6II− recipients. (D) Hematoxylin and eosin staining (100x) staining of B6 or B6II− lungs grafted into B6/B6II− F1 recipients with arrow depicting perivascular cuffing in B6II-graft. (E) Total number of CD4+ T cells and percent CD4+CD62LloCD44hi effector memory T cells in B6 and B6II− grafts transplanted into B6/B6II− F1 recipients. (F) Absolute CD8+ T cell numbers (top) and percentage of CD62LloCD44hi CD8+ T cells (bottom) in lung grafts of CD4+ T cell-depleted B6 recipients. (G) Percentages of CD4+ T cells expressing Foxp3 and CD8+: CD4+ Foxp3+ T cell ratios in B6 and B6II− grafts. (H) Percentages of CD4+ T cells expressing Foxp3 (top) and perivascular and peribronchial infiltrate (arrows) of B6 grafts two weeks after transplantation into hosts treated with 1mg PC61 i.p day −2, and 250 μg day −1 and every three days thereafter (bottom) (100x). (I) Representative plots demonstrating adoptively transferred cells with de novo expression of Foxp3. (J) Graphic representation of CD4+Foxp3GFP− cells converting to CD4+Foxp3GFP+ cells in B645.1 (blue) and B6II−45.1 (red) grafts (top) and similar data demonstrating de novo Foxp3 expression in adoptively transferred OT-II CD4+Foxp3− T cells after instillation of saline (left) or 1 mg of ovalbumin (right) intratracheally into B645.1 recipients of lung grafts (bottom). Analyses representative of at least eight mice per group except where indicated in the graph.
Treatment of recipient mice with the CD4+ T cell-depleting antibody GK1.5 prior to transplantation eliminated differences in CD8+ T cell infiltration or activation (Fig. 2F). CD8+ T cells of animals treated with control rat IgG had a similar phenotype to unmanipulated mice depicted in Fig. 2B (data not shown) and the use of CD4+ T cell knock-out recipients yielded results similar to those depicted in Fig. 2F. Collectively, these data suggest that MHCII expression on pulmonary non-hematopoietic cells plays a role in controlling T cell responses in a CD4+ T cell-dependent fashion.
Based on the finding that absence of MHC II on non-hematopoietic cells resulted in the activation of both CD4+ and CD8+ T cells, as well as our previous in vitro data demonstrating the importance of MHC II expression on vascular endothelium for the generation of CD4+Foxp3+ Tregs, we next focused on evaluating regulatory T cells in our lung grafts (2). Notably, fewer CD4+ T cells express Foxp3 in B6II− compared to B6 grafts (6.1±0.5% vs. 9.4±0.6%; P=0.009) and the CD8+:CD4+Foxp3+ T cell ratio is significantly altered (Fig. 2G). No such differences were evident in the mediastinal lymph nodes or native right lungs suggesting a local response (Supp. Fig. 3A). We did not detect differences in Treg activation markers such as GITR or CTLA4 (Supp. Fig. 3B) and Tregs isolated from B6 and B6II− lungs were equally potent in inhibiting CD3-stimulated T cell proliferation in vitro (Supp. Fig. 3C). To examine the role of Tregs in controlling pulmonary inflammation we treated B6 recipients of B6 lung grafts with PC61. Such treatment resulted in the decrease of CD4+Foxp3+ T cells in the B6 grafts to percentages comparable to those observed in untreated B6II− transplants (5.2±0.8 vs. 6.1±0.5 respectively; P=.43) (Fig. 2H vs. 2G) and was associated with inflammatory changes in B6 grafts similar to those seen in B6II− transplants (Fig. 2H vs. 2A). Thus, MHCII expression by lung non-hematopoietic cells is important in regulating the balance between regulatory and effector T cells. It is also conceivable that such inflammatory changes would be more accentuated in grafts derived from mice that lack all four of the classical MHC II genes (15).
We next set out to define a mechanism for the relative deficiency of Tregs in B6II− lungs. We were unable to detect differences in survival, proliferation or homing patterns of CD4+Foxp3+ Tregs in lungs deficient and sufficient in non-hematopoietic MHCII (Supp. Figs. 4A–C). To assess whether peripheral generation of Tregs was responsible for the observed differences, we utilized T cells isolated form mice carrying a GFP-Foxp3 fusion protein-reporter knockin allele (CD4+Foxp3GFP+) (9). We transferred CD4+Foxp3GFP− cells into B6CD45.1 recipients of B6CD45.1 or B6II−CD45.1 lungs and found that a significantly higher percentage of graft-infiltrating transferred CD4+Foxp3− had acquired Foxp3 expression in B645.1 compared to B6II−45.1 lungs (Fig. 2I+J). We also observed similar differences in Treg generation between B6 and B6II− grafts in a system of defined nominal antigen presentation (Fig. 2J-bottom graph). Collectively, these data indicate that MHCII expression on pulmonary non-hematopoietic cells plays a role in shaping immune responses through the local generation of Tregs in vivo.
Tumor Growth is Attenuated in Lungs Deficient in Non-Hematopoietic MHCII
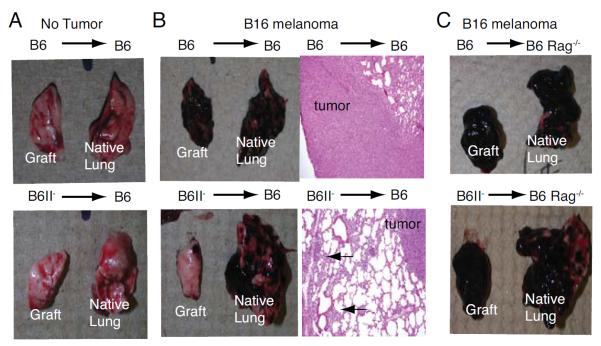
Despite the differences in inflammatory infiltrate (Fig. 2A) both B6 and B6II− grafts remained ventilated and had identical weights one month (Fig. 3A) or six months post-transplantation (data not shown). When injected with B16 melanoma significantly more tumor grew in B6 grafts compared to B6II− grafts as evaluated by gross inspection, lung weight and histology (Fig. 3B). Such differences disappeared if B6 Rag−/− mice were used as recipients demonstrating that the observed differences in tumor growth were due to alterations in adaptive immune responses (Fig. 3C). We observed a higher number of CD4+ and CD8+ T cells in B6II− grafts compared to B6 grafts, a lower percentage of CD4+ T cells expressing Foxp3 (Fig. 3D) and a higher percentage of melanoma antigen-specific CD8+ T cells (Fig. 3E). Collectively, our data provide evidence that MHCII expression by pulmonary non-hematopoietic cells plays a critical role in the local regulation of tumor growth.
Figure 3.
Tumor immune response. Gross appearance and weights of B6 and B6II− grafts and native right lungs (A) without and (B) after injection of B16 melanoma. Hematoxylin and eosin staining of grafts indicating melanoma (labeled as tumor) and inflammatory infiltrates (arrows). (C) Gross appearance and weights of B6 and B6II− grafts four weeks after transplantation into B6 Rag−/− mice injected with B16 melanoma. (D) Absolute numbers of graft-infiltrating CD4+CD90+ T cells and percentage of graft-infiltrating CD4+ T cells expressing Foxp3 in B6 or B6II− grafts at same time points as shown in (B). (E) Absolute numbers of CD8+CD90+ T cells and CD8+ T cells with T cell receptor-specificity for tyrosinase-related protein 2 in B6 or B6II-grafts at same time points as described in (B). Figure summarizes sixteen and nineteen transplanted mice per group in B6 recipients of B6 and B6II− grafts, respectively and seven transplanted mice per group in B6 Rag−/− recipients.
While it has been shown that peripheral expression of MHCII is important for CD4+ T cell homeostasis, most studies have focused on antigen presentation in lymphoid tissue (16, 17). This is in large part due to the fact that secondary lymphoid organs are widely considered the predominant, or even exclusive, site for productive interactions between hematopoietic APCs and CD4+ T cells. Unlike hematopoietic APCs that can traffic from non-lymphoid tissue to secondary lymphoid organs, non-hematopoietic cells are a permanent non-migratory structural component of the lung. Our study now provides compelling in vivo evidence that pulmonary non-hematopoietic cells, through their expression of MHCII, also play a critical role in downregulating CD4+ T cell-mediated immune responses. Our results have implications for the development of therapeutic approaches for multiple pulmonary disease processes.
Supplementary Material
Acknowledgments
supported by NIH R01HL094601, KO8HL083983 and KO8CA131097, AATS, Melanoma Research Foundation and Siteman Cancer Center ACS Internal Research Grant
References
- 1.Wolf AJ, Desvignes L, Linas B, Banaiee N, Tamura T, Takatsu K, Ernst JD. Initiation of the adaptive immune response to Mycobacterium tuberculosis depends on antigen production in the local lymph node, not the lungs. J Exp Med. 2008;205:105–115. doi: 10.1084/jem.20071367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Krupnick AS, Gelman AE, Barchet W, Richardson S, Kreisel FH, Turka LA, Colonna M, Patterson GA, Kreisel D. Murine vascular endothelium activates and induces the generation of allogeneic CD4+25+Foxp3+ regulatory T cells. J Immunol. 2005;175:6265–6270. doi: 10.4049/jimmunol.175.10.6265. [DOI] [PubMed] [Google Scholar]
- 3.Londei M, Lamb JR, Bottazzo GF, Feldmann M. Epithelial cells expressing aberrant MHC class II determinants can present antigen to cloned human T cells. Nature. 1984;312:639–641. doi: 10.1038/312639a0. [DOI] [PubMed] [Google Scholar]
- 4.Katz SC, Pillarisetty VG, Bleier JI, Shah AB, DeMatteo RP. Liver sinusoidal endothelial cells are insufficient to activate T cells. J Immunol. 2004;173:230–235. doi: 10.4049/jimmunol.173.1.230. [DOI] [PubMed] [Google Scholar]
- 5.Jiang G, Yang HR, Wang L, Wildey GM, Fung J, Qian S, Lu L. Hepatic stellate cells preferentially expand allogeneic CD4+ CD25+ FoxP3+ regulatory T cells in an IL-2-dependent manner. Transplantation. 2008;86:1492–1502. doi: 10.1097/TP.0b013e31818bfd13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gereke M, Jung S, Buer J, Bruder D. Alveolar type II epithelial cells present antigen to CD4(+) T cells and induce Foxp3(+) regulatory T cells. Am J Respir Crit Care Med. 2009;179:344–355. doi: 10.1164/rccm.200804-592OC. [DOI] [PubMed] [Google Scholar]
- 7.Krupnick AS, Lin X, Li W, Okazaki M, Lai J, Sugimoto S, Richardson SB, Kornfeld CG, Garbow JR, Patterson GA, Gelman AE, Kreisel D. Orthotopic mouse lung transplantation as experimental methodology to study transplant and tumor biology. Nat Protoc. 2009;4:86–93. doi: 10.1038/nprot.2008.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grusby MJ, Johnson RS, Papaioannou VE, Glimcher LH. Depletion of CD4+ T cells in major histocompatibility complex class II-deficient mice. Science. 1991;253:1417–1420. doi: 10.1126/science.1910207. [DOI] [PubMed] [Google Scholar]
- 9.Fontenot JD, Rasmussen JP, Williams LM, Dooley JL, Farr AG, Rudensky AY. Regulatory T cell lineage specification by the forkhead transcription factor foxp3. Immunity. 2005;22:329–341. doi: 10.1016/j.immuni.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 10.Kreisel D, Krupnick AS, Gelman AE, Engels FH, Popma SH, Krasinskas AM, Balsara KR, Szeto WY, Turka LA, Rosengard BR. Non-hematopoietic allograft cells directly activate CD8+ T cells and trigger acute rejection: an alternative mechanism of allorecognition. Nat Med. 2002;8:233–239. doi: 10.1038/nm0302-233. [DOI] [PubMed] [Google Scholar]
- 11.Gelman AE, Li W, Richardson SB, Zinselmeyer BH, Lai J, Okazaki M, Kornfeld CG, Kreisel FH, Sugimoto S, Tietjens JR, Dempster J, Patterson GA, Krupnick AS, Miller MJ, Kreisel D. Cutting edge: Acute lung allograft rejection is independent of secondary lymphoid organs. J Immunol. 2009;182:3969–3973. doi: 10.4049/jimmunol.0803514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gelman AE, Okazaki M, Lai J, Kornfeld CG, Kreisel FH, Richardson SB, Sugimoto S, Tietjens JR, Patterson GA, Krupnick AS, Kreisel D. CD4+ T lymphocytes are not necessary for the acute rejection of vascularized mouse lung transplants. J Immunol. 2008;180:4754–4762. doi: 10.4049/jimmunol.180.7.4754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Constant SL, Brogdon JL, Piggott DA, Herrick CA, Visintin I, Ruddle NH, Bottomly K. Resident lung antigen-presenting cells have the capacity to promote Th2 T cell differentiation in situ. J Clin Invest. 2002;110:1441–1448. doi: 10.1172/JCI16109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Laufer TM, Fan L, Glimcher LH. Self-reactive T cells selected on thymic cortical epithelium are polyclonal and are pathogenic in vivo. J Immunol. 1999;162:5078–5084. [PubMed] [Google Scholar]
- 15.Madsen L, Labrecque N, Engberg J, Dierich A, Svejgaard A, Benoist C, Mathis D, Fugger L. Mice lacking all conventional MHC class II genes. Proc Natl Acad Sci U S A. 1999;96:10338–10343. doi: 10.1073/pnas.96.18.10338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brocker T. Survival of mature CD4 T lymphocytes is dependent on major histocompatibility complex class II-expressing dendritic cells. J Exp Med. 1997;186:1223–1232. doi: 10.1084/jem.186.8.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bhandoola A, Tai X, Eckhaus M, Auchincloss H, Mason K, Rubin SA, Carbone KM, Grossman Z, Rosenberg AS, Singer A. Peripheral expression of self-MHC-II influences the reactivity and self-tolerance of mature CD4(+) T cells: evidence from a lymphopenic T cell model. Immunity. 2002;17:425–436. doi: 10.1016/s1074-7613(02)00417-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.