Abstract
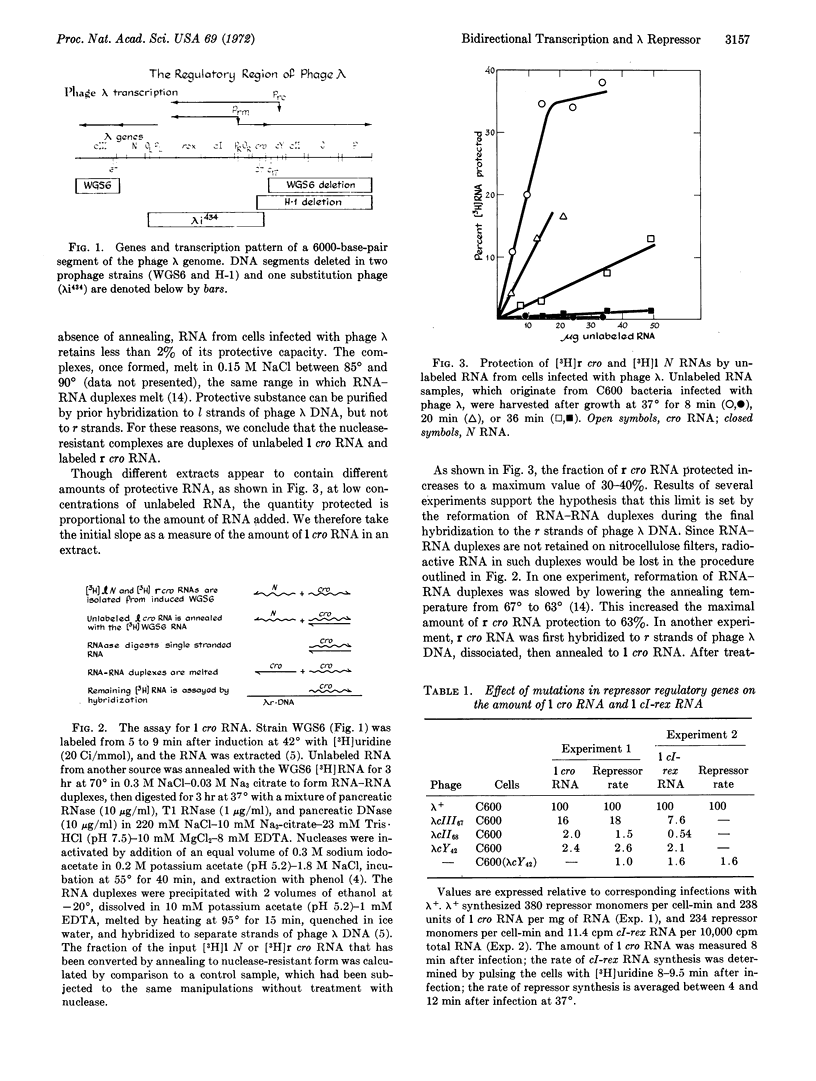
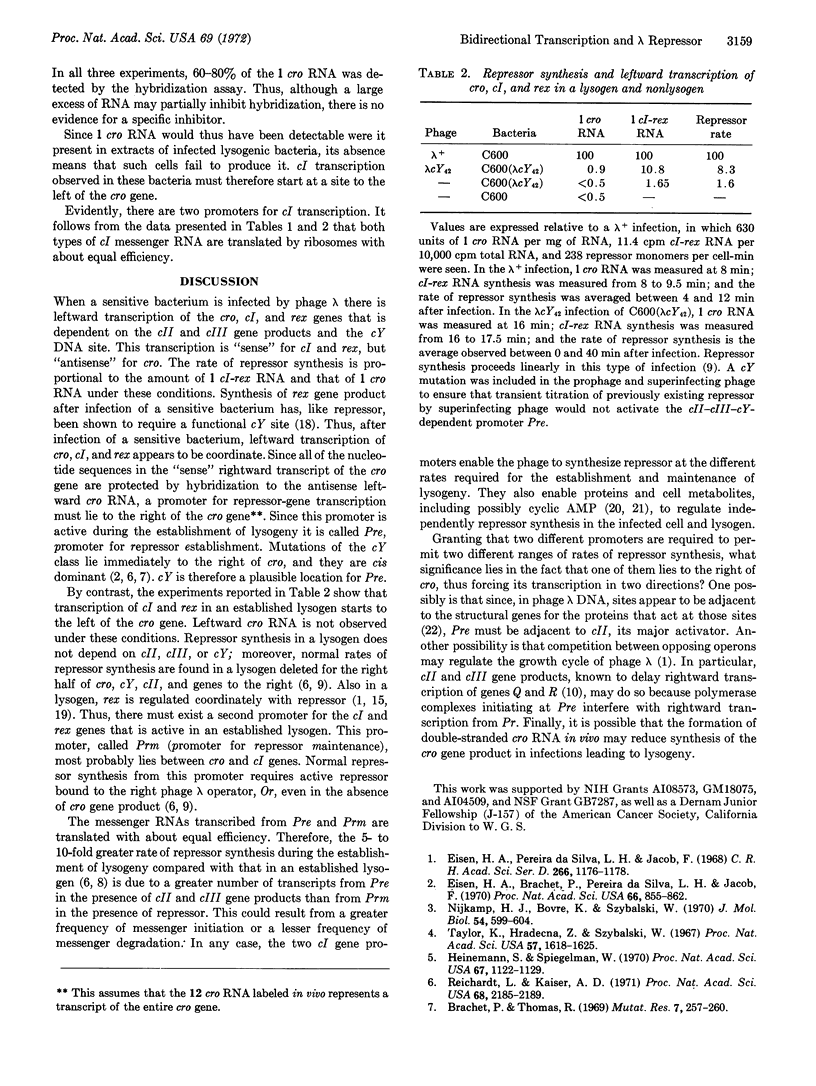
There are two promoters for transcription of gene cI in phage λ, the gene that codes for phage repressor. The promoters, called pre and prm, are located on the distal (pre) and proximal (prm) sides of gene cro, which itself is adjacent to cI. Since cI and cro are transcribed in opposite directions, cI transcription initiating at pre gives rise to an antisense transcript of cro, while cI transcription initiating at prm does not. Pre, active after infection of a sensitive cell, is stimulated by products of phage genes cII and cIII, and may be located at the site defined by the mutant cY. Prm is active in an established lysogen. These conclusions are based on measurements of the rates of synthesis of antisense cro RNA, cI RNA, and repressor protein in infected and lysogenic cells. To measure antisense RNA, an assay based on the formation of nuclease-resistant, double-stranded RNA, specific to the cro region, was developed. These results raise the possibility that bidirectional transcription of cro has a regulatory function in phage λ.
Keywords: antisense RNA, double-stranded RNA, overlapping operons, Escherichia coli
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Astrachan L., Miller J. F. Regulation of lambda rex expression after infection of Escherichia coli K by lambda bacteriophage. J Virol. 1972 Mar;9(3):510–518. doi: 10.1128/jvi.9.3.510-518.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brachet P., Thomas R. Mapping and functional analysis of y and CII mutants. Mutat Res. 1969 Mar-Apr;7(2):257–260. doi: 10.1016/0027-5107(69)90041-4. [DOI] [PubMed] [Google Scholar]
- Echols H., Green L. Establishment and maintenance of repression by bacteriophage lambda: the role of the cI, cII, and c3 proteins. Proc Natl Acad Sci U S A. 1971 Sep;68(9):2190–2194. doi: 10.1073/pnas.68.9.2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisen H., Brachet P., Pereira da Silva L., Jacob F. Regulation of repressor expression in lambda. Proc Natl Acad Sci U S A. 1970 Jul;66(3):855–862. doi: 10.1073/pnas.66.3.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisen H., Pereira da Silva L., Jacob F. Sur la régulation précoce du bactériophage lambda. C R Acad Sci Hebd Seances Acad Sci D. 1968 Mar 11;266(11):1176–1178. [PubMed] [Google Scholar]
- Grodzicker T., Arditti R. R., Eisen H. Establishment of repression by lambdoid phage in catabolite activator protein and adenylate cyclase mutants of Escherichia coli. Proc Natl Acad Sci U S A. 1972 Feb;69(2):366–370. doi: 10.1073/pnas.69.2.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinemann S. F., Spiegelman W. G. Control of transcription of the repressor gene in bacteriophage lambda. Proc Natl Acad Sci U S A. 1970 Nov;67(3):1122–1129. doi: 10.1073/pnas.67.3.1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong J. S., Smith G. R., Ames B. N. Adenosine 3':5'-cyclic monophosphate concentration in the bacterial host regulates the viral decision between lysogeny and lysis. Proc Natl Acad Sci U S A. 1971 Sep;68(9):2258–2262. doi: 10.1073/pnas.68.9.2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMacken R., Mantei N., Butler B., Joyner A., Echols H. Effect of mutations in the c2 and c3 genes of bacteriophage lambda on macromolecular synthesis in infected cells. J Mol Biol. 1970 May 14;49(3):639–655. doi: 10.1016/0022-2836(70)90288-3. [DOI] [PubMed] [Google Scholar]
- Neubauer Z., Calef E. Immunity phase-shift in defective lysogens: non-mutational hereditary change of early regulation of lambda prophage. J Mol Biol. 1970 Jul 14;51(1):1–13. doi: 10.1016/0022-2836(70)90265-2. [DOI] [PubMed] [Google Scholar]
- Nijkamp H. J., Bovre K., Szybalski W. Controls of rightward transcription in coliphage lambda. J Mol Biol. 1970 Dec 28;54(3):599–604. doi: 10.1016/0022-2836(70)90130-0. [DOI] [PubMed] [Google Scholar]
- Reichardt L., Kaiser A. D. Control of lambda repressor synthesis. Proc Natl Acad Sci U S A. 1971 Sep;68(9):2185–2189. doi: 10.1073/pnas.68.9.2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts J. W. Termination factor for RNA synthesis. Nature. 1969 Dec 20;224(5225):1168–1174. doi: 10.1038/2241168a0. [DOI] [PubMed] [Google Scholar]
- Spiegelman W. G. Two states of expression of genes cl, rex, and N in lambda. Virology. 1971 Jan;43(1):16–33. doi: 10.1016/0042-6822(71)90220-0. [DOI] [PubMed] [Google Scholar]
- Szybalski W. Initiation and patterns of transcription during phage development. Proc Can Cancer Conf. 1969;8:183–215. [PubMed] [Google Scholar]
- Taylor K., Hradecna Z., Szybalski W. Asymmetric distribution of the transcribing regions on the complementary strands of coliphage lambda DNA. Proc Natl Acad Sci U S A. 1967 Jun;57(6):1618–1625. doi: 10.1073/pnas.57.6.1618. [DOI] [PMC free article] [PubMed] [Google Scholar]




